Abstract
Purpose
Evaluate effects of prematurity on early optic nerve (ON) development and potential utility of ON parameters as indicators of central nervous system (CNS) development and pathology.
Design
Prospective cross-sectional and longitudinal study.
Participants and Controls
Forty-four preterm infants undergoing retinopathy of prematurity (ROP) screening and fifty-two term infants.
Methods
We analyzed optic nerves from portable handheld spectral domain optical coherence tomography (SDOCT) images (Bioptigen Inc., Research Triangle Park, NC) of 44 preterm and 52 term infants. The highest quality ON scan from either eye was selected for quantitative analysis. Longitudinal analysis was performed at both 31–36 and 37–42 weeks postmenstrual age (PMA). Preterm ON parameters were also assessed for correlation with indicators of cognitive, language and motor development, and CNS pathology.
Main Outcome Measures
Vertical cup diameter (vCupDiam), disc diameter (vDiscDiam), cup-to-disc ratio (vC:D), cup depth, and indicators of neuro-cognitive development and CNS pathology.
Results
At 37–42 weeks PMA, preterm infants had larger vCupDiam and vC:D than term infants (908 vs. 700 μm, p<0.001; 0.68 vs. 0.53 μm, p<0.001), while cup depth and vDiscDiam were not significantly different. Longitudinal changes (n=26 preterm eyes, mean interval 4.7 weeks) in vDiscDiam and in vC:D were an increase of 74 μm (p=0.008) and decrease of 0.05 (p=0.015), respectively. In preterm infants (n=44), periventricular leukomalacia was associated with larger vCupDiam (1084 vs. 828 μm, p=0.005) and vC:D (0.85 vs. 0.63, p<0.001), post-hemorrhagic hydrocephalus was associated with shallower cup (331 vs. 456 μm, p=0.030), and clinical magnetic resonance imaging (MRI) was associated with larger vC:D (0.73 vs. 0.64, p=0.023). In 23 preterm infants with Bayley Scales of Infant Development scores, larger vC:D was associated with lower cognitive scores (p=0.049).
Conclusions
This is the first analysis of ON parameters in premature infants using SDOCT. It demonstrated that by age of “term birth,” vCupDiam and vC:D are larger in preterm infants who were screened for ROP than in term infants. In this prospective pilot study, ON parameters in these preterm infants appear to weakly associate with CNS pathology and future cognitive development. Future prospective studies with larger numbers are necessary before further conclusions can be made.
To date, our understanding of perinatal optic nerve (ON) development comes from histopathology studies, which have shown that the in-utero ON axonal count peaks around 16–17 weeks gestational age and decreases until around 32 weeks.1 Additional histopathology studies have shown that the optic disc and retrobulbar nerve reach 75% of adult size by term birth,2 that both correlate with globe anteroposterior diameter,2 and that the retro-bulbar nerve grows during infancy due to myelination.2, 3
Imaging technologies such as digital fundus photography and optical coherence tomography (OCT) have allowed for in vivo studies of the living optic nerve. OCT studies in school-aged children suggest that history of and characteristics common to prematurity are associated with decreased optic neuronal tissue.4–6 Other studies have found racial variation, with black children having larger cup-to-disc ratios and thicker retinal nerve fiber layer (RNFL).7 Additionally, both adult and pediatric studies have shown intracranial pathologies to be associated with thinner RNFL.8–10 The only study comparing infant ON parameters to measurements associated with birth status has been a Retcam (Clarity Medical Systems, Inc., Pleasanton, CA) study assessing the effect of low birth weight in term infants.11 To date, we are not aware of OCT studies that address how prematurity affects ON development during infancy (PubMed MeSH terms infant AND optical coherence tomography AND optic nerve).
In the present study, we use spectral domain OCT (SDOCT) to explore whether differences exist during infancy between preterm and term infant ON measurements and to assess the relationship between these parameters and indicators of central nervous system (CNS) pathology.
METHODS
This Health Insurance Portability and Accountability Act-compliant, prospective study was approved by the Duke University Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. From April 2009 to October 2012, SDOCT images were obtained in 90 preterm infants at the Duke Neonatal Intensive Care Unit (NICU) and 60 term infants in the Duke Birthing Center. Preterm infants were eligible if undergoing retinopathy of prematurity (ROP) screening, which required either birth at ≤ 30 weeks gestational age or a birth weight of ≤ 1500 grams. Term infants born at ≥ 36 weeks gestational age and without known medical problems were eligible. Fifty-eight of these sixty term infants were also in a report by Allingham et al.12
Birth weight, gestational age, race and ethnicity, sex, and ROP status were recorded at the initial imaging session. Subjects were defined as Asian, black, Hispanic, and white. Similar to a study by Knight et al,13 Hispanic was considered a racial group, though subjects of Hispanic ethnicity and black race were considered to be black.
SDOCT volumes consisting of multiple vertical B-scans were captured with an 840-nm wavelength portable SDOCT system (Bioptigen Inc., Research Triangle Park, NC).14 Scans with sufficient focus and alignment to allow identification of Bruch’s membrane opening (BMO) and the deepest point of the optic cup were eligible; based on a subjective assessment of focus, resolution, centering of the optic nerve, and lack of tilt, the highest quality volume scan of the ON from one eye of each subject was selected for quantitative analysis. From the volume, B-scans with the largest vertical cup diameter (vCupDiam), disc diameter (vDiscDiam), and cup depth were selected and de-identified for analysis (up to 3 B-scans from the same volume if necessary). For the primary analysis of term vs. preterm infants, subjects had at least one adequate scan from 37–42 weeks postmenstrual age (PMA). Analysis of longitudinal ON growth included eyes of 26 preterm infants from the primary analysis who also had imaging at 31–36 weeks PMA (selecting the earliest adequate imaging session if there were multiple).
