Genetic alterations of the cytosolic, phosphorylating glyceraldehyde-3-phosphate dehydrogenase, GAPC, have substantial impacts on the overall cellular production of reductants, energy, and carbohydrate metabolites as well as seed production. Increased GAPC expression contributes to enhanced seed oil accumulation.
Abstract
The cytosolic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPC) catalyzes a key reaction in glycolysis, but its contribution to plant metabolism and growth are not well defined. Here, we show that two cytosolic GAPCs play important roles in cellular metabolism and seed oil accumulation. Knockout or overexpression of GAPCs caused significant changes in the level of intermediates in the glycolytic pathway and the ratios of ATP/ADP and NAD(P)H/NAD(P). Two double knockout seeds had ∼3% of dry weight decrease in oil content compared with that of the wild type. In transgenic seeds under the constitutive 35S promoter, oil content was increased up to 42% of dry weight compared with 36% in the wild type and the fatty acid composition was altered; however, these transgenic lines exhibited decreased fertility. Seed-specific overexpression lines had >3% increase in seed oil without compromised seed yield or fecundity. The results demonstrate that GAPC levels play important roles in the overall cellular production of reductants, energy, and carbohydrate metabolites and that GAPC levels are directly correlated with seed oil accumulation. Changes in cellular metabolites and cofactor levels highlight the complexity and tolerance of Arabidopsis thaliana cells to the metabolic perturbation. Further implications for metabolic engineering of seed oil production are discussed.
INTRODUCTION
The reactions of glycolysis provide carbon, reduced cofactors, and ATP for cellular operation and sustain seed oil biosynthesis (Plaxton, 1996; Schwender et al., 2003; Andre et al., 2007). Plants possess two separate glycolytic pathways located in the cytosol and plastid (Plaxton, 1996; Schwender et al., 2003). Transcriptomic analyses indicate that mRNAs encoding glycolytic enzymes are generally more abundant in the cytosol than plastid with the primary exception of pyruvate kinase (White et al., 2000; Ruuska et al., 2002). The conversion of glyceraldehyde-3-phosphate (GAP) to 1,3-bisphosphoglycerate by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a central step in metabolism linking the energy consuming with the energy producing steps of glycolysis and through triose phosphate translocators connecting plastidic and cytosolic metabolism. Nucleotide cofactors are a cosubstrate for this enzyme leading to production of NADH and NADPH along with cellular intermediates (Plaxton, 1996). The Arabidopsis thaliana genome contains four phosphorylating glycolytic GAPDH genes (GAPC1, GAPC2, GAPCp1, and GAPCp2) and one nonphosphorylating glycolytic GAPDH gene (NP-GAPDH) (Rius et al., 2008; Muñoz-Bertomeu et al., 2009). Two GAPCp are targeted to the plastids whereas NP-GAPDH and the other two GAPCs are located in the cytosol (Rius et al., 2008; Muñoz-Bertomeu et al., 2009). Prior studies indicate that the loss of cytosolic NP-GAPDH causes delayed growth and altered morphology (Rius et al., 2006). Knockout (KO) of both GAPCps results in severe developmental and growth defects, including arrested root development, dwarfism, and male sterility (Rius et al., 2006; Muñoz-Bertomeu et al., 2009, 2010), but KO or overexpression (OE) of GAPCps did not affect seed oil content (Muñoz-Bertomeu et al., 2009). These results demonstrate the importance of the plastidic GAPDHs and cytosolic NP-GAPDH in plant metabolism and growth and indicate that other GAPDHs, such as GAPCs, cannot compensate for their function.
The metabolic function of the cytosolic phosphorylating GAPCs remains unclear. One report indicated that the cytosolic GAPC1 KO, gapc-1, or suppression of this gene through RNA interference delayed growth and changed morphology of siliques resulting in lower seed numbers (Rius et al., 2006). However, recent studies have been unable to confirm these findings; in both single and double GAPC KO lines, growth was maintained at normal rates in Arabidopsis (Guo et al., 2012). Suppression of GAPC in potato (Solanum tuberosum) also produced normal plant and tuber morphology (Hajirezaei et al., 2006). The contribution of GAPCs to plant function and development is complicated because they have a dual role in regulation. Several reports have linked GAPCs to the Arabidopsis response to oxidative and water stresses (Hancock et al., 2005; Holtgrefe et al., 2008; Guo et al., 2012), and two recent studies demonstrate that GAPC can move into the nucleus in plants (Kim et al., 2013; Vescovi et al., 2013). Furthermore, GAPCs have been found to interact with phosphatidic acid (PA), a lipid mediator and key intermediate in glycerolipid biosynthesis (Kim et al., 2013; McLoughlin et al., 2013). The PA-GAPC interaction may provide a signaling link to coordinate carbohydrate and lipid metabolism.
Studies on transcription factors such as wri1 have linked the regulation of oil production with glycolytic activity. OE of WRI1 enhances the expression of genes involved in glycolysis and fatty acid biosynthesis, resulting in enhanced seed oil content (Focks and Benning, 1998; Cernac and Benning, 2004; Baud et al., 2009). The GAPDH reaction generates reducing equivalents NADPH or NADH and is an intermediate step in the glycolytic production of phosphoenolpyruvate (PEP), pyruvate, and acetyl-CoA that are precursors for most biosynthetic building blocks including fatty acids. It has been proposed that during Arabidopsis seed development, cytosolic, glycolytic intermediate PEP enters the plastid where it is converted to pyruvate that can be used for fatty acid synthesis (White et al., 2000; Ruuska et al., 2002; Andre et al., 2007). Thus, the enhanced production of oil that is a highly reduced form of carbon must be coordinated with sources of reductant and energy as well as carbon, resources that are provided by GAPDH and glycolysis. However, as two GAPCs and one NP-GAPDH occur in the cytosol, the role of specific cytosolic GAPDHs in glycolysis and lipid production is unknown.
This study probes the metabolic function of the two cytosolic GAPCs, particularly in relation to their role in seed oil production. We investigated the relationship between the GAPCs and oil accumulation in developing seeds of Arabidopsis by knocking out and overexpressing two isoforms to better understand the reductant, energy, and carbon limitations on oil biosynthesis including their regulation. The results demonstrate that these two GAPC genes have overlapping functions and manipulation of GAPC genes significantly impact the glycolysis pathway and seed oil production. In addition, the study shows that despite significant changes in metabolite and cofactor levels, GAPC-altered plants displayed no overt changes in vegetative growth, highlighting the complexity and tolerance of Arabidopsis cells to the metabolic perturbation.
RESULTS AND DISCUSSION
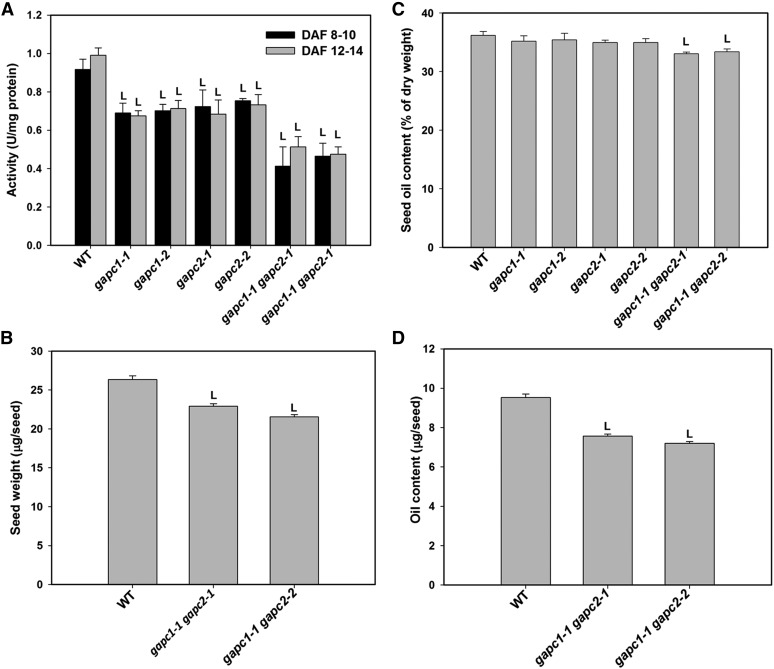
Knockout of GAPC1 and GAPC2 Decreases Seed Weight and Seed Oil Accumulation
Two GAPC1 single KOs (gapc1-1 and gapc1-2), two GAPC2 single KOs (gapc2-1 and gapc2-2), and two GAPC1/GAPC2 double KOs (gapc1-1 gapc2-1 and gapc1-1 gapc2-2) were isolated (Guo et al., 2012). NAD-dependent GAPDH activity was measured in developing seeds harvested in two stages: 8 to 10 d after flowering (DAF) and 12 to 14 DAF. In all cases, the variation in GAPDH activity between the two developmental states was small; however, the wild type was distinct from modified lines. Within the transgenic lines, GAPDH activity was reduced 22 to 25% in single KOs and 43 to 48% in double KOs at both ages (Figure 1A). Previous studies suggest that KO of GAPCps does not decrease the total GAPDH activity (Muñoz-Bertomeu et al., 2009); therefore, compensation in activity for GAPCps apparently is specific to plastid and incapable of functionally compensating for cytosolic forms. The remaining GAPDH activity is likely to come from other GAPDH isoforms including NP-GAPDH and plastidic GAPA/B involved in photosynthetic carbon fixation (Rius et al., 2006, 2008). Under normal growth conditions, GAPC1 or 2 single KO or double KO mutant plants grew and developed normally as wild-type plants. However, seed weight of the double mutants was decreased from 26.3 μg/seed for the wild type to 22.9 and 21.6 μg/seed for gapc1-1 gapc2-1 and gapc1-1 gapc2-2, respectively (Figure 1B).
