Abstract
A previous clinical trial demonstrated that four months of treatment with intranasal insulin improves cognition and function for patients with Alzheimer’s disease (AD) or mild cognitive impairment (MCI), but prior studies suggest that response to insulin treatment may differ by sex and ApoE ε4 carriage. Thus, responder analyses using repeated measures analysis of covariance were completed on the trial’s 104 participants with MCI or AD who received either placebo or 20 or 40 IU of insulin for 4 months, administered by a nasal delivery device. Results indicate that men and women with memory impairment responded differently to intranasal insulin treatment. On delayed story memory, men and women showed cognitive improvement when taking 20 IU of intranasal insulin, but only men showed cognitive improvement for the 40 IU dose. The sex difference was most apparent for ApoE ε4 negative individuals. For the 40 IU dose, ApoE ε4 negative men improved while ApoE ε4 negative women worsened. Their ApoE ε4 positive counterparts remained cognitively stable. This sex effect was not detected in functional measures. However, functional abilities were relatively preserved for women on either dose of intranasal insulin compared with men. Unlike previous studies with young adults, neither men nor women taking intranasal insulin exhibited a significant change in weight over 4 months of treatment.
Keywords: Alzheimer’s disease, insulin, intranasal drug administration, mild cognitive impairment, randomized clinical trials
INTRODUCTION
Insulin is a prolific hormone whose roles within the central nervous system (CNS) have been elaborated over the past several decades. Insulin receptors in the brain are heavily represented in the olfactory bulb, cerebral cortex, hippocampus, hypothalamus, amygdala, and septum [1, 2], areas that influence declarative memory, maintain energy homeostasis, and influence central control of weight [3]. Acutely raising peripheral insulin levels results in elevated insulin levels in the brain and cerebrospinal fluid (CSF), whereas chronically elevated peripheral insulin levels results in a downregulation of insulin transport across the blood-brain barrier that may lead to deficient CNS insulin [4]. Data from animal and human studies have linked peripheral insulin abnormalities with cognitive decline and Alzheimer’s disease (AD) pathology [5–7]. Specifically, disorders of chronic hyperinsulinemia and insulin resistance are associated with less effective insulin within the CNS, which may then lead to impaired neuronal function in memory-dependent areas of the brain and promote AD pathology [8–11].
Evidence from animal and human studies suggests that by supplementing insulin levels in the CNS, the cognitive decline associated with AD is slowed and markers of AD pathology such as amyloid-β (Aβ) levels may be modulated. Long-Evans rats underwent intracerebroventricular (ICV) administration of insulin, heat-deactivated insulin, or saline after a passive avoidance task. Rats who had taken insulin performed with increased latency on the following day, consistent with the theory that CNS insulin improves memory function [12]. When insulin is administered to humans intranasally, insulin is thought to rapidly access the brain, circumventing the blood-brain barrier and bypassing the periphery (see Benedict et al. [13] for a review of intranasal delivery of insulin to the brain). In healthy adults, insulin administered intranasally was reliably detected in CSF, peaking at 30 minutes after administration, with CSF insulin levels still elevated 80 minutes later [14]. In studies of older adults with AD, mild cognitive impairment (MCI), or normal memory, intranasal insulin has been shown to reliably increase verbal memory for memory-impaired adults when dosed acutely [15] and when taking intranasal insulin daily across 21 days [16]. In a follow-up study of older adults with early AD or MCI [17], subjects received intranasally-administered placebo, 20 IU of insulin, or 40 IU of insulin daily for 4 months. Subjects treated with either dose of insulin improved in caregiver-rated daily functioning, and those taking the lower dose (20 IU) improved on delayed verbal memory. Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) [18] scores improved on both doses of insulin, with greater effects observed for younger subjects. These analyses indicate that the intranasal insulin treatment may be a viable treatment for memory disorders, yet some people may benefit more than others. Identification of characteristics that predict which subjects are most likely to respond to treatment and at what doses would greatly facilitate the development of tailored therapeutic regimens.