For the analysis of ON parameters and markers of CNS pathology, we reviewed medical records of preterm infants in the primary study for: Bayley Scales of Infant and Toddler Development-Third Edition (Bayley) scores at 18–22 months corrected age, drop off in head circumference growth from NICU discharge till follow-up at 18–22 months corrected age, receipt of magnetic resonance imaging (MRI), presence and grade of intraventricular hemorrhage (IVH) on ultrasound, presence of periventricular leukomalacia (PVL) by ultrasound or MRI, head circumference at NICU admission and discharge, and 5 minute APGARs. Bayley scores were chosen to measure CNS development because they are standardized, norm-referenced measures shown to have adequate reliability and validity, and are considered the gold standard of infant and toddler development assessment tools.15
Quantitative analysis required a MATLAB script, which allowed the masked grader (AYT) to mark the vertical disc diameter (vDiscDiam), vertical cup diameter (vCupDiam), and cup depth (Figure 1). The vertical cup-to-disc ratio (vC:D) was then calculated. A senior masked grader and faculty audited all scans to confirm the markings. Vertical rather than horizontal ON parameters were measured since vertical scans were prioritized to image retinal vessels for assessment of plus disease in ROP,16 and since they are more commonly used in clinical assessments for glaucoma17 and less affected by nerve tilt. Lateral measurements within the scans were corrected using an age-based estimate of the infant eye’s axial length.14 To evaluate inter-grader measurement reliability, two graders (AYT and ALR) measured the same ten randomly-chosen masked scans. To evaluate inter-scan measurement reliability, 1 grader (AYT) measured 2 randomly-chosen masked scans from 10 unique imaging sessions.
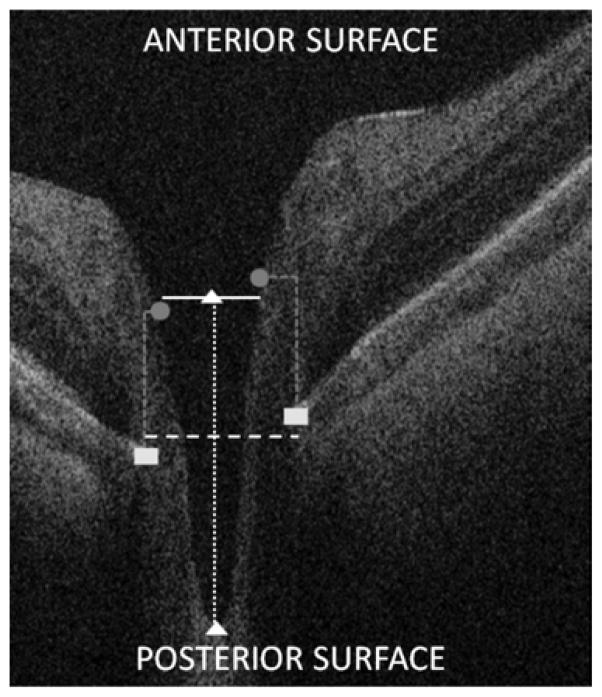
Figure 1.
Measurement of optic nerve parameters in a spectral domain optical coherence tomography (SDOCT) scan of an infant optic nerve. Using cross-sectional SDOCT B-scans, vertical disc diameter (vDiscDiam, dashed white line) and vertical cup diameter (vCupDiam, solid white line) were measured parallel to the anterior and posterior surface of the scan (not on the diagonal); vertical cup-to-disc ratio (vC:D) was calculated; cup depth (dotted white line) was measured parallel to the A-scans within the B-scan from the plane of the cup to the top of the lamina cribrosa (white triangles). To accommodate for image tilt in the B-scans, the plane of the disc (dashed white line) was defined as the plane halfway between the two points (white rectangles) defining Bruch’s membrane opening (BMO), which can be visualized as the outer edge of the retinal pigmented epithelium. The plane of the cup (solid white line) was defined as the plane halfway between the 2 points (gray circles) marking the cup border, set at a plane 200 μm superior to the BMO markings; the same 200 μm offset was used in the Cirrus Optic Disc Cube protocol for SDOCT.13 To assess the effect a change in offset between cup and disc planes would have on our measurements, we conducted secondary analyses of the same scans using 150 instead of 200 μm as the offset.
Intraclass correlation coefficients (ICC) were calculated to assess inter-grader and inter-scan reliability. Two-tailed Wilcoxon rank-sums tests assessed for ON parameter variation by gender and eye in preterm and term groups. Two-way analysis of variance models addressing 1) ROP status and race and 2) term status and race assessed for ON parameter variation; models without interaction terms were used as the terms were insignificant, indicating that neither the difference in ROP status nor term status was different for the races. Kruskal-Wallis and Tukey’s tests assessed for variation in gestational age and ON parameters between racial groups. Bivariate analyses assessed for correlation between ON parameters and gestational age as well as birth weight. One-tailed Wilcoxon signed-rank tests assessed for ON longitudinal development. One-tailed Wilcoxon rank-sums tests assessed for associations between ON parameters and indicators of CNS pathology. Linear models assessed for correlation between ON parameters and Bayley scores. A p-value of <0.05 was considered significant.
RESULTS
Demographics
For the primary analysis, 26 of 90 preterm and 4 of 60 term infants imaged were excluded due to lack of imaging at 37–42 weeks PMA and 20 preterm and 4 term infants due to lack of adequate scans, leaving 44 preterm and 52 term infants. Preterm infants had lower gestational age (26.4 vs. 39.1 weeks) and birth weight (872 vs. 3345 grams) than term infants (Table 1). The term group had significantly more Hispanics (36.5% vs. 6.8%) and female infants (59.6% vs. 36.4%).
Table 1.