Figure 1.
KO of GAPCs Decreases Arabidopsis Seed Oil Accumulation.
(A) NAD-dependent GAPDH activity in developing seeds. Total protein was extracted from seeds in two developing stages (8 to 10 DAF and 12 to 14 DAF). Values are means ± sd (n = 3).
(B) Seed weight of wild-type and GAPC double KOs. Seed weight was average weight based on calculation of the total weight (∼3 mg) and total number of seeds. Values are means ± sd (n = 3).
(C) Seed oil content of wild-type and GAPC mutants. Seed oil content was analyzed after seeds were harvested and dried under room temperature for at least 2 weeks. Seed oil content was calculated as the percentage of oil over the seed weight. Values are means ± sd (n = 3).
(D) Oil content per seed. Values are means ± sd (n = 3).
“L” in (A) to (D) indicates significantly lower than the wild type (Student’s t test, P < 0.05).
Seed oil content was decreased in the double mutant lines from measured wild-type levels of 36.2% to gapc1-1 gapc2-1 and gapc1-1 gapc2-2 lines with 33.0 and 33.4% of seed weight, respectively (Figure 1C). Compounded with decreased seed weight, the overall reduction in oil was ∼20% per seed due to the absence of both GAPCs (Figure 1D). In addition, seeds from the double KO lines had decreased levels of C18:0 and C18:3 with concomitant increases in C18:2 (Supplemental Figure 1). The data suggest that GAPCs play an important role in seed oil accumulation; however, as the single KO lines did not show distinct phenotypes, the enzymes have overlapping functions.
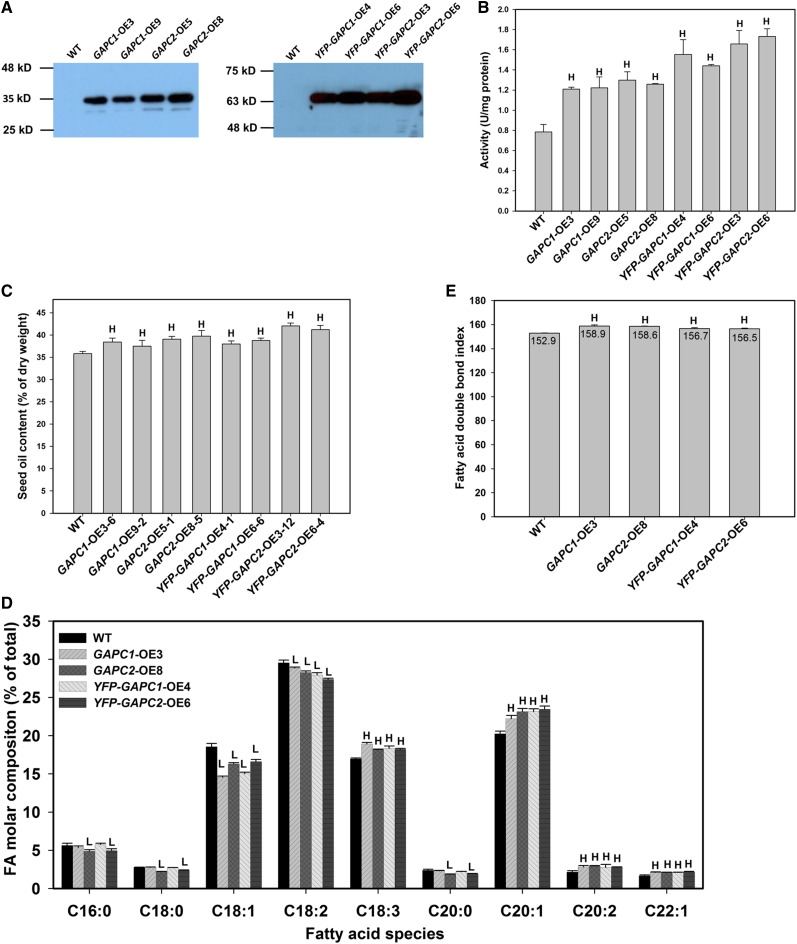
Constitutive Overexpression of GAPC Increases Seed Oil but Decreases Seed Production
Constitutive expression of the GAPC1 and GAPC2 open reading frames was driven by the 35S promoter, resulting in OE lines of GAPC1 and GAPC2 by insertion into binary vector p35S-FAST (Pro35S:GAPC-OE, GAPC fused with the FLAG octapeptide) and p35S-FAST/eYFP (Pro35S:YFP-GAPC-OE, GAPC fused with both yellow fluorescent protein [YFP] and the FLAG octapeptide). The FLAG octapeptide and YFP tags were used for ease of purification and visualization. OE of GAPCs was confirmed by immunoblotting in four Pro35S:GAPC and four Pro35S:YFP-GAPC lines (Figure 2A). GAPC1 and GAPC2 were most abundant in lines that contained YFP. OE of GAPC in developing seeds (12 DAF) resulted in increases in GAPDH activity by over 40% for Pro35S:GAPC1/2 lines and more than 80% in Pro35S:YFP-GAPC1/2 lines (Figure 2B). Both YFP-GAPC1 and YFP-GAPC2 were localized in the cytosol in leaves and developing seeds (Supplemental Figures 2A to 2C). It has been reported that a small portion of GAPCs in leaves could be translocated into the nucleus under stress conditions, such as cadmium and PA treatments (Kim et al., 2013; Vescovi et al., 2013). Whereas the control YFP was only present in the cytosol and nucleus as expected, no apparent accumulation of YFP-GAPC1 and YFP-GAPC2 was detected in the nucleus in either leaves or developing seeds under the conditions tested (Supplemental Figures 2A and 2C).
Figure 2.
Increased Seed Oil Content in GAPC OE Seeds.
(A) Immunoblotting of overexpressed GAPC1 and GAPC2 in Arabidopsis seeds (>12 DAF). GAPCs were either fused with a FLAG tag (GAPC-OE) or with both YFP and FLAG tags (YFP-GAPC-OE) under the control of the cauliflower mosaic virus 35S promoter. Total protein was extracted from the seeds of T3 homozygous lines and the wild type was used as negative control. Total protein (10 μg) was separated on a 10% SDS-PAGE gel, transferred to a membrane, and blotted with anti-FLAG antibody. Two independent lines were examined for each transformation.
(B) GAPDH activity assay using total proteins extracted from developing seeds (12 to 14 DAF) of T3 lines. Values are means ± sd (n = 3).
(C) Seed oil analyses of GAPC OE seeds (T3). Seed oil content was calculated as the percentage of oil over the seed weight. Values are means ± sd (n = 3).
(D) Fatty acid composition was altered in GAPC OE seeds. Fatty acid composition was calculated as mol %. Values are means ± sd (n = 3).
(E) Fatty acid double bond index was increased in OE seeds. Fatty acid double bond index was calculated as DBI = mol % of unsaturated fatty acids × number of double bonds of each unsaturated fatty acid. Values are means ± sd (n = 3).
“H” and “L” in (B) to (E) indicate significantly higher and lower than the wild type, respectively (Student’s t test, P < 0.05).
[See online article for color version of this figure.]