Animal and human studies utilizing mostly young adults provide some evidence that intranasal insulin treatment response may differ by sex. For instance, male and female rats were found to have differential sensitivity to ICV leptin and insulin [19]. Human studies suggest sex differences in both cognitive and anorexigenic effects of intranasal insulin. Hallschmid et al. [20] administered placebo or 160 IU (4×40 IU) intranasal insulin per day to 40 healthy young adults for 8 weeks each. Men receiving intranasal insulin lost weight and body fat, waist circumference decreased, and plasma leptin levels decreased 27%; alternatively, women gained weight, which the authors attributed to an increase in extracellular water. They posited that CNS insulin acts as a negative feedback signal in the regulation of adiposity in a sex-specific manner. Benedict et al. [21] reported that women, but not men, noted significant improvement on memory and working memory tasks after acute dosing with intranasal insulin. Alternatively, only men had reduced food consumption following intranasal insulin. To explain this finding, Krug et al. [22] studied 14 postmenopausal women in the same study design in order to determine whether low estrogen levels were a prerequisite for the anorexigenic effects of intranasal insulin. The results were identical to those reported by Benedict [21]; thus it was concluded that the differential findings based on sex were not mediated by estrogen.
Previous studies have also shown treatment response to be moderated by ApoE ε4 carriage [15, 23, 24]. It has been found that the link between AD pathology and insulin abnormalities is strongest for ApoE ε4 negative individuals [25]. Accordingly, treatment response was strongest for ApoE ε4 negative individuals in a previous study of intranasal insulin treatment for older adults with mild memory problems or AD [15]. Other studies have found interactions between ApoE ε4 carriage and central insulin or glucose action [25, 26].
This paper examines each of these previously reported moderators of treatment response in secondary analyses of a recently conducted intrasanasal insulin trial [17]. First, we examine whether cognition is differentially facilitated in males versus females, as some previous evidence suggests that females are more sensitive to the cognitive effects of insulin. Then, we examine whether the weight of males or females significantly changes over the course of 16 weeks of intranasal insulin treatment, as some previous evidence suggests that males are more sensitive to the anorexigenic effects of insulin. Finally, we explore whether ApoE ε4 carriage predicts treatment response to intranasal insulin for participants who completed our previous clinical trial, as some evidence suggests treatment response is strongest in ApoE ε4 negative individuals.
MATERIALS AND METHODS
Participants
Methods have been detailed in a previous publication [17]. The primary trial was conducted over a 4-year period and approved by the Institutional Review Boards at the University of Washington and the VA Puget Sound. Informed consent was obtained from all participants. A total of 104 older adults enrolled in the study (64 with amnestic MCI and 40 with mild-moderate AD, Mini-Mental Status Exam (MMSE) >15). Eligibility was determined by a consensus of expert physicians and neuropsychologists following cognitive testing, evaluation of medical history, clinical laboratory screening, and a physical examination. Cognitive diagnoses were made according to modified Petersen criteria for the diagnosis of amnestic MCI [16, 27] and National Institute for Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRA) criteria for AD [28]. For participants with amnestic MCI, cognitive scores were compared with an age- and education-adjusted estimate of the participant’s premorbid ability (Shipley Vocabulary test). Participants whose delayed memory scores deviated at least 1.5 SD from this estimate were considered for the diagnosis of amnestic MCI, which was then determined by expert consensus using all available data, following published criteria. Participants were free from psychiatric disorders, alcoholism, severe head trauma, hypoxia, neurologic disorders other than MCI or AD, renal or hepatic disease, diabetes, chronic obstructive pulmonary disease, and unstable cardiac disease. Participants, study partners, and all study personnel involved with data collection or analysis were blinded to treatment assignment.
Procedures
Participants were randomly assigned to receive a daily dosage of 20 IU insulin (n = 36), 40 IU insulin (n = 38), or placebo (n = 30) administered intranasally for 4 months. Participants were stratified by whether or not they were carriers of the ApoE ε4 allele, a known risk factor for AD that has predicted insulin response in previous studies. ApoE ε4 negative status was defined as the absence of an ε4 allele (95% of subjects who were ApoE ε4 negative were homozygous for ε3, and 5% had one ε2 allele); ApoE ε4 positive status was defined as being homozygous (17%) or heterozygous (83%) for the ε4 allele. Saline or insulin (Novolin R; Novo Nordisk, Princeton, New Jersey) was administered after breakfast and again after dinner with a ViaNase® nasal drug delivery device (Kurve Technology, Bothell, Washington). The device was designed to deliver drugs to the olfactory region to maximize transport into the CNS. It released a metered dose of the study medication into a chamber covering the participant’s nose, which was then inhaled as the participant breathed regularly for two minutes until the prescribed dose was delivered.
