Abstract
Inflammatory myofibroblastic tumor (IMT) is a rare neoplasm, most commonly seen in children and adolescents. It can occur in nearly every part of the body. Imaging properties and the clinical presentation of IMT can mimic malignant process. A 41-year-old female presented with cough of 3 months duration. Chest X-ray showed a coin shadow in the right upper lobe. Positron emission tomography/computed tomography scan showed a 3.2 × 2.4 cm lesion with homogeneous appearance with a very high fluorodeoxyglucose uptake value, suggesting a neoplastic process. She underwent lobectomy and the final diagnosis was IMT.
Keywords: Inflammatory myofibroblastic tumor, lung, plasma cell granuloma, xanthogranulma
Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare neoplasm of mesenchymal origin, most commonly seen in the lungs of children and adolescents and it occurs in nearly every part of the body. Imaging properties and the clinical presentation of IMT can mimic malignant process. The patient presented with a mass lesion in the right upper lobe of the lung, which was showing homogeneous appearance on computed tomography (CT) scan with a very high fluorodeoxyglucose (FDG) uptake value, suggesting a malignant process. She underwent lobectomy and the final diagnosis was IMT.
Case Report
The case we present here is about a 41-year-old female patient presented with cough of 3 months duration. She had no history of hemoptysis or weight loss. Physical examination was normal. Chest X-ray [Figure 1] showed a coin shadow in the right upper lobe. CT showed a homogenously enhancing spiculated soft tissue lesion of size 3.2 × 2.4 cm, in apical segment of right upper lobe. On the whole-body 18FDG positron emission tomography (PET)/CT, the lesion had a high focal fluorodeoxyglucose-uptake (maximum standardized uptake value [SUVmax] 16.8 g/ml) [Figure 2]. In the absence of fever, the first possibility considered was neoplastic. Fine-needle aspiration cytology was inconclusive. Hence, she underwent upper lobectomy. Gross examination showed partly circumscribed yellowish white firm lesion with vague lobulation measuring 2.7 × 2.8 × 2.5 cm. Sections studied showed an ill-defined neoplasm consisting of cellular as well as hypocellular areas, consisting of spindle cells with elongated vesicular nuclei having small nucleoli and eosinophilic cytoplasm with indistinct borders. Admixed with the spindle cells were a dense infiltrate of lymphoplasmacytic cells along with histiocytes. No mitoses or necrosis was noted. Immunohistochemistry showed positvity for vimentin, smooth muscle actin and desmin and negative for CD117 confirming the diagnosis of IMT. She is doing well and is on regular follow-up.
Figure 1.
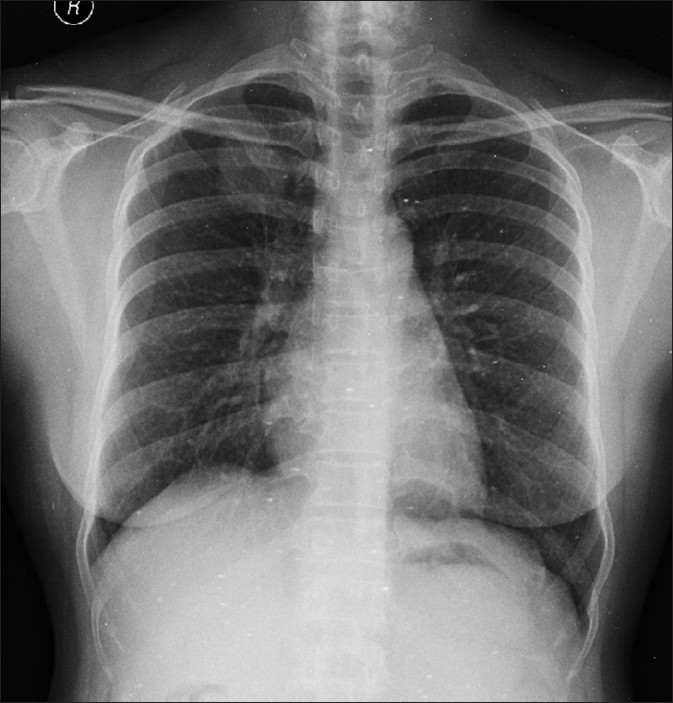
Chest X-ray showing coin shadow
Figure 2.