The constitutive OE of GAPC1 and GAPC2 led to increased seed oil content. Comparison of wild-type and OE lines indicated a change in oil from 36% (wild type) to between 38 and 42% of seed weight depending on the line (eight independent lines; Figure 2C). Fatty acid composition was also significantly altered in the OE lines (Figure 2D), favoring the production of elongated fatty acids (average increase of 14, 32, and 27% for C20:1, C20:2, and C22:1, respectively) and more unsaturated acyl chains (i.e., unsaturation index increased from 153 in the wild type to 157 to 159 for OEs [Figure 2E]). Plants perform fatty acid biosynthesis in the plastid, producing acyl chains up to 18 carbons in length. Elongation to greater lengths and a significant amount of desaturation occurs extraplastidially in the endoplasmic reticulum and requires a nonplastidic source of ATP, NAD(P)H, and acetyl-CoA (Ohlrogge and Browse, 1995; Chapman and Ohlrogge, 2012). The increase in C20 fatty acids and the fatty acid double bond index in the GAPC OE seeds suggest a greater supply of reducing power, carbon, and energy to support elongation and desaturation outside of the plastid.
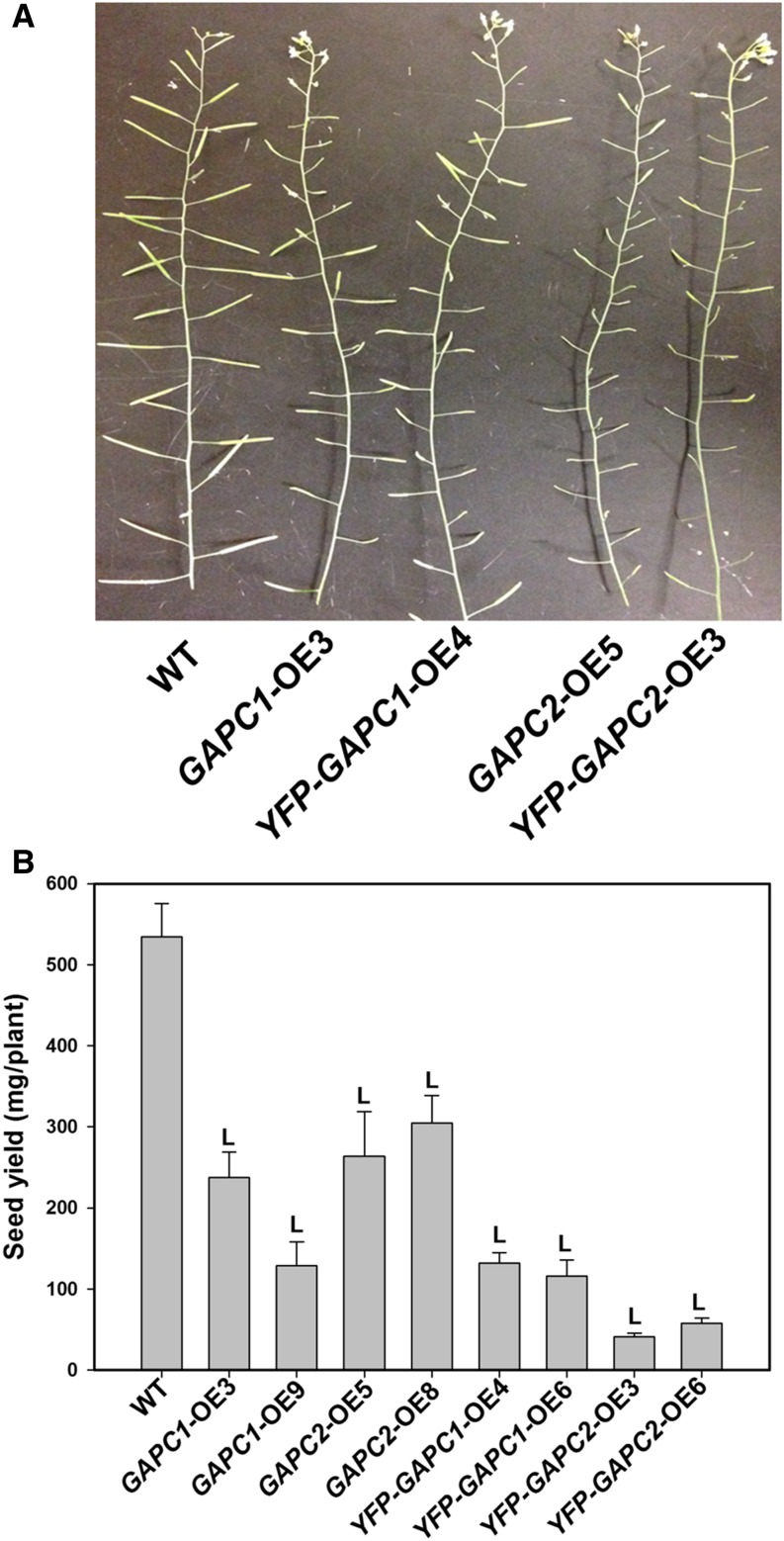
However, the constitutively expressed transgenic lines produced abnormal siliques. The number of siliques was reduced and many were incomplete or shorter than the wild type (Figure 3A), resulting in dramatically reduced seed yield in the OE lines (Figure 3B). For the four Pro35S:GAPC OE lines, seed yield was decreased by 40 to 76% and in the four Pro35S:YFP-GAPC OE lines the reduction was even more pronounced (75 to 92% reduced seed yield). The reduced seed biomass resulted from decreases in seed number, though individual seed weights increased 53 to 88% relative to the wild type (Supplemental Figures 3A and 3B). Low seed numbers were not the consequence of general flower development as plants did not exhibit changes in flower morphology (Supplemental Figure 4A). However, anthers did not dehisce and, thus, pollen failed to be released after flowers opened in OE plants (Supplemental Figure 4B). Double staining by fluorescein diacetate and propidium iodide indicated that the majority of pollen was not viable (∼10% viable) in OE plants, which might limit the production of seeds in OE plants (Supplemental Figures 4C and 4D). Normal seed production was restored when OE stigmas were fertilized with pollen from the wild type or gapc1-1 gapc2-1 (Supplemental Figures 5A and 5B). Thus, the perturbation of metabolism results in defective development of pollen and anther dehiscence in GAPC1- or GAPC2-OE plants. The male sterility phenotype in 35S promoter-driven GAPC OE lines is similar to that displayed by fad3-2 fad7-2 fad8 mutants that failed to produce jasmonate (McConn and Browse, 1996; Mandaokar and Browse, 2009; Song et al., 2011). However, further studies would be required to determine if OE of GAPCs in flowers is linked with jasmonate production or signaling.
Figure 3.
OE of GAPCs under the Control of the 35S Promoter Reduced Fertility.
(A) P35S-driven OE of GAPCs reduced fertility and silique size in transgenic plants.
(B) Seed yield per plant for eight independent T3 lines compared with the wild type. Values are means ± se (n = 8). “L” indicates significantly lower than the wild type (Student’s t test, P < 0.05).
[See online article for color version of this figure.]