Population and baseline characteristics of preterm and term infants at 37–42 weeks PMA
| Parameter | Preterm Infants (n=44) | Term Infants (n=52) |
|---|---|---|
| PMA at Imaging (weeks), mean (SD) | 39.0 (1.8) | 39.2 (1.1) |
| Gestational Age (weeks), mean (SD)* | 26.4 (2.4) | 39.1 (1.3) |
| Birth Weight (grams), mean (SD)* | 872 (245) | 3345 (447) |
| Gender, n (%)* | ||
| Male | 28 (63.6%) | 21 (40.4%) |
| Female | 16 (36.4%) | 31 (59.6%) |
| Eye, n (%) | ||
| Right | 21 (47.7%) | 25 (48.1%) |
| Left | 23 (52.3%) | 27 (51.9%) |
| Stage 3 ROP/requiring laser, n (%)* | 18 (40.9%) | 0 (0.0%) |
| Race, n (%)* | ||
| Black | 18 (40.9%) | 13 (25.0%) |
| Asian | 0 (0.0%) | 1 (1.9%) |
| White | 23 (52.3%) | 19 (36.5%) |
| Hispanic | 3 (6.8%) | 19 (36.5%) |
PMA, post-menstrual age; ROP, retinopathy of prematurity
SD, standard deviation
Statistically significant difference between preterm vs. term infants
Forty-four preterm infants in the primary analysis were included in the correlation of ON parameters with indicators of CNS pathology. Analysis of Bayley scores and head circumference growth included 23 of 44 preterm infants (11 lost to follow-up; 10 not yet 18–22 months corrected age); two infants were too neurologically impaired for Bayley assessment; their scores were recorded as 1 point below the lowest possible.
Reproducibility
Between two readers (AYT and ALR), ICC for vDiscDiam was 0.75 (95% confidence interval (CI), 0.30–0.93), vCupDiam was 0.97 (0.89–0.99), vC:D was 0.90 (0.68–0.98), and cup depth was 0.89 (0.64–0.97). ICC of same-day scans read by one grader (AYT) for vDiscDiam was 0.89 (0.65–0.97), vCupDiam was 0.99 (0.97–1.00), vC:D was 0.97 (0.88–0.99), and cup depth was 0.81 (0.44–0.95).
Optic nerve parameters in preterm versus term infants and by race
Neither preterm nor term ON parameters differed significantly when stratified by gender or eye. In the preterm group, ON parameters did not significantly correlate with birth weight (Table 2, Tong, available at www.aaojournal.org) or gestational age (p=0.427). Gestational age did not differ significantly between races for preterm (p=0.171) or for term infants (p=0.448).
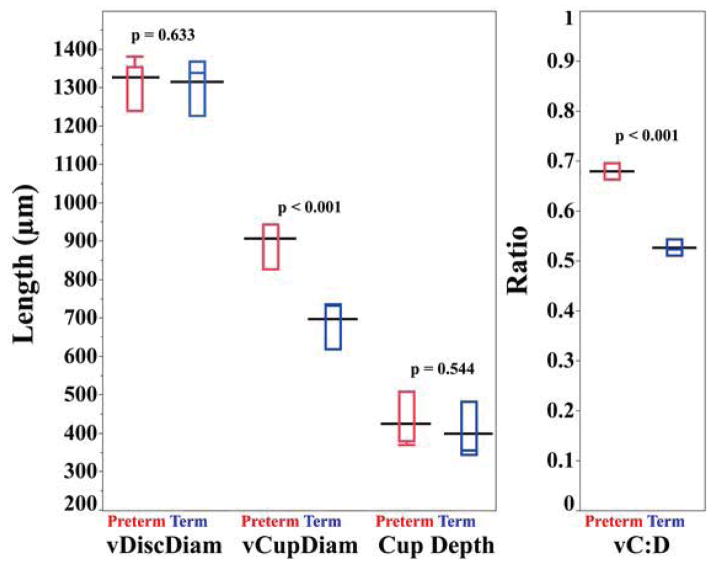
Preterm infants had larger vCupDiam than term infants at 37–42 weeks (adjusted mean ± standard error (SE); 908 ± 43 vs. 700 ± 37 μm, p<0.001) while vDiscDiam did not differ significantly (1327 ± 21 vs. 1313 ± 18 μm, p=0.633). Thus, vC:D was larger in preterm than term infants (0.68 ± 0.03 vs. 0.53 ± 0.02, p<0.001) (Figure 2). Cup depth did not differ significantly between preterm and term infants (423 ± 32 vs. 398 ± 27 μm, p=0.544); however, preterm infants with ROP stage 3 or treatment had a shallower cup than those without (403 ± 46 vs. 509 ± 44 μm, p=0.049). No other parameters differed significantly by ROP status.
Figure 2.
Comparison of optic nerve parameters in preterm (red) vs. term (blue) infants at 37–42 weeks postmenstrual age. The black line represents the adjusted mean for each group. The red (blue) box and whisker plots represent the median, quartiles, maximums and minimums of each optic nerve parameter for the preterm (term) group. Vertical cup diameter (vCupDiam) and vertical cup-to-disc ratio (vC:D) are significantly larger in preterm than in term infants. There was no significant difference in vertical disc diameter (vDiscDiam) and cup depth between the two groups. The repeat analysis with cup offset 150 μm (instead of 200 μm) above the disc plane revealed the same significant findings by term status and race (data not shown).
When term and preterm infants were combined, both vDiscDiam and cup depth significantly differed when comparing across black, Hispanic, and white races (1348 ± 23 vs. 1376 ± 28 vs. 1236 ± 19 μm, p<0.001; 500 ± 35 vs. 361 ± 44 vs. 372 ± 30 μm, p=0.011, respectively). In preterm infants alone (insufficient Hispanics to analyze), vDiscDiam and cup depth differed significantly between black and white preterm infants (mean ± standard deviation (SD); 1352 ± 117 vs. 1244 ± 144, p=0.023 and 515 ± 168 vs. 366 ± 184 μm, p=0.019, respectively) (Table 3). In the term infants alone, vDiscDiam alone was significantly different between black, Hispanic, and white term infants (Table 3); using Tukey’s test, vDiscDiam significantly differed between black and white (1346 ± 102 vs. 1227 ± 95 μm, p=0.017) as well as between Hispanic and white term infants (1369 ± 141 vs. 1227 ± 95 μm, p=0.001); no other ON parameters differed significantly by race.
Table 3.