Parallel versions of the cognitive testing were administered at baseline, 2 and 4 months of treatment, and 2 months post treatment. The primary analysis compared changes from baseline to 4 months, which will comprise the timeframe for our analyses. Testing was completed in the morning after participants consumed a standard meal. The 4 month testing was completed the morning after the participants’ last dose of study medication, thereby ensuring at least 12 hours had passed since their last dosing. The cognitive protocol contained two co-primary outcome measures: delayed story recall, and the Dementia Severity Rating Scale (DSRS). In delayed story recall [24, 29, 30], participants listened to a recording of a story that contained 44 units of information. They were asked to repeat the story both immediately and after a 20-minute delay. The delayed story recall score was obtained as a sum of the informational bits that were recalled verbatim at the 20-minute delay. The DSRS [31] score was determined after a questionnaire was completed by the study partner. The questionnaire was used to rate the change in the participant’s cognitive, functional, and social status over a specific period of time, with higher scores indicating greater impairment. Two secondary measures were administered: the ADAS-Cog and the Alzheimer’s Disease Cooperative Study-Activities of Daily Living Subscale (ADCS-ADL) The ADAS-Cog [18] includes measures of memory, praxis, orientation, and language, with higher scores indicating greater impairment. The ADSC-ADL scale [32] was completed by the study partner and used to rate the participant’s ability to perform daily activities within the past month, with lower scores indicating greater impairment.
Statistical analyses
Data were analyzed with SPSS version 18. Primary (delayed story recall and DSRS) and secondary (ADAS-Cog and ADCS-ADL) cognitive and functional outcome scores were analyzed; thus, there were four outcomes measured at baseline and after 4-months of treatment. Non-normal outcome distributions were log-transformed. Age, diagnosis (MCI or AD), education, and Modified Mini-Mental State Exam (3MSE) were included as covariates in all analyses; only significant covariates were included in the final models.
To test the hypothesis that treatment response would be moderated by sex, each outcome was subjected to mixed-model repeated-measures analysis of covariance (ANCOVA), including all treatment groups (placebo, 20 IU of insulin, or 40 IU of insulin) and sex groups (male or female) as between-subjects factors and time (baseline and 4 months) as the repeated factor, using the general linear model procedure, type III sums of squares. If a significant 3-way interaction between treatment group, sex, and time emerged, each of the two insulin groups was compared in separate models with the placebo group using repeated measures ANCOVA. ApoE ε4 allele carriage and body mass index (BMI) were explored as potential moderators for treatment response and also as potential moderators for the sex interaction. For BMI, t-tests were utilized to determine whether baseline sex differences were present. Chi-square analyses were utilized for ApoE 4 carriage. Then, ApoE ε4 carriage and BMI were entered into mixed-model ANCOVAs identical to the ones described above.
RESULTS
The means and standard deviations of demographic variables and cell sizes for each group are reported in Table 1. T-tests revealed sex differences for age and BMI, in that men were older and had higher BMIs than women. Age and BMI were examined as covariates in all analyses. For ease of interpretation, the change in adjusted means from Time 1 (pre-treatment) to Time 2 (post-treatment) is graphed to illustrate significant results (Figs. 1–3). The means and standard deviations of the cognitive outcome measures are reported in Supplementary Table 1.
Table 1.
Mean and standard deviation of demographic variables and weight by sex and treatment
| Placebo | Low dose | High dose | ||||
|---|---|---|---|---|---|---|
| Females (n = 13) | Males (n = 17) | Females (n = 14) | Males (n = 22) | Females (n = 18) | Males (n = 20) | |
| Age1 | 72.8 (9.7) | 76.4 (10.4) | 71.1 (8.1) | 73.9 (7.1) | 67.3 (10.0) | 72.2 (7.8) |
| Education | 13.9 (3.0) | 16.3 (3.1) | 14.9 (2.4) | 16.0 (4.1) | 16.9 (2.8) | 15.6 (2.8) |
| 3MSE | 77.8 (19.9) | 89.0 (10.3) | 88.6 (10.2) | 80.6 (15.9) | 80.3 (17.3) | 87.9 (11.4) |
| ApoE ε4 (%yes) | 41.7% | 47.1% | 50.0% | 50.0% | 44.4% | 40.0% |
| BMI1 | 26.2 (3.0) | 28.3 (5.0) | 24.7 (3.1) | 28.0 (4.2) | 26.0 (4.9) | 27.7 (5.0) |
| Plasma insulin1 (ng/ml) | 14.46 (4.10) | 15.59 (7.09) | 15.00 (4.80) | 14.41 (4.92) | 15.00 (5.98) | 16.47 (5.54) |
| Weight: Baseline1 | 146.8 (8.50) | 199.9 (7.81) | 148.7 (8.44) | 185.4 (6.58) | 138.1 (7.36) | 186.0 (6.93) |
| Weight: 4 months1 | 147.0 (8.53) | 201.53 (7.83) | 148.1 (8.47) | 184.8 (6.60) | 141.0 (7.38) | 187.4 (6.95) |
Sex difference, males greater than females, p < 0.05;
BMI, body mass index; 3MSE, Modified Mini-Mental State Exam.