Seed-Specific OE of GAPCs Increases Seed Oil Content without Compromising Seed Yield
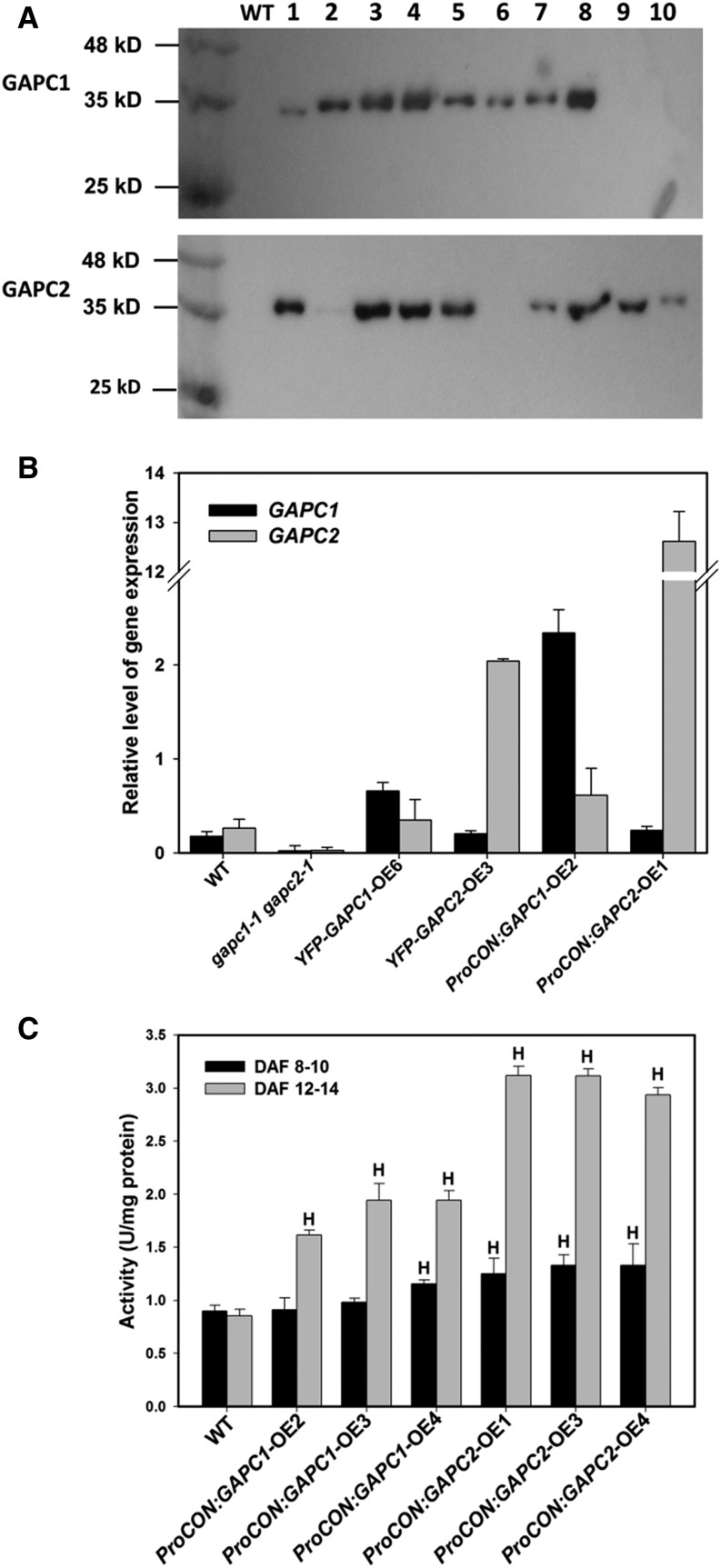
To overcome penalties in seed production due to constitutive expression, seed-specific OE Arabidopsis lines of GAPC1 and GAPC2 were generated by placing these genes under the control of the soybean (Glycine max) β-conglycinin seed-specific promoter (ProCON:GAPC-OE). Eight positive transgenic lines for both GAPC1 and GAPC2 were identified by immunoblotting and gene expression tests in developing seeds (12 to 14 DAF) (Figure 4A). Transcripts of both GAPC1 and GAPC2 were almost undetectable in the gapc1-1 gapc2-1 double KO, whereas the transcript level of GAPC1 and GAPC2 was elevated in both 35S OE lines (e.g., 2.6-fold in YFP-GAPC1-OE6 and 6.8-fold in YFP-GAPC2-OE3) as well as lines with seed-specific OE (12-fold in ProCON:GAPC1-OE2 and 48-fold in ProCON:GAPC2-OE1) (Figure 4B).
Figure 4.
Seed-Specific OE of GAPC1 and GAPC2.
(A) Immunoblotting of overexpressed GAPC1 and GAPC2. GAPCs were fused with a FLAG tag under the control of a seed-specific soybean β-conglycinin promoter (ProCON:GAPC). Total proteins were extracted from the developing seed of T1 plants and the wild type was used as a negative control. Total protein (10 μg) was separated on a 10% SDS-PAGE gel, transferred to a membrane, and blotted with an anti-FLAG antibody. Ten lines were examined for each transformation.
(B) Transcript level of GAPCs in developing seeds. YFP-GAPC1-OE6 and YFP-GAPC2-OE3 lines driven by the 35S promoter were transformed into the wild type. ProCON:GAPC1-OE2 and ProCON:GAPC2-OE1 lines driven by a seed-specific soybean β-conglycinin promoter were also transformed into the wild type. gapc1-1 gapc2-1, GAPC double knockout. Values are means ± sd (n = 3).
(C) GAPDH activity assay using total proteins extracted from developing seeds (8 to 10 DAF and 12 to 14 DAF) of T1 lines. Values are means ± sd (n = 3). H indicates significantly higher than the wild type (Student’s t test, P < 0.05).
GAPDH activity was assessed in developing plant seeds at 8 to 10 and 12 to 14 DAF. At 8 to 10 DAF, GAPDH activity increased by 28.4 to 47.9% in four lines and remained unchanged in two lines. However, up to a 3-fold increase was observed in the seeds of ProCON:GAPC1/2 OE lines at 12 to 14 DAF, indicating the temporal nature of seed-specific expression (Figure 4C). The plants grew normally in terms of silique development, seed yield, and seed weight (Supplemental Figures 6A to 6D), but ProCON:GAPC seeds produced more oil (37.6 to 39.1% for OE versus 35.6% for the wild type [Figure 5A]) and had altered fatty acid compositions that were similar to those of the Pro35S:GAPC OE lines (Supplemental Figure 7). The increased oil content was confirmed in the second generation as propagated T2 seeds had on average a 3.3 and 3.5% increase in oil for GAPC1 and GAPC2 lines, respectively (Figure 5B) without changes in seed yield or weight (Supplemental Figures 6C and 6D). GAPDH activity in ProCON:GAPC2-OE1 seeds was ∼50% higher than that in ProCON:GAPC1-OE2 seeds (12 to 14 DAF), but the increased GAPDH activity did not result in a much higher oil content in GAPC2-OE1 seeds. Given that the differences in GAPC2 and GAPC1 maximal in vitro activity in the OE seeds do not correspond with correspondingly high increases in oil, the GAPC2 lines may not be operating at the same level of activation because OE is not optimized. This could mean that other steps downstream of GAPDH in glycolysis might limit production of more downstream metabolites due to the regulation of key enzymes or substrate availability in GAPC2-OE seeds. In addition, other steps in oil biosynthetic processes might become limiting at a certain threshold of increased GAPC2 expression.
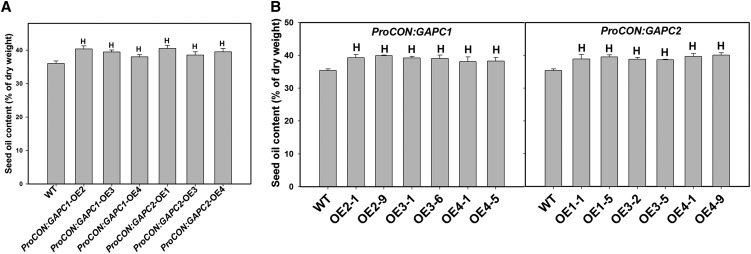
Figure 5.
Seed Oil Content Is Increased in GAPC Seed-Specific OE Seeds.
(A) Seed oil content of WT and T1 seed-specific OE seeds. Values are means ±sd (n = 3). Data of three independent lines are presented.
(B) Seed oil content of the wild type and T2 seed-specific OE seeds. Heterozygous (OE2-1, OE3-1, and OE4-1 for ProCON:GAPC1; OE1-1, OE3-2, and OE4-1 for ProCON:GAPC2) and homozygous (OE2-9, OE3-6, and OE4-5 for ProCON:GAPC1; OE1-5, OE3-5, and OE4-9 for ProCON:GAPC2) seeds for each independent line were analyzed. Values are means ± sd (n = 3).
“H” in (A) and (B) indicates significantly higher than the wild type (Student’s t test, P < 0.05).