Characterization of optic nerve parameters by race in preterm and term infants at 37–42 weeks PMA
| Parameter | Preterm (n=41*) | Term (n=51) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Black (n=18) Mean (SD) |
White (n=23) Mean (SD) |
P-Value ** | Black (n=13) Mean (SD) |
Hispanic (n=19) Mean (SD) |
White (n=19) Mean (SD) |
P-Value ** | |
| vDiscDiam (μm) | 1352 (117) | 1244 (144) | 0.023 | 1346 (102) | 1369(141) | 1227 (95) | 0.001 |
| vCupDiam (μm) | 942 (241) | 805 (261) | 0.118 | 747 (211) | 699 (341) | 655 (168) | 0.430 |
| vC:D | 0.69 (0.14) | 0.65 (0.19) | 0.408 | 0.55 (0.13) | 0.50 (0.22) | 0.53 (0.13) | 0.838 |
| Cup Depth (μm) | 515 (168) | 366 (184) | 0.019 | 483 (204) | 328 (220) | 381 (195) | 0.121 |
3 Hispanic subjects excluded due to insufficient numbers to analyze
Kruskal-Wallis test
vDiscDiam, vertical disc diameter; vCupDiam, vertical cup diameter; vC:D, vertical cup-to-disc ratio
SD, standard deviation
Optic nerve longitudinal growth in preterm infants
Between 31–36 weeks and 37–42 weeks PMA (n=26, mean interval of 4.7 weeks), mean vDiscDiam significantly increased by (mean ± SE) 74 ± 28 μm (p=0.008) while vC:D decreased by 0.05 ± 0.02 (p=0.015) (Table 4, available at www.aaojournal.org, and Table 5). vCupDiam and cup depth did not significantly change.
Table 5.
Optic nerve parameters in 26 preterm infants when aged 31–36 weeks vs. 37–42 weeks PMA*
| Parameters | 31–36 weeks PMA Mean (SD) |
37–42 weeks PMA Mean (SD) |
Mean Difference Overall (SE)* | % Change Overall | Estimated Mean Difference per Week (μm) | Estimated % Change per Week | P-Value** |
|---|---|---|---|---|---|---|---|
| vDiscDiam (μm) | 1254 (193) | 1328 (137) | 74 (28) | 5.9% | 16 | 1.3% | 0.008*** |
| vCupDiam (μm) | 918 (219) | 915 (267) | −4 (21) | −0.4% | −1 | −0.1% | 0.431 |
| vC:D | 0.74 (0.16) | 0.69 (0.17) | −0.05 (0.02) | −6.8% | −0.01 | −1.4% | 0.015*** |
| Cup Depth (μm) | 432 (148) | 461 (170) | 28 (26) | 6.5% | 6 | 1.4% | 0.858 |
Average weeks between imaging sessions = 4.7
Wilcoxon Rank Sums test, 1-tailed
Repeat analysis with cup offset 150 μm above the disc plane; p-values for vDiscDiam and vC:D changed to p=0.012 and p=0.053, respectively
vDiscDiam, vertical disc diameter; vCupDiam, vertical cup diameter; vC:D, vertical cup-to-disc ratio
SD, standard deviation; SE, standard error
Optic nerve parameters and indicators of CNS pathology
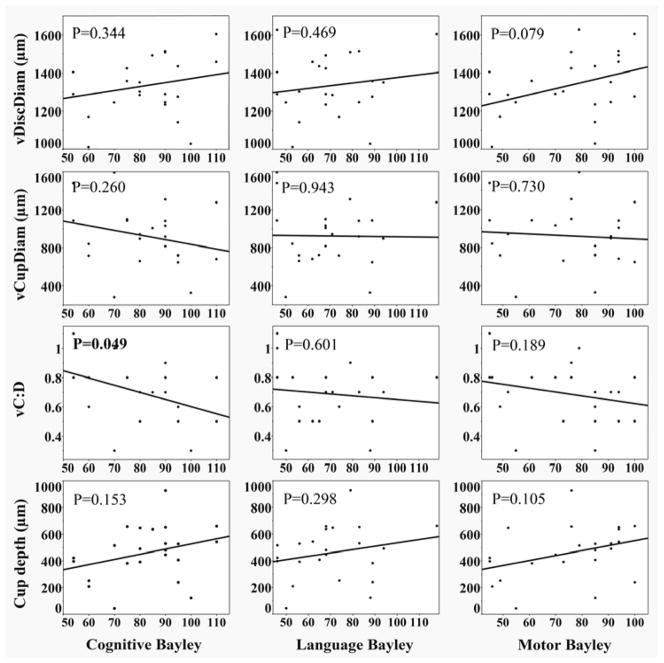
Among 44 preterm infants, presence of post-hemorrhagic hydrocephalus was associated with less cup depth (Table 6). Diagnosis of PVL alone (n=10; 7/10 cystic, 3/10 undocumented severity) as well as PVL or post-hemorrhagic hydrocephalus (n=12) was associated with larger vCupDiam and larger vC:D (Table 6). Infants who received an MRI (n=19) had larger vC:D (Table 6). Lower 5 minute APGAR scores were weakly associated with larger vCupDiam and vC:D (slope= −55.5, R2=0.125, p=0.019; slope=−0.04, R2=0.178, p=0.004, respectively). Smaller head circumference at NICU admission was associated with larger vDiscDiam and cup depth (slope= −14.4, R2=0.101, p=0.036; slope= −18.5, R2=0.104, p=0.033, respectively). In the 23 infants with Bayley scores, larger vC:D was associated with lower cognitive Bayley scores (R2=0.172, p=0.049); no associations of language or motor Bayley scores and ON parameters were significant (Figure 3).
Table 6.