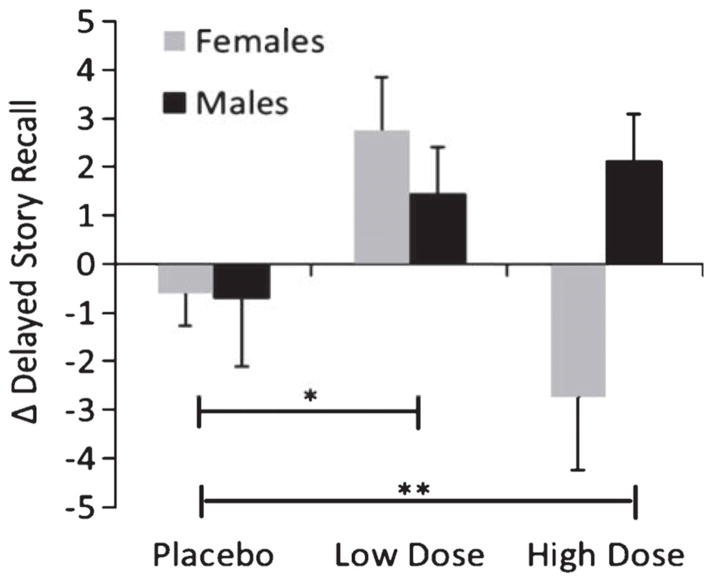
Figure 1.
Mean change scores for delayed story recall from baseline to month 4, with standard errors of the mean (error bars), by sex and treatment group. Placebo indicates saline condition, Low Dose indicates 20 IU intranasal insulin, High Dose indicates 40 IU intranasal insulin. Note: A higher score represents cognitive improvement over time. *Significant treatment effect, p = 0.02. **Significant sex×treatment Interaction, p = 0.05.
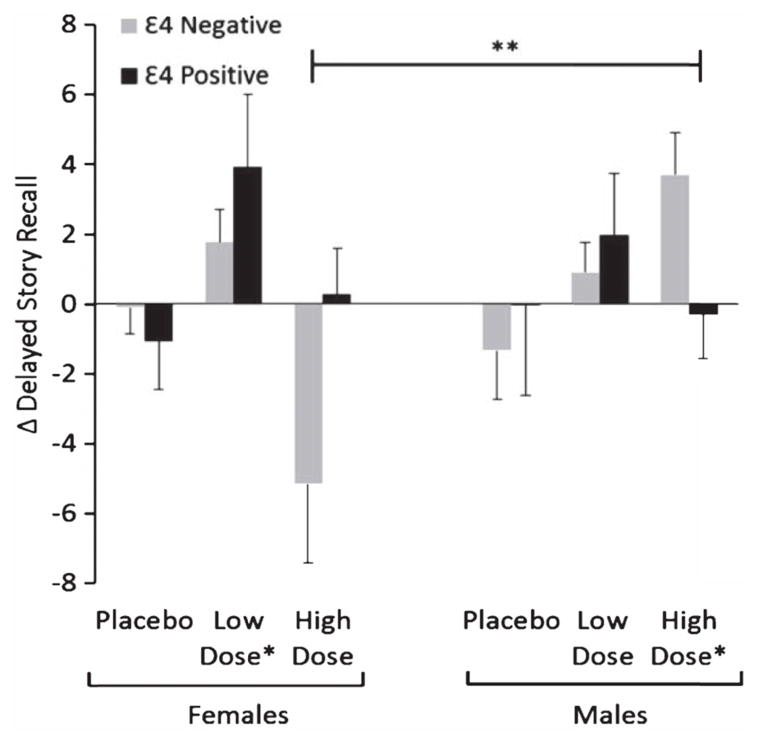
Figure 3.