Expression of Many Genes Related to Lipid Production Is Altered in KO and OE Seeds
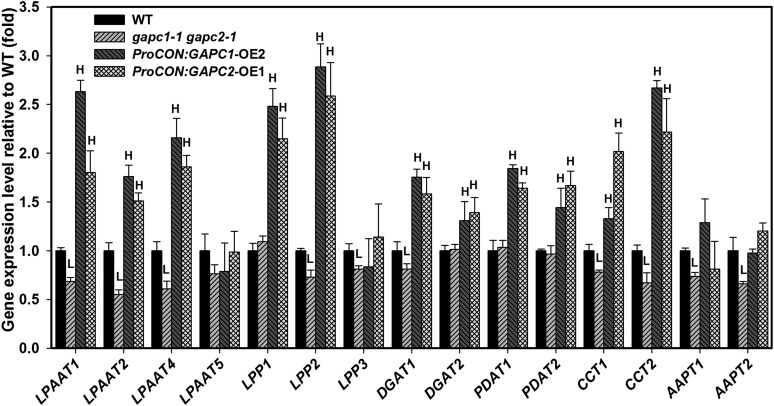
To understand how manipulations of GAPCs affect seed oil biosynthesis, expression levels of selected genes involved in triacylglycerol (TAG) biosynthesis were measured by real-time PCR in developing seeds. In the Kennedy pathway of TAG biosynthesis, lysophosphatidic acid acyltransferase (LPAAT) catalyzes the conversion of lysophosphatidic acid to PA, which is then dephosphorylated by lipid phosphate phosphatase (LPP) to form DAG. Diacylglycerol acyltransferase (DGAT) catalyzes the final step of TAG formation from DAG and acyl-CoA. In plants, there are other enzymatic steps to produce intermediates for TAG biosynthesis, and some utilize PC that is a primary substrate for desaturation. PC can be made from DAG by the combination of choline-phosphate:CTP cytidylyltransferase (CCT) and aminoalcohol-phosphotransferase (AAPT) that synthesize CDP-choline and subsequently transfer the phosphocholine head group to DAG, respectively. PC can also be used with DAG to generate TAG by phospholipid:diacylglycerol acyl transferase (PDAT) (Chapman and Ohlrogge, 2012). KO of both GAPCs resulted in a decrease in transcript levels of lipid assembly genes, including LPAATs, LPPs, DGAT1, CCTs, and AAPTs, and the converse was true for OE lines (Figure 6). Changes in expressed levels of genes were consistent with altered production of oil and in some cases approached a 50% change relative to the wild type. Nearly all genes showed a positive correlation with the OE of GAPC genes, except for LPAAT5, LPP3, and APPT (Figure 6). In the GAPDH-deficient lines most transcripts were reduced; however, DGAT and PDAT were largely unaffected (Figure 6).
Figure 6.
Manipulation of GAPCs Alters the Expression Level of Genes Involved in Oil Biosynthesis in Developing Seeds.
KO of GAPCs decreases while OE of GAPCs increases the expression level of genes involved in oil biosynthesis in developing seeds. RNA was extracted from T2 developing seeds (12 to 14 DAF), and the transcript level was determined by real-time PCR normalized to UBQ10. Values are means ± sd (n = 3). “H” and “L” indicate significantly higher and lower than the wild type (Student’s t test, P < 0.05).
GAPC KO and OE Have Significant Effects on Metabolism
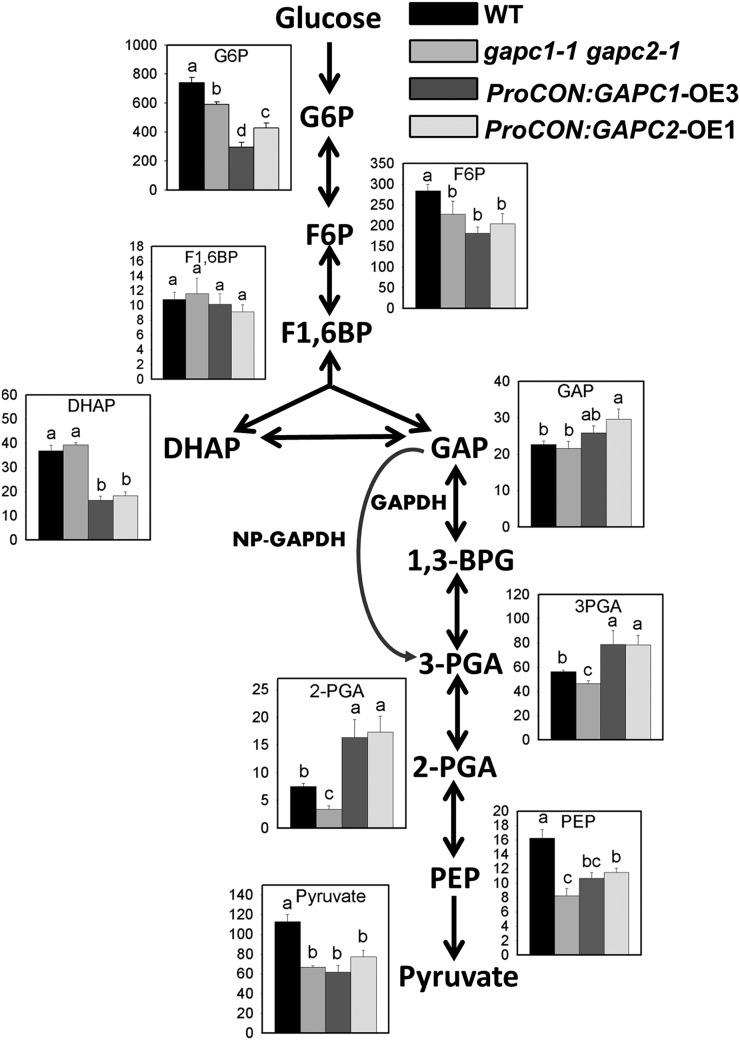
To examine the impact of GAPCs on central metabolites, a liquid chromatography–tandem mass spectrometry (LC-MS/MS) method was used for quantification of targeted metabolites involved in glycolysis, the tricarboxylic acid (TCA) cycle, and other metabolic pathways (Luo et al., 2007; Arrivault et al., 2009). Moderate changes occurred for most glycolytic intermediates in the developing seeds of gapc1-1 gapc2-1 (Figure 7). In gapc1-1 gapc2-1 seeds, the levels of metabolites downstream of GAPDH were reduced, possibly indicating the partial depletion of metabolic pools when the seed glycolysis pathway is downregulated as a consequence of reduced seed growth compounded by reduced oil production (Figures 1B and 1C). OE lines indicated a partial depletion of intermediates that were upstream of GAPDH and the increased levels of 3-PGA and 2-PGA that are the downstream products of GAPDH. PEP and pyruvate that are further downstream were reduced in all KO and OE lines, reflecting altered steady state levels necessary for the repartitioning of carbon, including enhanced oil (Figure 7). However, compared with wild-type seeds, GAP, the substrate of GAPC, is significantly increased in the ProCON:GAPC2-OE1 line. The increase in GAP in OE seeds is consistent with rebalancing of triose phosphate by triose phosphate isomerase (TPI), which catalyzes the interconversion of GAP and DHAP. TPI in Trypanosoma brucei brucei is known to be inhibited by PEP and the recently resolved crystal structure of TPI ties PEP to regulation of this enzyme (Lambeir et al., 1987; Grüning et al., 2014). Thus, a reduced level of DHAP in OE seeds indicates the shift in enzymatic equilibrium favoring GAP production and is consistent with lower PEP levels that regulate TPI. However, the levels of DHAP and GAP in gapc1-1 gapc2-1 remained the same as the wild type, although PEP was lower in gapc1-1 gapc2-1. This leads to another possibility, namely, that increased GAP level in OE seeds was at the expense of upstream metabolites including G6P and F6P.
Figure 7.
Altered Metabolite Level in the Glycolysis Pathway in Developing Seeds of gapc1-1 gapc2-1 and Seed-Specific OEs.
Metabolites were extracted from T2 developing seeds (12 to 14 DAF) and determined and quantified by comparison with authentic standards. The y axis is metabolite content in nmol/g fresh weight (FW). 1,3-BPG was below the detection level and thus not measured. Values are means ± se (n = 4). One-way ANOVA was performed, and different lowercase letters mark significant differences among the wild type, gapc1-1 gapc2-1, and OEs (P < 0.05). G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; F1,6BP, fructose-1,6-diphosphate; DHAP, dihydroxy-acetone-phosphate; 1,3-BPG, 1,3-bisphosphoglycerate; 3PGA, glycerate-3-phosphate; 2PGA, glycerate-2-phosphate.