Relationship between preterm optic nerve parameters at 37–42 weeks with indicators of central nervous system pathology
| Parameters | vDiscDiam (μm) | vCupDiam (μm) | vC:D | Cup Depth (μm) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean (SD) | P-value* | Mean (SD) | P-value* | Mean (SD) | P-value* | Mean (SD) | P-value* | |
|
|
|
|
|
|||||
| IVH | ||||||||
| Y (24/44) | 1301 (145) | 0.786 | 916 (189) | 0.396 | 0.70 (0.19) | 0.444 | 430 (207) | 0.671 |
| N (20/44) | 1294 (150) | 852 (257) | 0.65 (0.15) | 443 (159) | ||||
| Post-hemorrhagic | ||||||||
| Hydrocephalus | ||||||||
| Y (7/44) | 1239 (106) | 0.149 | 1006 (297) | 0.344 | 0.81 (0.20) | 0.112 | 331 (59) | 0.030 |
| N (37/44) | 1309 (151) | 864 (267) | 0.65 (0.16) | 456 (194) | ||||
| PVL | ||||||||
| Y (10/44) | 1275 (152) | 0.674 | 1084 (222) | 0.005 | 0.85 (0.13) | <0.001 | 447 (209) | 0.654 |
| N (34/44) | 1304 (146) | 828 (262) | 0.63 (0.15) | 433 (180) | ||||
| PVL or post-hemorrhagic | ||||||||
| Hydrocephalus | ||||||||
| Y (12/44) | 1261 (141) | 0.280 | 1022 (248) | 0.042** | 0.81 (0.15) | 0.004 | 421 (199) | 0.292 |
| N (32/44) | 131 (147) | 836 (269) | 0.63 (0.16) | 442 (182) | ||||
| MRI performed | ||||||||
| Y (19/44) | 1305 (162) | 0.713 | 966 (314) | 0.066 | 0.73 (0.19) | 0.023 | 425 (212) | 0.485 |
| N (25/44) | 1292 (136) | 827 (227) | 0.64 (0.15) | 445 (165) | ||||
| Head Circumference | ||||||||
| Drop Off | ||||||||
| Y (5/23) | 1349 (141) | 0.823 | 941 (262) | 0.551 | 0.70 (0.16) | 0.8815 | 527 (254) | 0.602 |
| N (18/23) | 1331 (172) | 917 (342) | 0.68 (0.20) | 442 (188) | ||||
Wilcoxon Rank Sums test, 1-tailed
On repeat analysis with cup offset 150 μm above the disc plane, p=0.069; all other statistical values remain unchanged in significance
Y, present; N, absent
IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; MRI, magnetic resonance imaging
vDiscDiam, vertical disc diameter; vCupDiam, vertical cup diameter; vC:D, vertical cup-to-disc ratio
SD, standard deviation
Figure 3.
Correlation between optic nerve parameters in preterm infants at 37–42 weeks postmenstrual age (PMA) (n=23) and cognitive, language, and motor Bayley scores (Bayley Scales of Infant and Toddler Development-Third Edition15). The Bayley assesses infant and toddler development across cognitive, language and motor domains, and composite standard scores can be obtained for each of these domains. These scores are based on a mean of 100 and a standard deviation of 15. Scores ranging from 85 to 115 are considered within normal limits. Mild delay is defined as a score between 85 and 70 and significant delay is defined as a score below 70.
DISCUSSION
To our knowledge, this is the first study using handheld SDOCT to characterize preterm infant ON development in the NICU. We found that vCupDiam and vC:D are larger in preterm compared to term infants by 37–42 weeks PMA; these findings remained true in repeat analyses using a cup plane set 150 μm (instead of 200 μm) above the disc plane. In addition, vDiscDiam increases while vC:D decreases from 31–36 to 37–42 weeks PMA. Furthermore, in a limited pilot analysis, ON parameters weakly correlate with indicators of CNS development and pathology. Larger vCupDiam correlated with a diagnosis of PVL, while larger vC:D correlated with diagnosis of PVL, receipt of an MRI, lower 5 minute APGAR scores, and lower cognitive Bayley scores.
Preterm vs. Term Comparison
Several studies have assessed ON parameters in school-aged children for correlation with preterm birth and characteristics associated with prematurity, such as low birth weight and small head circumference (Table 2, available at www.aaojournal.org).4–6, 17–21 The three studies published using fundus photographs have reported conflicting results.18, 19, 21 Although the older study by Hellstrom et al18 found no correlation between preterm birth and optic disc area, a follow-up study by the same group19 and one by Wikstrand et al21 found preterm birth to be associated with smaller optic disc area and less neuronal rim. On the other hand, both Hellstrom et al studies18, 19 found no difference in optic cup area between term and preterm groups, while Wikstrand et al21 noted a significant association between preterm birth and larger cup area. While not entirely in agreement, the four studies using OCT4–6, 20 have found more consistent results suggesting that preterm birth, low birth weight and small head circumference are associated with measures reflecting less ON axonal tissue (Table 2). Akerblom et al4 and Wang et al5 found a significant correlation between preterm birth and thinner RNFL, while Wang et al20 did not. Although these studies add to our understanding of infant ON development, more research is needed to address whether the differences found in school-aged children are present at birth or are a result of later growth impairment.
Of the four published studies on infant ON parameters,2, 11, 22, 23 none used OCT. Only Kandasamy et al11 (n=35 term infants) assessed for relationships between ON measurements and parameters associated with preterm birth using Retcam, and found no association between low birth weight and disc area (Table 2). However, cup areas were not measured.11 A study by Hackl et al22 also attempted such measures but reported a high possibility of cup measurement error using Retcam photos.
Our study is the first to use OCT to examine the relationships between ON parameters and preterm birth, low birth weight, and gestational age in infants. Our data, which showed larger vCupDiam and vC:D in preterm compared to term infants by age of term birth, strongly suggests that preterm birth negatively affects ON development at a very early age. This is likely due to an interruption of ON ganglion cell maturation, as proposed by Samarawickrama et al.6 Hellstrom et al19 postulated that this might be due to post-natal differences in environment during the 16–33 week gestational age window as the optic nerve undergoes an axonal count increase and then apoptosis.1 For the preterm infant, there may be more than average axonal apoptosis. Furthermore, as suggested by Jacobson et al,24 for preterm infant with CNS development abnormalities, further ON axonal loss could result from trans-synaptic degeneration. Other as-yet undescribed mechanisms, such as ON hypoplasia,25 may also participate in disruption of ON development in these preterm infants.