Mean change scores for delayed story recall from baseline to month 4, with standard errors of the mean (error bars), by sex, treatment group, and ApoE 4 carriage. Placebo indicates saline condition, Low Dose indicates 20 IU intranasal insulin, High Dose indicates 40 IU intranasal insulin. Note: A higher score represents improvement over time. *Significantly improved over placebo (treatment effect). **Significant treatment×sex×ApoE status interaction, p < 0.01.
Sex and treatment response
For delayed story recall, a treatment by sex interaction was observed (p = 0.02; see Fig. 1), along with the previously reported overall treatment effect (p = 0.04). The lower and higher insulin dose groups were then each compared to placebo. Those receiving the low dose of insulin demonstrated improved story recall over time compared with placebo-assigned participants (p = 0.02) and this pattern did not differ for males and females (p = 0.59). In contrast, when the high dose group was compared with placebo, there was no overall treatment effect (p = 0.78), but there was a significant sex interaction (p = 0.05) such that men on the high dose improved, but not women (Fig. 1). For partner-rated functional scores on the DSRS, there were marginally significant treatment effects (p = 0.05 for low dose, p = 0.07 for high dose) but no sex interactions (p = 0.92 for low dose, p = 0.81 for high).
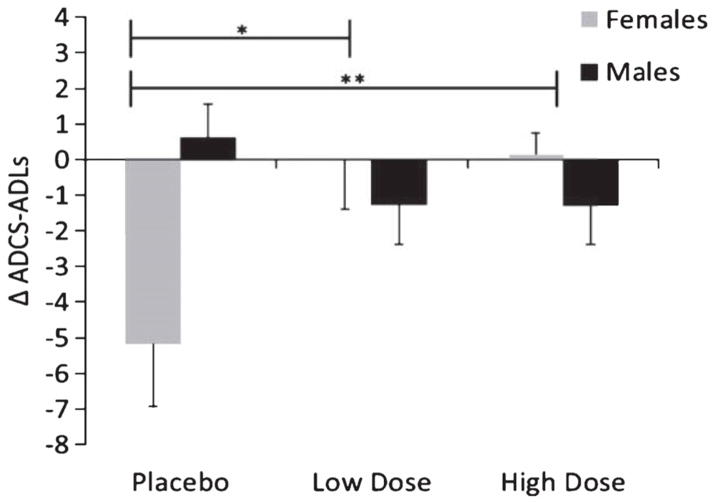
In secondary analyses, no significant sex interactions were observed for the ADAS-Cog (p = 0.47). For the ADCS-ADL scale, there was no overall treatment effect (p = 0.29), but a significant treatment by sex interaction was observed (p < 0.01, see Fig. 2). Follow-up analyses reveal the sex interaction when each insulin group is compared to placebo (p = 0.01 for low dose, p < 0.01 for high). Females but not males benefitted from intranasal insulin.
Figure 2.
Mean change scores for ADCS-ADL from baseline to month 4, with standard errors of the mean (error bars), by sex and treatment group. Placebo indicates saline condition, Low Dose indicates 20 IU intranasal insulin, High Dose indicates 40 IU intranasal insulin. Note: A higher score represents functional improvement over time; scores are adjusted for age. *Significant sex×treatment interaction, p = 0.01. **Significant sex×treatment interaction, p < 0.01.
Apolipoprotein ε4 (ApoE ε4)
ApoE ε4 carriage was not a significant predictor of treatment response for any cognitive or functional outcome. Next, we examined whether the ApoE ε4 carriage interacted with sex to predict study outcomes. Frequency of ApoE ε4 carriage did not differ between males and females (27 of 59 men and 20 of 45 women were ε4 positive). A significant treatment by sex by ApoE ε4 carriage interaction was observed for delayed story recall (p = 0.04; Fig. 3). Follow-up analyses indicate that the sex/allele carriage interaction was specific to the high dose group. ApoE ε4 negative males benefited from the high dose of insulin, whereas ApoE ε4 negative females declined over time on the high dose of insulin (p < 0.01). In co-primary and secondary outcome analysis, ApoE ε4 carriage did not moderate the treatment response or the treatment by sex interaction for the DSRS, ADAS-Cog, or ADCS-ADL.