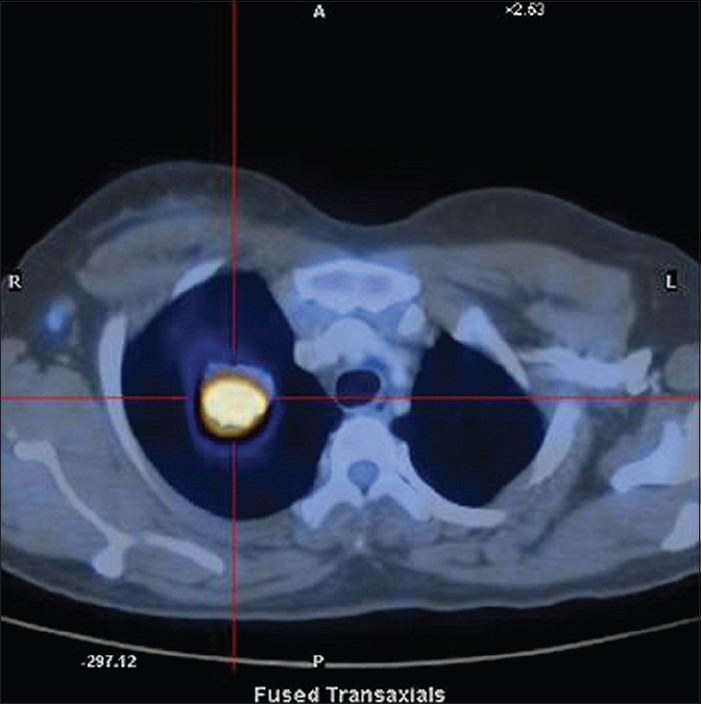
18Fluorodeoxyglucose Positron emission tomography/computed tomography scan showing a homogenously enhancing spiculated soft tissue lesion in the upper lobe with a high focal fluorodeoxyglucose-uptake (maximum standardized uptakevalue 16.8 g/ml)
Discussion
Inflammatory myofibroblastic tumor is an idiopathic benign mass lesion composed of fibrous tissues, myofibroblasts and marked inflammatory infiltration, predominantly plasma cells. IMT is also known as plasma cell granuloma, xanthogranuloma, inflammatory pseudotumor, and fibrous histiocytoma.[1] IMT is very rare with an incidence of approximately 0.04-1% of all the pulmonary neoplasms. Other common sites are mesentery, omentum, larynx, liver spleen, and breast. The clinical and imaging characteristics of this lesion are similar to that of a neoplasm. The biological behavior and the tendency of spontaneous regression is suggestive of a benign nature. Some reports suggest IMT may be a neoplastic disorder and few cases of metastasizing IMT have also been reported.[2]
Pathogenesis of IMT is not known. Microscopically, IMT is characterized by abundant inflammatory infiltrate consisting of predominantly plasma cells, lymphocytes, histiocytes, admixed with a variable proportion of fibroblasts and myofibroblasts. On immunohistochemistry tumor cells exhibit strong diffuse positivity with smooth muscle actin and vimentin, and are negative for cytokeratin, CD34 and S100.[3] Pulmonary IMT is divided into two types: One is invasive and another one is noninvasive . Invasive IMT usually occurs among younger patients and may reach large size invading surrounding structures.