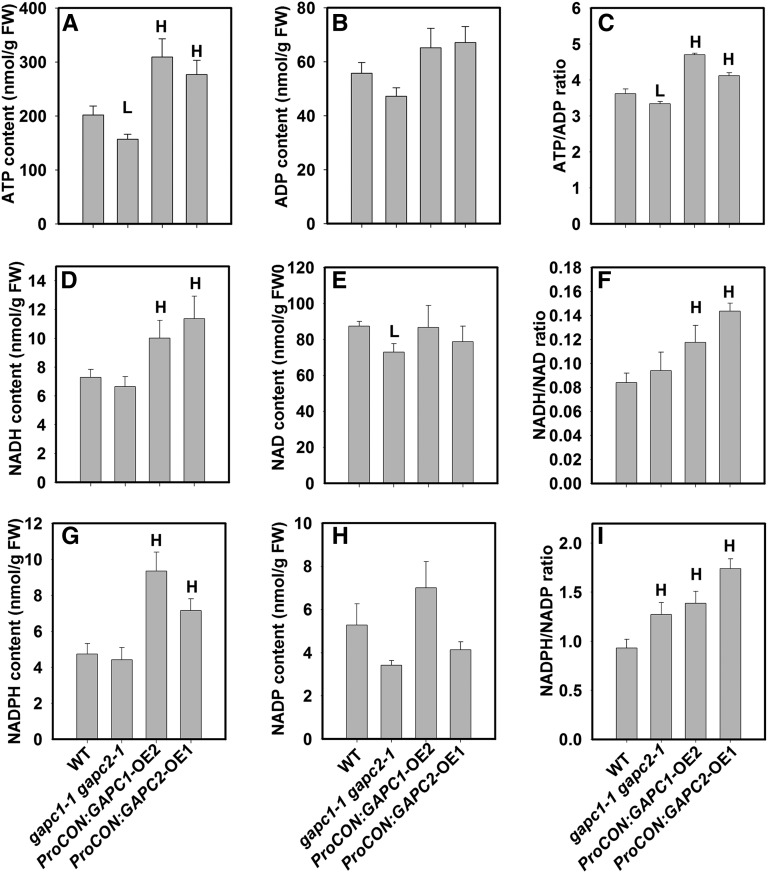
Glycolysis is a significant source of ATP, NADH, and NADPH for protein and oil biosynthesis, which are energetically costly processes, in seeds and transgenic alterations impact the levels of energy metabolites as well as storage reserve production (Vigeolas et al., 2004; Elhiti et al., 2012). The levels of ATP, ADP, NAD(P), and NAD(P)H were determined and their ratios calculated as a proxy for energy charge and reducing power in wild-type and transgenic lines. The levels of metabolites, ATP, and ADP and their ratio in the wild type were comparable with previous reports (Gibon et al., 2002; Arrivault et al., 2009). ATP levels were reduced in gapc1-1 gapc2-1 and increased in OEs relative to the wild type, while ADP levels were not significantly altered (Figures 8A and 8B). As a result, the ATP/ADP ratio was decreased in gapc1-1 gapc2-1 and increased in OE lines, which is consistent with a role for GAPDH in decreasing or increasing the glycolytic activity (Figure 8C). GAPDH directly produces reduced nucleotide cofactors and as a consequence maintains the net production of reducing equivalents for glycolysis. Oxidized but not reduced forms of nucleotides were sufficiently changed to increase the ratios of NAD(P)H/NAD(P) in gapc1-1 gapc2-1 lines (Figures 8D to 8I). Increases in NAD(P)H for GAPC OE lines resulted in further enhancements in the reducing power ratios (Figures 8D, 8G, 8F, and 8I).
Figure 8.
Metabolic Impact of GAPCs on ATP/ADP, NADH/NAD+, and NADPH/NADP+ in Developing Seeds.
(A) ATP content.
(B) ADP content.
(C) ATP/ADP ratio.
(D) NADH content.
(E) NAD+ content.
(F) NADH/NAD+ ratio.
(G) NADPH content.
(H) NADP+ content.
(I) NADPH/NADP+ ratio.
Compounds were extracted from T2 developing seeds (12 to 14 DAF) and quantified by comparison with authentic standards. Values are means ± se (n = 4). “H” and “L” indicate significantly higher and lower than the wild type (Student’s t test, P < 0.05).
The TCA cycle provides an additional source of NADH coupled to electron transport resulting in ATP production. The TCA cycle respires carbon provided from pyruvate and is therefore tied to glycolysis during aerobic metabolism. Organic acids in the TCA cycle were quantified to better understand the consequences of GAPC manipulation. All measured organic acids, including citrate, aconitrate, isocitrate, 2-oxoglutarate, succinate, fumarate, and malate, were significantly decreased in gapc1-1 gapc2-1 (Table 1). OE of GAPC2 also decreased the levels of succinate and fumarate, whereas the level of citrate, aconitate, isocitrate, 2-oxoglutarate, and malate were comparable to the wild type (Table 1). The reduction in KO lines likely reflects depletion of intermediates that accompanies reduced growth and TCA activity. The decreases in OE lines of specific metabolites is less clear but could be related to the use of malate or citrate for fatty acid metabolism (Schwender et al., 2006; Allen et al., 2009; Alonso et al., 2011; Allen and Young, 2013), as OE lines have increased fatty acid elongation (Figure 2D, requiring a cytosolic form of acetyl-CoA, reductant, and energy (Schwender et al., 2006). Other metabolites in the pentose phosphate pathway and starch biosynthesis pathway were only modestly altered, as summarized in Supplemental Table 1.
Table 1. Content of Organic Acids in Developing Seeds (12 to 14 DAF).
| Metabolite | Wild Type | gapc1-1 gapc2-1 | ProCON:GAPC1-OE2 | ProCON:GAPC2-OE1 |
|---|---|---|---|---|
| Citrate | 9437.6 ± 879.6a | 6247.2 ± 625.8b | 10328.7 ± 933.1a | 10576.8 ± 1185.5a |
| Aconitate | 4.9 ± 0.4a | 3.5 ± 0.4b | 4.6 ± 0.4a | 5.5 ± 0.4a |
| Isocitrate | 49.5 ± 5.1a | 31.2 ± 3.1b | 48.6 ± 2.1a | 42.7 ± 3.4a |
| 2-Oxoglutarate | 67.9 ± 5.5a | 44.34 ± 4.0b | 61.5 ± 2.3a | 64.1 ± 5.0a |
| Succinate | 207.0 ± 17.1a | 111.6 ± 12.1b | 49.8 ± 8.3c | 36.3 ± 3.0c |
| Fumarate | 71.2 ± 5.2a | 39.5 ± 1.2b | 56.6 ± 6.9a | 29.9 ± 3.7b |
| Malate | 2437.0 ± 124.8a | 1843.5 ± 216.1b | 2266.3 ± 255.8a,b | 1934.2 ± 269.9a,b |
Values (nmol/g fresh weight) are means ± se (n = 4). One-way ANOVA was performed, and different lowercase letters mark significant difference between means (P < 0.05).
The decrease in reduced forms of nucleotide cofactors was modest for KO lines, but increased significantly in OE lines. Oxidized nucleotide cofactors were also reduced for the KO lines, but quantitatively similar for OE; thus, the ratios of reduced to oxidized forms increased most significantly in the OE lines. In the case of increased oil production, GAPDH OE in the cytosol would create more reduced nucleotides, which are necessary for fatty acid biosynthesis or possibly elongation and desaturation that occur outside of the plastid. Thus, the increased ratio of reduced cofactors may represent an incompletely tapped cytosolic source of reducing power for altered fatty acid profiles in the OE lines (Figures 2D and 2E).
In conclusion, this study provides significant and novel information in several ways: First, the data of the single and double GAPC KOs clearly show that these two genes have overlapping functions and single and double KOs do not have growth defects. Second, because of the presence of seven different GAPDHs, the function of GAPCs in plant metabolism is unclear. This study demonstrates that GAPCs impact glycolysis. In addition, our data indicate a potential feedback regulation of TPI by PEP levels in Arabidopsis. Third, this study shows that KO and OE of GAPCs have opposite effects on seed oil accumulation, connecting GAPCs with seed oil production. The production of oil and fatty acid profiles are consistent with enhanced GAPDH and glycolytic activity in the cytosol, suggesting that the balance of reductant, energy, and carbon are all important contributors to the final levels of oil in developing seeds. Given that GAPDH isoforms are an integral enzymatic step in glycolytic pathways that are found in multiple subcellular locations, these enzymes tie ATP and reductant status in the cell and link central carbon metabolism and lipid production. The changes in oil levels indicate that this class of enzymes is an important biotechnological target for metabolic engineering efforts aimed at increasing energy dense compounds in plants. Furthermore, changes in cellular metabolism characterized through metabolite and cofactor levels highlight the complexity and tolerance of Arabidopsis cells to the metabolic perturbation. The results also describe a clear male sterility phenotype that occurs with overexpression of either GAPC, raising intriguing questions for further investigation.