Weight and BMI
Four months of treatment with intranasal insulin did not significantly alter subjects’ weight, regardless of sex (p = 0.12; Table 1).BMI did not moderate treatment response or the treatment by sex interaction for any cognitive or functional outcome.
Safety
As previously reported, there were no treatment related serious adverse events (SAEs) in the trial, and most AEs were minor, such as mild rhinitis and infrequent nose bleeds [17].
DISCUSSION
Our results indicate that there may be differences in how men and women respond to intranasal insulin treatment for adults with AD and MCI, differences that appear to be dose-dependent. On delayed story memory, both men and women showed cognitive improvement when taking the low dose (20 IU) of intranasal insulin daily for 16 weeks, but only men showed cognitive improvement on the high dose (40 IU). The sex difference was especially distinct for ApoE ε4 negative individuals. On the high dose, ApoE ε4 negative men improved while their ApoE ε4 positive counterparts did not. The opposite was true for women in the high dose group; ApoE ε4 negative women had the poorest cognitive outcome, while ApoE ε4 positive women remained stable. ApoE ε4 positive men and women remained stable on the high dose, without significant improvement or decline. This sex effect was not detected in functional measures. However, functional abilities were relatively preserved for women on either dose of intranasal insulin versus that of men. Unlike previous studies on young adults, neither men nor women taking intranasal insulin exhibited a significant change in weight over 4 months of treatment.
This study has provided additional evidence that the cognitive benefits of intranasal insulin may differ between sexes. Sex-specific findings varied between outcomes, possibly because the cognitive and functional outcome measures tap into different aspects of brain anatomy and function. When taking the high dose of intranasal insulin, men outperformed women on delayed story recall. This primary cognitive outcome is sensitive to hippocampal degeneration [33, 34] and is therefore sensitive to the cognitive decline associated with amnestic MCI and early AD. Sex differences were not observed on the ADAS-Cog, which measures a wider variety of cognitive functions. Women taking intranasal insulin tended to perform better versus placebo on the functional scales, which are most sensitive to later stages of the disease process, when functional changes are observed with greater frequency. Thus, different optimal doses of intranasal insulin may selectively benefit specific brain regions in men versus women. A larger study including brain imaging is needed to speak definitively to these sex differences.
Another interpretation of these data is that a fundamental difference in central insulin sensitivity exists between men and women. The many actions of CNS insulin are fairly recently elucidated and sex differences remain largely unexplored, but there is evidence of sex differences in peripheral insulin metabolism from which we can posit. Although whole-body insulin clearance is greater in women than men, splanchnic insulin extraction is greater in men. Therefore, greater insulin clearance in other peripheral organs accounts for women’s greater whole-body insulin clearance [35]. This finding has led to speculation that women more effectively regulate insulin metabolism in peripheral organs, which is consistent with epidemiological evidence that despite the higher rates of obesity in females, men have higher rates of impaired glucose tolerance and higher incidence of diabetes [36]. There is also evidence that men are more sensitive to the cognitive consequences of peripheral insulin abnormalities. In a recent epidemiological study on individuals with MCI, Cholerton et al. [37] found a greater association between hyperinsulinemia and MCI status in men than in women. Thus, not only do men have more insulin abnormalities, but they are also more sensitive to the cognitive effects of such abnormalities, and therefore these differences may explain why men benefit from the corrective actions of higher doses of intranasal insulin.
There are other health-related factors that may have differed on average between men and women in the study that could account for the sex/dose interaction found in this study. Men were heavier on average, although BMI did not explain the sex differences noted in treatment response on the high dose of insulin. Other possible factors that may vary by sex include the distance from nasal cavity to brain, undetected metabolic differences, and brain size.
In previous work, ApoE ε4 status has been an important predictor of intranasal insulin response [15]. There has been evidence that ApoE ε4 negative individuals are more sensitive to the cognitive consequences of insulin resistance [15, 24] and that a relationship between insulin resistance and dementia risk may be most commonly observed for adults without an ε4 allele [38, 39]. Our current results further extend this finding to suggest that the ApoE ε4 gene interacts with sex to moderate treatment response. As noted above, men have a stronger relationship between insulin abnormalities and MCI status [37], and men have less efficient peripheral insulin clearance than women [35]. Therefore it is possible that the ApoE ε4 negative males benefited from the higher dose of intranasal insulin because they were both more sensitive to intranasal insulin and had greater pre-existing insulin abnormalities. The ApoE ε4 negative females were less sensitive to changes in CNS insulin levels and had fewer pre-existing insulin abnormalities; therefore ApoE ε4 negative women did not benefit from the high dose of intranasal insulin.