Inflammatory myofibroblastic tumor can occur at any age, but is most commonly seen in the second decade of life. Patients present with cough, fever, and hemoptysis. On imaging, IMT usually appears as a single peripheral, lobulated mass, predominantly occurring in the lower lobe, however it can be multiple in 5% of cases. Calcification can occur and is more common in children than in adults. On CT scan, the mass shows heterogeneous enhancement.[4] However in our patient, the lesion was located in the upper lobe and it showed homogenous enhancement on CT scan, which is rare.
Inflammatory myofibroblastic tumor can also be FDG-avid on PET/CT images. Few authors have reported the use of FDG-PET/CT in IMT, some of them showed very high SUV values. The range of reported SUV values varies from 5 to >35 g/ml.[5,6,7] This makes differentiation of IMT difficult from other neoplasms. The possible reason for such a high uptake in these benign tumors is probably the associated intense inflammation. This leads to increased metabolic activity, which in turn leads to high uptake on FDG-PET/CT scan. Few workers have described increased uptake on somatostatin receptor imaging (like 111In octreotide and 68Ga DOTATOC) and this has been attributed to increased expression of somatostatin receptors in the inflammatory cells.[8,9]
The mainstay of treatment is radical resection with negative margins. In case of invasive IMT surgical removal of adjacent structures may be necessary. For patients who cannot undergo surgery, radiation and corticosteroids have been used. Long-term surveillance is important because local and distant recurrence and sarcomatous degeneration has also been reported in IMT. Long-term survival is usually good (5 years survival 91%). Here, we report a 41-year-old female with pulmonary IMT with high FDG uptake mimicking malignancy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Takeda S, Onishi Y, Kawamura T, Maeda H. Clinical spectrum of pulmonary inflammatory myofibroblastic tumor. Interact Cardiovasc Thorac Surg. 2008;7:629–33. doi: 10.1510/icvts.2007.173476. [DOI] [PubMed] [Google Scholar]
- 2.Fabre D, Fadel E, Singhal S, de Montpreville V, Mussot S, Mercier O, et al. Complete resection of pulmonary inflammatory pseudotumors has excellent long-term prognosis. J Thorac Cardiovasc Surg. 2009;137:435–40. doi: 10.1016/j.jtcvs.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–68. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 4.Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory pseudotumor: The great mimicker. AJR Am J Roentgenol. 2012;198:W217–27. doi: 10.2214/AJR.11.7288. [DOI] [PubMed] [Google Scholar]
- 5.Sato M, Takasaka I, Okumura T, Shioyama Y, Asato Y, Yoshimi F, et al. F-18 fluorodeoxyglucose accumulation in an inflammatory pseudotumor of the spleen. Ann Nucl Med. 2007;21:521–4. doi: 10.1007/s12149-007-0055-6. [DOI] [PubMed] [Google Scholar]
- 6.Reddy MP, Menda Y, Floresca J, Juweid M, Graham MM. FDG positron emission tomographic imaging of pseudo-pseudo tumor. Clin Nucl Med. 2002;27:445–6. doi: 10.1097/00003072-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Huellner MW, Schwizer B, Burger I, Fengels I, Schläpfer R, Bussmann C, et al. Inflammatory pseudotumor of the lung with high FDG uptake. Clin Nucl Med. 2010;35:722–3. doi: 10.1097/RLU.0b013e3181ea33d0. [DOI] [PubMed] [Google Scholar]
- 8.Calabrese F, Zuin A, Brambilla E, Zucchetta P, Lunardi F, Valente M, et al. Pulmonary inflammatory myofibroblastic tumour with unusual octreoscan uptake: Two reports. Eur Respir J. 2010;35:448–50. doi: 10.1183/09031936.00053609. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Jindal T, Dutta R, Kumar R. Functional imaging in differentiating bronchial masses: An initial experience with a combination of (18)F-FDG PET-CT scan and (68) Ga DOTA-TOC PET-CT scan. Ann Nucl Med. 2009;23:745–51. doi: 10.1007/s12149-009-0302-0. [DOI] [PubMed] [Google Scholar]