Data from this study may serve as the beginning of a normative dataset for ON parameters in preterm infants using SDOCT. Our average preterm vDiscDiam (mean ± SD, 1327 ± 21 μm; average gestational age, 26.4 weeks) is comparable to Rimmer et al’s2 results from 20 cadaver eyes of infants born at < 20 weeks PMA. In contrast, DeSilva et al26 found slightly larger mean vDiscDiam in their 51 term and preterm subjects (1410 ± 19 μm, average gestational age, 30.1 weeks) using Retcam. However, as no average preterm gestational age was reported, it is difficult to compare their findings to our own. More normative data about ON development is needed, which will help differentiate between pathologies such as optic pathway pathology, pediatric glaucoma, and the normal effects of prematurity.9, 27–29
Our interest in assessing cup depth stemmed from previous reports of increased “cupping” in children with history of prematurity,21 and of heaped optic nerves visible on SDOCT images but not on fundus exam in children with ocular albinism.23 While we found no difference in preterm vs. term cup depth, we did find a shallower cup in infants with history of hydrocephalus and eyes with more advanced ROP. Although vascular congestion of plus disease may impact cup depth, advanced ROP has been associated with more serious CNS disease. No comparison data exists since cup depth could not be measured by previous imaging modalities. However, the reproducibility of our significant findings upon repeat analyses at two different offsets of the cup to disc plane supports the validity of our results.
We found no significant correlation between ON parameters and birth weight in the preterm group (Table 2). This differs from Samarawickrama et al6 and Wang et al’s5 findings, which correlated lower birth weight with smaller disc diameters and areas, larger cup diameters and areas, and thinner RNFL (Table 2). However, their analyses of ON parameters by low birth weight were analyzed with an unspecified number of term versus preterm children.5, 6 As preterm children tend to have low birth weights, analysis without correcting for preterm status is a significant confounding factor.
Previous reports in adults,30–34 school-aged children,6, 7 and term infants12 have found racial variations in ON parameters. Whereas Rimmer et al’s2 histopathology study of subjects ages 4.8 months gestation to 21.9 years found no significant racial differences in vDiscDiam and El-Dairi et al’s7 OCT study of school-aged children found larger vC:D and thicker RNFL in black versus white children, our findings differed from both in that vDiscDiam (p<0.001) and cup depth (p=0.011), but not vC:D, differed significantly across a comparison of all races. Our vDiscDiam measurements, when grouped by race, were fairly consistent with those found by Allingham et al,12 who used a subset of the term infant eyes analyzed in our study. Our finding of a larger vDiscDiam in Hispanic versus white term infants (p=0.001) was also true in Allingham et al’s12 study (p=0.02). Our finding of larger vDiscDiam in black versus white term infants reached significance (p=0.017), while Allingham et al12 showed a trend (p=0.07), which could be due to a wide vDiscDiam ICC confidence interval and small sample size when the groups are subdivided by race. Additionally, Allingham et al12 reported a larger vCupDiam in black versus white term infants (mean ± SD; 560 ± 230 vs. 440 ± 150 μm, p=0.03) while our study did not (747 ± 211 vs. 655 ± 168 μm, p=0.578), which is likely due to different image analysis methods (they not seek the B-scan with the largest cup as we did, but rather measured disc and cup in the same B-scan). These differences all point towards a need for caution in infant optic nerve analysis by small subgroups and different analysis methodologies, as well as a need for further larger studies.
This pilot study did not address the potential relationships between ON microanatomy and CNS pathology and visual function. Current analyses of visual function in preterm children recognize that ON and CNS abnormalities may confound the assessment of ocular causes of limited vision.35, 36 Early precise assessment of the optic nerve in future research in preterm infants will likely begin to unravel the contribution of ON and CNS pathology versus ocular pathology to the limitation of visual function in preterm infants.
Longitudinal analysis
Until now, our understanding of ON development prior to the age of term birth has come entirely from two histopathology studies, which suggested that ON axonal tissue increases throughout gestation and infancy, likely due to an increase in neuronal, glial, and septal elements.2, 37 Rimmer et al2 found that the vDiscDiam reaches 50% of adult size by 19 weeks gestational age, 75% by 39 weeks gestational age, and 95% by 10 months after birth, consistent with our findings that the vDiscDiam in a preterm infant grows by a mean of 74 μm (5.9%) over a 4.7 week period while the vC:D decreases by a mean of 0.05 (6.8%) over the same period.
However, our finding of a statistically significant increase in vDiscDiam and decrease in vC:D must be interpreted cautiously. The magnitude of change per week was very small and could have been impacted by noise or sampling bias; the ICC confidence interval for inter-grader vDiscDiam and intra-grader cup depth was relatively wide (0.30–0.93 and 0.44–0.95, respectively), and only 26 of 90 subjects had multiple imaging sessions needed for inclusion in the longitudinal analysis.
Neurologic correlation
Several reports have associated CNS abnormalities with ON pathology.21, 24, 38–39 In a study of 46 children (32 term birth, 14 preterm birth) with diagnoses of bilateral ON hypoplasia on fundus exam, 69.5% of the children had some type of neurodevelopmental handicap.38 Wikstrand et al21 reported that in fundus photos, 6 children born preterm and with known brain lesions had more cupping than those without. Similarly, a diagnosis of PVL was associated with larger optic cups in fundus photos of school-aged children24, thought to be due to synaptic degeneration of axons in the optic radiation.40 In a study of 104 preterm infants with Retcam imaging at 33–34 weeks PMA, McLoone et al39 found significantly smaller horizontal disc diameter, disc area, and cup area for subjects with grade 4 IVH, and a trend towards the same with increasing IVH severity.
OCT measures of ON parameters in preterm infants may have utility in evaluating CNS pathology and neurocognitive development. Better methods of doing so are particularly important for preterm infants as 58% who survive to 2.5 years have some disability and 27% go on to have severe disability.41 In our study, infants diagnosed with PVL had larger vCupDiam and vC:D during infancy than those without PVL, which is consistent with Jacobson et al’s24 findings in school-aged children. Preterm infants who received a head MRI, a marker for suspicion of CNS pathology, were also significantly, but weakly associated with larger vC:D. In our pilot analysis of ON parameters and Bayley scores (n=23), low cognitive Bayley scores were correlated with larger vC:D (p=0.049). We recognize the need to exercise caution in interpreting these results despite their statistical significance. The small sample size and inclusion of only half of the preterm infants in the Bayley score analysis could have resulted in unintentional selection bias. Nonetheless, these preliminary findings continue to support the need for further study to establish the utility of SDOCT optic nerve analysis as a potential non-contact, low risk, efficient, and portable tool to assess infant CNS development.