There is growing evidence that ApoE ε4 carriers are different than non-carriers in how they respond to treatments such as exercise [40], insulin-sensitizing drugs [41], and many other health-related interventions [42]. However, many studies examining the ApoE ε4 gene do not examine sex as a potential modifier, but instead sex is only utilized as a control variable. Unfortunately, the current pilot trial was underpowered to fully examine these questions; a larger trial is necessary to really be able to speak to treatment response interactions based upon the ApoE ε4 allele.
Subjects’ weight did not significantly change throughout the duration of the treatment. This is an important finding, as several studies administering ICV insulin to rats [19] and intranasal insulin to healthy young adults have found insulin to have an anorexigenic effect, especially for men [20, 21]. This finding is consistent with the work of Hallschmid et al. [43], who determined that obesity creates central resistance to the adiposity signal in intranasal insulin. Thus, this central resistance was likely the reason that older and more overweight subjects would be less likely to respond to the body weight regulating aspects of insulin. It is also possible that the dosage utilized in the current study did not meet the minimum threshold required to significantly alter subjects’ weight. The studies that have noted weight changes in healthy younger adults typically used doses of intranasal insulin exceeding 100 IU per day, which was well above those dosages used in this study.
This study should be viewed in light of its limitations. As a pilot study, the sample size was small, which made interactions more difficult to detect due to lack of power. These analyses were post-hoc and exploratory, and due to small cell sizes (see Table 1), the findings should be interpreted with caution. Finally, although this is the longest trial for intranasal insulin to date, 16 weeks remains a relatively brief duration of treatment and thus it is uncertain what the long-term effects of intranasal insulin administration would be.
CONCLUSIONS
There has been evidence that intranasal insulin is a safe and effective treatment for the memory loss associated with MCI and AD. The current paper suggests that treatment response may vary by sex and ApoE ε4 carriage. The current study justifies further examination by these variables in future work. Learning about differences in treatment response not only provides information that may be helpful in specifying which individuals will be most likely to benefit from this intervention, but it also provides additional information about the possible mechanisms of action for intranasal insulin.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Aging grants AG027415 (to Dr. Craft) and T32 AG000258 (to Dr. Claxton), and the Department of Veterans Affairs. This material is the result of work supported in part by resources from the Veterans Affairs Puget Sound Health Care System, Seattle, Washington.
References
- 1.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 2.Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1990;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- 3.Porte D, Jr, Baskin DG, Schwartz MW. Insulin signaling in the central nervous system: A critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 2005;54:1264–1276. doi: 10.2337/diabetes.54.5.1264. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MR, Porte D., Jr Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides. 1990;11:467–472. doi: 10.1016/0196-9781(90)90044-6. [DOI] [PubMed] [Google Scholar]
- 5.Cole AR, Astell A, Green C, Sutherland C. Molecular connexions between dementia and diabetes. Neurosci Biobehav Rev. 2007;31:1046–1063. doi: 10.1016/j.neubiorev.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Gasparini L, Gouras GK, Wang R, Gross RS, Beal MF, Greengard P, Xu H. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci. 2001;21:2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: Link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 8.Hoyer S, Lee SK, Loffler T, Schliebs R. Inhibition of the neuronal insulin receptor. An in vivo model for sporadic Alzheimer disease? Ann N Y Acad Sci. 2000;920:256–258. doi: 10.1111/j.1749-6632.2000.tb06932.x. [DOI] [PubMed] [Google Scholar]
- 9.Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: Relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- 10.Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, de Leon MJ. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36:811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Biophys Acta. 2009;1792:482–496. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive avoidance task. Physiol Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 13.Benedict C, Frey WH, 2nd, Schioth HB, Schultes B, Born J, Hallschmid M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Exp Gerontol. 2011;46:112–115. doi: 10.1016/j.exger.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: A transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 15.Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH, 2nd, Craft S. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 17.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 19.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- 20.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes. 2004;53:3024–3029. doi: 10.2337/diabetes.53.11.3024. [DOI] [PubMed] [Google Scholar]