METHODS
Plant Growth Conditions
Arabidopsis thaliana wild-type (ecotype Columbia-0) and T-DNA insertion KO lines were obtained from ABRC at Ohio State University. The mutants were screened and the homozygotes were obtained previously (Guo et al., 2012). Plants were grown in soil in a growth chamber with cool white light of 160 μmol m−2 s−1 under 16 h light/8 h dark and 23°C/19°C cycles.
Generation of GAPC Transgenic Arabidopsis
To overexpress GAPC under the control of the cauliflower mosaic virus 35S promoter, GAPC1 and GAPC2 cDNA were cloned into p35S-FAST (Ge et al., 2005) or p35S-FAST/eYFP (derived from p35S-FAST by introducing eYFP into the XmaI and BamHI restriction sites). To generate seed-specific OE of GAPC, GAPC1 and GAPC2 cDNA were first cloned into the NotI site of the BetaConSoyHyg plasmid (kindly provided by Edgar Cahoon at University of Nebraska-Lincoln). The BetaConSoyHyg plasmid contains the soybean (Glycine max) β-conglycinin promoter and the coding sequence of a hygromycin B phosphotransferase gene. The DNA cassette containing the promoter, GAPC1 or 2 coding sequence, and terminator was released by AscI digestion and then inserted into the AscI site of binary vector pYZ101-Asc (kindly provided by Zhanyuan Zhang at University of Missouri-Columbia). pYZ101-Asc had the BASTA resistant gene for transgenic plant selection. The primers used for cloning of GAPC are listed in Supplemental Table 2. The sequences of constructs were verified by sequencing. The above constructs were transformed into the C58C1 Agrobacterium tumefaciens strain and transformed into Arabidopsis to obtain transgenic plants. Transgenic plants were screened by antibiotics and PCR. Expression of GAPC proteins was determined by immunoblotting with anti-FLAG antibody.
GAPDH Enzyme Activity Assay
NAD-dependent GAPDH activity was assayed using spectrophotometric quantification (340 nm) of NADH with modification according to the method described previously (Rius et al., 2008). Arabidopsis developing seeds were collected and ground in a 1.5-mL centrifuge tube by a pestle. Total protein was extracted from ground seeds using chilled buffer A containing 50 Tris-HCl, pH 7.5, 10 mM KCl, 1 mM EDTA, 0.5 mM PMSF, and 2 mM DTT (Fan et al., 1999). The homogenate was centrifuged at 10,000g for 20 min and the supernatant containing 15 to 30 μg of protein was used for each activity assay.
RNA Extraction and Real-Time PCR Analyses
Developing seeds were ground in liquid nitrogen and total RNA was extracted using RNeasy Plant Mini Kit (Qiagen). RNA was digested with RNase-free DNase I on a column and 0.5 µg RNA was used for synthesis of the first-strand cDNA using an iScript cDNA synthesis kit (Bio-Rad). Real-time PCR was performed as described previously (Li et al., 2006). UBQ10 (At4g05320) was used as a control gene. The expression level of each gene was normalized to that of UBQ10. PCR was performed with a MyiQ System (Bio-Rad) using SYBR Green. The following program was used for real-time PCRs: 95°C for 3 min and 50 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 30 s.
Fatty Acid Composition and Oil Content
Dried Arabidopsis seeds (3 to 5 mg/sample) were placed in glass tubes with Teflon-lined screw caps. Then, 2 mL methanol with 5% (v/v) H2SO4 and 0.01% butylated hydroxyl toluene was added to the glass tube. The tubes were incubated for 90 min in an 85°C water bath for transmethylation. Fatty acid methyl esters (FAMEs) were extracted with hexane. FAMEs were quantified using gas chromatography supplied with a hydrogen flame ionization detector and a capillary column SUPELCOWAX-10 (30 m; 0.25 mm) with helium carrier at 20 mL/min. The oven temperature was maintained at 170°C for 1 min and then increased in steps to 210°C, raising the temperature by 3°C every min. FAMEs from TAG were identified by comparing their retention times with known standards. Heptacanoic acid (17:0) was used as the internal standard to quantify the amounts of individual fatty acids. Fatty acid composition was calculated as mol %.
Metabolite Analyses by LC-MS/MS
Metabolite extraction and analyses was performed by LC-MS/MS using a modified method described previously (Luo et al., 2007; Arrivault et al., 2009). About 30 mg of developing seeds was harvested and ground in liquid nitrogen. Two milliliters of methanol/chloroform (7:3, v/v; −20°C) was added and mixed by vortex followed by the addition of 0.9 μg PIPES as the internal standard. The mixtures were maintained for 2 h at −20°C with occasional vortexing. Polar metabolites were extracted from the methanol/chloroform phase by the addition of 1.6 mL water to each sample and then centrifuged at 1000 rpm after vigorous vortexing. The methanol-water phase was then transferred to a new tube. Another 1.6 mL of water was added to each sample to extract polar metabolites one more time. Two extracts were pooled and dried by N2 aspiration at room temperature. The dried extract was redissolved with 100 μL water and filtered with 0.45 μM cellulose acetate centrifuge tube filters and then diluted by 10- or 100-fold for metabolite analyses by LC-MS/MS. The standard curve for each metabolite was generated using authentic standards for quantification of targeted metabolites. Sugar phosphates, organic acids, ATP/ADP, and NAD(P)/NAD(P)H were determined by interpolating from the linear relationship between peak area and standard concentration.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL databases under the following accession numbers: GAPC1, At3g04120; GAPC2, At1g13440; LPAAT1, At4g30580; LPAAT2, At3g57650; LPAAT4, At1g75020; LPAAT5, At3g18850; LPP1, At2g01180; LPP2, At1g15080; LPP3, At3g02600; DGAT1, At2g19450; DGAT2, At3g51520; PDAT1, At5g13640; PDAT2, At3g44830; CCT1, At2g32260; CCT2, At4g15130; AAPT1, At1g13560; AAPT2, At3g25585; and UBQ10, At4g05320.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Fatty Acid Composition of GAPC Double Knockouts Compared with the Wild Type.
Supplemental Figure 2. Subcellular Localization of GAPC.
Supplemental Figure 3. OE of GAPCs under the Control of 35S Promoter Resulted in Bigger and Heavier Seeds.
Supplemental Figure 4. OE of GAPCs under the Control of 35S Promoter Causes Male Sterility.
Supplemental Figure 5. Both Wild-Type and gapc1-1 gapc2-1 Pollen Rescued Silique Development of 35S Promoter–Driven OE Plants.
Supplemental Figure 6. Siliques Develop Normally on Plants of Seed-Specific OE of GAPC.
Supplemental Figure 7. Seed-Specific OE of GAPCs Alters Fatty Acid Composition in the Seeds.
Supplemental Table 1. Metabolites Involved in the Pentose Phosphate Pathway and Starch Biosynthesis Pathway.
Supplemental Table 2. Primer List.
Supplementary Material
Acknowledgments
This work was supported by the U.S. Department of Energy (DE-SC0001295 and DE-AR0000202), the USDA (2007-35318-18393), and the National Science Foundation (EF-1105249; IOS-0818740). Any product or trademark mentioned here does not imply a warranty, guarantee, or endorsement by the authors or their affiliations over other suitable products.
AUTHOR CONTRIBUTIONS
L.G., D.K.A., and X.W. designed the research. L.G., F.M., F.W., and B.F. performed experiments. L.G., D.K.A., and X.W. wrote the article. All authors commented on the writing of the article.