Several limitations in this study are inherent to the study design. Our study size was small as the protocol prioritized imaging of the macula and fovea over the optic nerve; as a result, we were unable to capture adequate ON scans during some imaging sessions. The small size of our Hispanic preterm cohort (n=3) limits our interpretation by race (Table 3). Our small study size also resulted in only 26 preterm infants with multiple imaging sessions, and data variability limited the study of longitudinal changes. Difficulty achieving outpatient follow-up also limited the number of subjects in the analysis correlating nerve parameters with Bayley scores. We studied only vertical ON parameters because vertical scans were prioritized in our imaging due to a goal of imaging the retinal vessels. Technological limitations also introduced variability into our measurements; the portability of the SDOCT probe allowed scans to have varying degrees of rotation and the imager to introduce unintended amounts of tilt; the large blood vessels around the optic nerve occasionally produced enough shadowing to obscure the location of BMO. A future direction would be to obtain quantitative peripapillary RNFL data from these images; this would lessen the technical limitations of rotation, tilt, and blood vessel shadowing proximal to the nerve. Finally, axial length was not measured, but instead estimated based on an equation published by Maldonado et al.14
This is the first SDOCT analysis to examine ON anatomy in preterm infants. This analysis revealed that the vCupDiam and vC:D are already larger in preterm compared to term infants by the age of “term birth”. In this limited pilot study, indicators of a difference in early ON development in preterm infants appear to weakly associate with CNS pathology and future cognitive development. However, due to the numerous technical and practical limitations of this study, future investigation with a larger cohort is necessary before any further conclusions can be made.
Supplementary Material
Optic nerve parameters as measured by spectral domain optical coherence tomography are significantly different between preterm versus term infants by 37–42 weeks postmenstrual age and may correlate with indicators of brain pathology and neurocognitive development.
Acknowledgments
Financial Support: The Hartwell Foundation; The Andrew Family Foundation, Grant Number 1UL1 RR024128-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. The sponsors or funding organizations had no role in the design or conduct of this research.
The authors would like to thank Du Tran-Viet for complex imaging assistance.
Footnotes
Meeting Presentation: This study was presented in part at the Association for Research in Vision and Ophthalmology Annual Meeting, Seattle, WA, May 9, 2013.
Conflict of Interest: Dr. Toth receives royalties through her university from Alcon and research support from Bioptigen, Genentech, and Physical Sciences Inc. She also has patents pending in OCT imaging and analysis. Dr. El-Dairi received consulting fees from Prana Pharmaceuticals. No other authors have financial disclosures. No authors have a propriety interest in the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Provis JM, van Driel D, Billson FA, Russell P. Human fetal optic nerve: overproduction and elimination of retinal axons during development. J Comp Neurol. 1985;238:92–100. doi: 10.1002/cne.902380108. [DOI] [PubMed] [Google Scholar]
- 2.Rimmer S, Keating C, Chou T, et al. Growth of the human optic disk and nerve during gestation, childhood, and early adulthood. Am J Ophthalmol. 1993;116:748–53. doi: 10.1016/s0002-9394(14)73476-2. [DOI] [PubMed] [Google Scholar]
- 3.Dolman CL, McCormick AQ, Drance SM. Aging of the optic nerve. Arch Ophthalmol. 1980;98:2053–8. doi: 10.1001/archopht.1980.01020040905024. [DOI] [PubMed] [Google Scholar]
- 4.Akerblom H, Holmstrom G, Eriksson U, Larsson E. Retinal nerve fibre layer thickness in school-aged prematurely-born children compared to children born at term. Br J Ophthalmol. 2012;96:956–60. doi: 10.1136/bjophthalmol-2011-301010. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Spencer R, Leffler JN, Birch EE. Characteristics of Peripapillary Retinal Nerve Fiber Layer in Preterm Children. American Journal of Ophthalmology. 2012;153:850–5. e1. doi: 10.1016/j.ajo.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Samarawickrama C, Huynh SC, Liew G, et al. Birth weight and optic nerve head parameters. Ophthalmology. 2009;116:1112–8. doi: 10.1016/j.ophtha.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 7.El-Dairi MA, Asrani SG, Enyedi LB, Freedman SF. Optical coherence tomography in the eyes of normal children. Arch Ophthalmol. 2009;127:50–8. doi: 10.1001/archophthalmol.2008.553. [DOI] [PubMed] [Google Scholar]
- 8.Moon CH, Hwang SC, Kim BT, et al. Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci. 2011;52:8527–33. doi: 10.1167/iovs.11-8034. [DOI] [PubMed] [Google Scholar]
- 9.Chang L, El-Dairi MA, Frempong TA, et al. Optical coherence tomography in the evaluation of neurofibromatosis type-1 subjects with optic pathway gliomas. J AAPOS. 2010;14:511–7. doi: 10.1016/j.jaapos.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Fjeldstad AS, Carlson NG, Rose JW. Optical coherence tomography as a biomarker in multiple sclerosis. Expert Opin Med Diagn. 2012;6:593–604. doi: 10.1517/17530059.2012.719496. [DOI] [PubMed] [Google Scholar]
- 11.Kandasamy Y, Smith R, Wright IM, Hartley L. Optic disc measurements in full term infants. Br J Ophthalmol. 2012;96:662–4. doi: 10.1136/bjophthalmol-2011-300950. [DOI] [PubMed] [Google Scholar]
- 12.Allingham M, Cabrera M, O’Connell RV, et al. Racial variation in optic nerve head parameters quantified in healthy newborns by handheld spectral domain optical coherence tomography. J AAPOS. 2013;17:501–6. doi: 10.1016/j.jaapos.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Knight OJ, Girkin CA, Budenz DL, et al. Effect of race, age, and axial length on optic nerve head parameters and retinal nerve fiber layer thickness measured by Cirrus HD-OCT. Arch Ophthalmol. 2012;130:312–8. doi: 10.1001/archopthalmol.2011.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci. 2010;51:2678–85. doi: 10.1167/iovs.09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albers CA, Grieve AJ. Test Review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development. 3. Vol. 25. San Antonio, TX: Harcourt Assessment Journal of Psychoeducational Assessment; 2007. pp. 180–90. [Google Scholar]