- 21.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93:1339–1344. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- 22.Krug R, Benedict C, Born J, Hallschmid M. Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory. J Clin Endocrinol Metab. 2010;95:E468–E472. doi: 10.1210/jc.2010-0744. [DOI] [PubMed] [Google Scholar]
- 23.Aisen PS, Berg JD, Craft S, Peskind ER, Sano M, Teri L, Mulnard RA, Thomas RG, Thal LJ. Steroid-induced elevation of glucose in Alzheimer’s disease: Relationship to gender, apolipoprotein E genotype and cognition. Psychoneuroendocrinology. 2003;28:113–120. doi: 10.1016/s0306-4530(02)00015-x. [DOI] [PubMed] [Google Scholar]
- 24.Craft S, Asthana S, Schellenberg G, Cherrier M, Baker LD, Newcomer J, Plymate S, Latendresse S, Petrova A, Raskind M, Peskind E, Lofgreen C, Grimwood K. Insulin metabolism in Alzheimer’s disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology. 1999;70:146–152. doi: 10.1159/000054469. [DOI] [PubMed] [Google Scholar]
- 25.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: Relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 26.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: Design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 30.Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 1996;17:123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 31.Clark CM, Ewbank DC. Performance of the dementia severity rating scale: A caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10:31–39. [PubMed] [Google Scholar]
- 32.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, Ferris S. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–S39. [PubMed] [Google Scholar]
- 33.Kilpatrick C, Murrie V, Cook M, Andrewes D, Desmond P, Hopper J. Degree of left hippocampal atrophy correlates with severity of neuropsychological deficits. Seizure. 1997;6:213–218. doi: 10.1016/s1059-1311(97)80008-8. [DOI] [PubMed] [Google Scholar]
- 34.Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proc Natl Acad Sci U S A. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen MD, Nielsen S, Gupta N, Basu R, Rizza RA. Insulin clearance is different in men and women. Metabolism. 2012;61:525–530. doi: 10.1016/j.metabol.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vistisen D, Witte DR, Tabak AG, Brunner EJ, Kivimaki M, Faerch K. Sex differences in glucose and insulin trajectories prior to diabetes diagnosis: The whitehall II study. Acta Diabetol. 2012 doi: 10.1007/s00592-012-0429-7. published online ahead of print Sept 16, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Cholerton B, Baker LD, Trittschuh EH, Crane PK, Larson EB, Arbuckle M, Saucedo HH, McCurry SM, Bowen JD, McCormick WC, Craft S. Insulin and sex interactions in older adults with mild cognitive impairment. J Alzheimers Dis. 2012;31:401–410. doi: 10.3233/JAD-2012-120202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craft S, Asthana S, Schellenberg G, Baker L, Cherrier M, Boyt AA, Martins RN, Raskind M, Peskind E, Plymate S. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer’s disease differ according to apolipoprotein-E genotype. Ann N Y Acad Sci. 2000;903:222–228. doi: 10.1111/j.1749-6632.2000.tb06371.x. [DOI] [PubMed] [Google Scholar]
- 39.Profenno LA, Faraone SV. Diabetes and overweight associate with non-APOE4 genotype in an Alzheimer’s disease population. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:822–829. doi: 10.1002/ajmg.b.30694. [DOI] [PubMed] [Google Scholar]
- 40.Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC. Exercise engagement as a moderator of the effects of APOE Genotype on Amyloid Deposition. Arch Neurol. 2012;69:636–643. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanFossen BT, Watson GS, Baker LD, Rhoads KW, Cholerton BA, Reger MA, Plymate SR, Schellenberg G, Craft S. Statin users without an APOE-epsilon4 allele have increased insulin resistance. J Alzheimers Dis. 2010;19:1149–1153. doi: 10.3233/JAD-2010-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nebes RD, Pollock BG, Perera S, Halligan EM, Saxton JA. The greater sensitivity of elderly APOE epsilon4 carriers to anticholinergic medications is independent of cerebrovascular disease risk. Am J Geriatr Pharmacother. 2012;10:185–192. doi: 10.1016/j.amjopharm.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes (Lond) 2008;32:275–282. doi: 10.1038/sj.ijo.0803722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.