Glossary
- GAP
glyceraldehyde-3-phosphate
- KO
knockout
- OE
overexpression
- PA
phosphatidic acid
- PEP
phosphoenolpyruvate
- DAF
days after flowering
- TAG
triacylglycerol
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- TCA
tricarboxylic acid
- TPI
triose phosphate isomerase
- FAME
fatty acid methyl ester
Footnotes
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Allen D.K., Young J.D. (2013). Carbon and nitrogen provisions alter the metabolic flux in developing soybean embryos. Plant Physiol. 161: 1458–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D.K., Ohlrogge J.B., Shachar-Hill Y. (2009). The role of light in soybean seed filling metabolism. Plant J. 58: 220–234. [DOI] [PubMed] [Google Scholar]
- Alonso A.P., Val D.L., Shachar-Hill Y. (2011). Central metabolic fluxes in the endosperm of developing maize seeds and their implications for metabolic engineering. Metab. Eng. 13: 96–107. [DOI] [PubMed] [Google Scholar]
- Andre C., Froehlich J.E., Moll M.R., Benning C. (2007). A heteromeric plastidic pyruvate kinase complex involved in seed oil biosynthesis in Arabidopsis. Plant Cell 19: 2006–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrivault S., Guenther M., Ivakov A., Feil R., Vosloh D., van Dongen J.T., Sulpice R., Stitt M. (2009). Use of reverse-phase liquid chromatography, linked to tandem mass spectrometry, to profile the Calvin cycle and other metabolic intermediates in Arabidopsis rosettes at different carbon dioxide concentrations. Plant J. 59: 826–839. [DOI] [PubMed] [Google Scholar]
- Baud S., Wuillème S., To A., Rochat C., Lepiniec L. (2009). Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in Arabidopsis. Plant J. 60: 933–947. [DOI] [PubMed] [Google Scholar]
- Cernac A., Benning C. (2004). WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J. 40: 575–585. [DOI] [PubMed] [Google Scholar]
- Chapman K.D., Ohlrogge J.B. (2012). Compartmentation of triacylglycerol accumulation in plants. J. Biol. Chem. 287: 2288–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhiti M., Yang C., Chan A., Durnin D.C., Belmonte M.F., Ayele B.T., Tahir M., Stasolla C. (2012). Altered seed oil and glucosinolate levels in transgenic plants overexpressing the Brassica napus SHOOTMERISTEMLESS gene. J. Exp. Bot. 63: 4447–4461. [DOI] [PubMed] [Google Scholar]
- Fan L., Zheng S., Cui D., Wang X. (1999). Subcellular distribution and tissue expression of phospholipase Dalpha, Dbeta, and Dgamma in Arabidopsis. Plant Physiol. 119: 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focks N., Benning C. (1998). wrinkled1: A novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol. 118: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X., Dietrich C., Matsuno M., Li G., Berg H., Xia Y. (2005). An Arabidopsis aspartic protease functions as an anti-cell-death component in reproduction and embryogenesis. EMBO Rep. 6: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y., Vigeolas H., Tiessen A., Geigenberger P., Stitt M. (2002). Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. Plant J. 30: 221–235. [DOI] [PubMed] [Google Scholar]
- Grüning N.M., Du D., Keller M.A., Luisi B.F., Ralser M. (2014). Inhibition of triosephosphate isomerase by phosphoenolpyruvate in the feedback-regulation of glycolysis. Open Biol. 4: 130232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Devaiah S.P., Narasimhan R., Pan X., Zhang Y., Zhang W., Wang X. (2012). Cytosolic glyceraldehyde-3-phosphate dehydrogenases interact with phospholipase Dδ to transduce hydrogen peroxide signals in the Arabidopsis response to stress. Plant Cell 24: 2200–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajirezaei M.R., Biemelt S., Peisker M., Lytovchenko A., Fernie A.R., Sonnewald U. (2006). The influence of cytosolic phosphorylating glyceraldehyde 3-phosphate dehydrogenase (GAPC) on potato tuber metabolism. J. Exp. Bot. 57: 2363–2377. [DOI] [PubMed] [Google Scholar]
- Hancock J.T., Henson D., Nyirenda M., Desikan R., Harrison J., Lewis M., Hughes J., Neill S.J. (2005). Proteomic identification of glyceraldehyde 3-phosphate dehydrogenase as an inhibitory target of hydrogen peroxide in Arabidopsis. Plant Physiol. Biochem. 43: 828–835. [DOI] [PubMed] [Google Scholar]
- Holtgrefe S., Gohlke J., Starmann J., Druce S., Klocke S., Altmann B., Wojtera J., Lindermayr C., Scheibe R. (2008). Regulation of plant cytosolic glyceraldehyde 3-phosphate dehydrogenase isoforms by thiol modifications. Physiol. Plant. 133: 211–228. [DOI] [PubMed] [Google Scholar]
- Kim S.C., Guo L., Wang X. (2013). Phosphatidic acid binds to cytosolic glyceraldehyde-3-phosphate dehydrogenase and promotes its cleavage in Arabidopsis. J. Biol. Chem. 288: 11834–11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeir A.M., Opperdoes F.R., Wierenga R.K. (1987). Kinetic properties of triose-phosphate isomerase from Trypanosoma brucei brucei. A comparison with the rabbit muscle and yeast enzymes. Eur. J. Biochem. 168: 69–74. [DOI] [PubMed] [Google Scholar]
- Li M., Qin C., Welti R., Wang X. (2006). Double knockouts of phospholipases Dzeta1 and Dzeta2 in Arabidopsis affect root elongation during phosphate-limited growth but do not affect root hair patterning. Plant Physiol. 140: 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B., Groenke K., Takors R., Wandrey C., Oldiges M. (2007). Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography-mass spectrometry. J. Chromatogr. A 1147: 153–164. [DOI] [PubMed] [Google Scholar]
- Mandaokar A., Browse J. (2009). MYB108 acts together with MYB24 to regulate jasmonate-mediated stamen maturation in Arabidopsis. Plant Physiol. 149: 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M., Browse J. (1996). The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell 8: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin, F., Arisz, S.A., Dekker, H.L., Kramer, G., de Koster, C.G., Haring, M.A., Munnik, T., and Testerink, C. (2013). Identification of novel candidate phosphatidic acid-binding proteins involved in the salt-stress response of Arabidopsis thaliana roots. Biochem. J. 450: 573–581. [DOI] [PubMed] [Google Scholar]
- Muñoz-Bertomeu J., Cascales-Miñana B., Mulet J.M., Baroja-Fernández E., Pozueta-Romero J., Kuhn J.M., Segura J., Ros R. (2009). Plastidial glyceraldehyde-3-phosphate dehydrogenase deficiency leads to altered root development and affects the sugar and amino acid balance in Arabidopsis. Plant Physiol. 151: 541–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Bertomeu J., Cascales-Miñana B., Irles-Segura A., Mateu I., Nunes-Nesi A., Fernie A.R., Segura J., Ros R. (2010). The plastidial glyceraldehyde-3-phosphate dehydrogenase is critical for viable pollen development in Arabidopsis. Plant Physiol. 152: 1830–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J., Browse J. (1995). Lipid biosynthesis. Plant Cell 7: 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W.C. (1996). The organization and regulation of plant glycolysis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 185–214. [DOI] [PubMed] [Google Scholar]
- Rius S.P., Casati P., Iglesias A.A., Gomez-Casati D.F. (2006). Characterization of an Arabidopsis thaliana mutant lacking a cytosolic non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase. Plant Mol. Biol. 61: 945–957. [DOI] [PubMed] [Google Scholar]
- Rius S.P., Casati P., Iglesias A.A., Gomez-Casati D.F. (2008). Characterization of Arabidopsis lines deficient in GAPC-1, a cytosolic NAD-dependent glyceraldehyde-3-phosphate dehydrogenase. Plant Physiol. 148: 1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska S.A., Girke T., Benning C., Ohlrogge J.B. (2002). Contrapuntal networks of gene expression during Arabidopsis seed filling. Plant Cell 14: 1191–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwender J., Ohlrogge J.B., Shachar-Hill Y. (2003). A flux model of glycolysis and the oxidative pentosephosphate pathway in developing Brassica napus embryos. J. Biol. Chem. 278: 29442–29453. [DOI] [PubMed] [Google Scholar]
- Schwender J., Shachar-Hill Y., Ohlrogge J.B. (2006). Mitochondrial metabolism in developing embryos of Brassica napus. J. Biol. Chem. 281: 34040–34047. [DOI] [PubMed] [Google Scholar]
- Song S., Qi T., Huang H., Ren Q., Wu D., Chang C., Peng W., Liu Y., Peng J., Xie D. (2011). The jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23: 1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi M., Zaffagnini M., Festa M., Trost P., Lo Schiavo F., Costa A. (2013). Nuclear accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase in cadmium-stressed Arabidopsis roots. Plant Physiol. 162: 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeolas H., Möhlmann T., Martini N., Neuhaus H.E., Geigenberger P. (2004). Embryo-specific reduction of ADP-Glc pyrophosphorylase leads to an inhibition of starch synthesis and a delay in oil accumulation in developing seeds of oilseed rape. Plant Physiol. 136: 2676–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.A., Todd J., Newman T., Focks N., Girke T., de Ilárduya O.M., Jaworski J.G., Ohlrogge J.B., Benning C. (2000). A new set of Arabidopsis expressed sequence tags from developing seeds. The metabolic pathway from carbohydrates to seed oil. Plant Physiol. 124: 1582–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.