- 16.Maldonado R, et al. Three-dimensional Assessment of Vascular and Perivascular Characteristics in Subjects with Retinopathy of Prematurity Ophthalmology. Ophthalmology. 2014 doi: 10.1016/j.ophtha.2013.12.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. British Journal of Ophthalmology. 2002;86:238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellstrom A, Hard AL, Chen Y, et al. Ocular fundus morphology in preterm children. Influence of gestational age, birth size, perinatal morbidity, and postnatal growth. Invest Ophthalmol Vis Sci. 1997;38:1184–92. [PubMed] [Google Scholar]
- 19.Hellstrom A, Hard AL, Svensson E, Niklasson A. Ocular fundus abnormalities in children born before 29 weeks of gestation: a population-based study. Eye (Lond) 2000;14:324–9. doi: 10.1038/eye.2000.81. [DOI] [PubMed] [Google Scholar]
- 20.Wang XY, Huynh SC, Rochtchina E, Mitchell P. Influence of Birth Parameters on Peripapillary Nerve Fiber Layer and Macular Thickness in Six-Year-Old Children. American Journal of Ophthalmology. 2006;142:505–7. doi: 10.1016/j.ajo.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 21.Wikstrand MH, Hard AL, Niklasson A, Hellstrom A. Birth weight deviation and early postnatal growth are related to optic nerve morphology at school age in children born preterm. Pediatr Res. 2010;67:325–9. doi: 10.1203/PDR.0b013e3181ca9f43. [DOI] [PubMed] [Google Scholar]
- 22.Hackl S, Zeman F, Helbig H, Oberacher-Velten IM. Optic disc morphology in premature infants. Br J Ophthalmol. 2013;97:314–7. doi: 10.1136/bjophthalmol-2012-302066. [DOI] [PubMed] [Google Scholar]
- 23.Chong G, Farsiu S, Freedman SF, et al. Abnormal foveal morphology in ocular albinism imaged with spectral-domain optical coherence tomography. Archives of Ophthalmology. 2009;127:37–44. doi: 10.1001/archophthalmol.2008.550. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson L, Hellstrom A, Flodmark O. Large cups in normal-sized optic discs: A variant of optic nerve hypoplasia in children with periventricular leukomalacia. Archives of Ophthalmology. 1997;115:1263–9. doi: 10.1001/archopht.1997.01100160433007. [DOI] [PubMed] [Google Scholar]
- 25.Glass LR, Cioffi GA, Blumberg DM. Retinal nerve fiber layer analysis of cupping in children born prematurely. J Glaucoma. 2014;23:e1–5. doi: 10.1097/IJG.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 26.De Silva DJ, Cocker KD, Lau G, et al. Optic disk size and optic disk-to-fovea distance in preterm and full-term infants. Invest Ophthalmol Vis Sci. 2006;47:4683–6. doi: 10.1167/iovs.06-0152. [DOI] [PubMed] [Google Scholar]
- 27.El-Dairi M, Holgado S, Asrani S, Freedman SF. Optical coherence tomography (OCT) measurements in black and white children with large cup-to-disc ratios. Exp Eye Res. 2011;93:299–307. doi: 10.1016/j.exer.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Avery RA, Liu GT, Fisher MJ, et al. Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151:542–9. e2. doi: 10.1016/j.ajo.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avery RA, Ferner RE, Listernick R, et al. Visual acuity in children with low grade gliomas of the visual pathway: implications for patient care and clinical research. J Neurooncol. 2012;110:1–7. doi: 10.1007/s11060-012-0944-y. [DOI] [PubMed] [Google Scholar]
- 30.Girkin CA, McGwin G, Jr, Sinai MJ, et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 2011;118:2403–8. doi: 10.1016/j.ophtha.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Girkin CA. Differences in optic nerve structure between individuals of predominantly African and European ancestry: Implications for disease detection and pathogenesis. Clin Ophthalmol. 2008;2:65–9. doi: 10.2147/opth.s1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol. 2010;128:541–50. doi: 10.1001/archophthalmol.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quigley HA, Brown AE, Morrison JD, Drance SM. The size and shape of the optic disc in normal human eyes. Arch Ophthalmol. 1990;108:51–7. doi: 10.1001/archopht.1990.01070030057028. [DOI] [PubMed] [Google Scholar]
- 34.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace DK, Bremer DL, Good WV, et al. Correlation of recognition visual acuity with posterior retinal structure in advanced retinopathy of prematurity. Arch Ophthalmol. 2012;130:1512–6. doi: 10.1001/archophthalmol.2012.2118. [DOI] [PubMed] [Google Scholar]
- 36.Kozeis N, Mavromichali M, Soubasi-Griva V, et al. Visual function in preterm infants without major retinopathy of prematurity or neurological complications. Am J Perinatol. 2012;29:747–54. doi: 10.1055/s-0032-1316446. [DOI] [PubMed] [Google Scholar]
- 37.Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Investigative Ophthalmology & Visual Science. 1988;29:1151–8. [PubMed] [Google Scholar]
- 38.Burke JP, O’Keefe M, Bowell R. Optic nerve hypoplasia, encephalopathy, and neurodevelopmental handicap. Br J Ophthalmol. 1991;75:236–9. doi: 10.1136/bjo.75.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLoone E, O’Keefe M, Donoghue V, et al. RetCam image analysis of optic disc morphology in premature infants and its relation to ischaemic brain injury. British Journal of Ophthalmology. 2006;90:465–71. doi: 10.1136/bjo.2005.078519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banker B, Larroche J. Periventricular leukomalacia of infancy: A form of neonatal anoxic encephalopathy. Archives of Neurology. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- 41.Serenius F, Kallen K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2. 5 years after active perinatal care in Sweden. JAMA. 2013;309:1810–20. doi: 10.1001/jama.2013.3786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.