Abstract
Background
The African American Heart Failure Trial (A-HeFT) and the FDA approval of BiDil for race-specific prescription have stirred the debate about the scientific and medical status of race. Yet there is no assessment of the potential fallouts of this dispute on physicians’ willingness to prescribe the drug. We present here an analysis of the factors influencing physicians’ prescription of BiDil and investigate whether exposure to the controversy has an impact on their therapeutic judgments about the drug.
Methods
We conducted an electronic survey with physicians in the department of internal medicine at the University of Cincinnati. Participants were randomly assigned to two groups, with one group receiving information about the controversy over BiDil. We used various statistical tests, including a linear mixed effects model, to analyze the results.
Results
27% of the participants reported using patients’ race as a major factor in making treatment decisions. 33% reported the inefficacy of standard therapies, 25% the severity of the disease, and 15% other unspecified factors as primary determining criteria in prescribing BiDil. With respect to the controversy, 68% of physicians reported that they were not aware of any controversy surrounding BiDil. Physicians’ willingness to prescribe BiDil as a therapy was associated with their awareness of the controversy surrounding A-HeFT (p < 0.003). But their willingness to prescribe the therapy along racial lines did not vary significantly with exposure to the controversy.
Conclusions
Overall, physicians prescribe and are willing to prescribe BiDil more to black patients than to white patients. However, physicians’ lack of awareness about the controversial scientific status of A-HeFT suggests the need for more efficient ways to convey scientific information about BiDil to clinicians. Furthermore, the uncertainties about the determination of clinical utility of BiDil for the individual patient raise questions about whether this specific race-based therapy is in patients’ best interest.
Keywords: African American Heart Failure Trial (A-HeFT), BiDil, clinical utility, mixed-model, race-based therapy, race instrumentalism
INTRODUCTION
The scientific status of race in human population genomics and the utility of race-based therapies in the clinic continue to divide researchers (Burchard et al. 2003; Collins 2004; Krieger 2005; Long, Li, and Healy 2009; Maglo 2011; Risch et al. 2002; Root 2010; Krieger 2005; Wilson et al. 2001). The introduction of BiDil in the clinic as a race-specific drug in 2005 has only exacerbated the controversy by giving the impression that “race” is a valid scientific category. However, careful scrutiny reveals that while race may indeed be a convenient problem-solving tool in well-defined situations (Kitcher 2007; Ossorio and Duster 2005; Maglo and Martin 2012; Krieger 2012a, 2012b; Krieger et al. 2013), the success of the African American Heart Failure Trial (A-HeFT) does not justify the attribution of a biological basis to race (Bloche 2004; Maglo 2012; Kahn 2013).
BiDil is currently commercialized by Arbor Pharmaceuticals but was initially commercialized by Nitromed and approved by the FDA in June 2005 following the completion of the A-HeFT study. Results of the trial demonstrated that a fixed dose combination of two generic drugs, isosorbide dinitrate and hydralazine hydrochloride, reduced first hospitalization by 33% and increased survival rate by 43% in self-identified African American patients with congestive heart failure (Taylor et al. 2004). However, critics sharply called into question the alleged race-based efficacy of BiDil and charged that the putative race-based benefits of BiDil were driven by non-clinical and non-scientific factors (Bloche 2004; Duster 2007; Kahn 2004; Sankar and Kahn 2005).
This dispute has also prompted growing concerns about racial profiling in clinics serving patients of diverse ethnic backgrounds (Kahn 2013; Satel 2002; Wolinsky 2011). Because of the poorly defined nature of race, its usefulness as a tool to facilitate therapeutic intervention is unclear. One such example is offered by Satel (2006), in which putative findings regarding the relationship between race and treatment response had to be retracted due to lack of corroboration in the literature.
However, not only are drugs being reported with greater levels of efficacy in certain populations, but targeted race-based therapies are also increasingly being brought to market and, ultimately, to the bedside (Exner et al. 2001; Grens 2007; Kahn 2013; Tate and Goldstein 2004). Whereas the controversy sparked by BiDil has focused mainly on theoretical issues (e.g., status of race in science, pitfalls of FDA regulatory decisions), other more practical issues (e.g., actual use of BiDil in clinics) have largely been overlooked (Akinniyi and Payne 2011; Frank et al. 2010). Despite BiDil’s use as a standard of care for self-identified black patients, few, if any, studies have documented the impact of the theoretical dispute over race on physicians’ prescription patterns.
The purpose of this study was threefold: first to evaluate the scientific context in which BiDil has emerged as a race-based therapy; second, to identify factors influencing physicians’ prescription patterns; and finally, to determine whether physicians’ willingness to prescribe the drug is associated with awareness of the theoretical dispute over BiDil as a race-based therapy.
From the Genomics Lab to the Cardiology Clinic
The Genomic Race Debate
The recent revival of the conflict over the relevance of race in the clinic originates partly in genomics. The Human Genome Project was predicated on its potential to lead to a new revolution in scientific medicine. Genomic studies of human evolutionary history and migration patterns have revealed the existence of population substructures within our species. Despite tremendous similarity among humans (99.9%),1 technological advancements increasingly allow analysis of population differences attributable to various factors, including genetic mutations, gene flow, mating patterns, and isolation by distance. Of the 1% genetic differences among humans, the Fst (which measures the genetic variation among populations) ranges from 3% to 5%, while individual variation accounts for 93% to 95% (Rosenberg et al. 2002). Continental genetic cluster, which is interpreted as race (Maglo 2011; Royal et al. 2010), accounts for less than 2% of the pairwise Fst compared to 77% for isolation by distance (Handley et al. 2007).
These findings initially raised expectations that studies of human populations might help identify both susceptibility- and benefit-related gene variants that modify human health, thus bridging the gap between human evolutionary history and genomic medicine (Jorde, Watkins, and Bamshad 2001). These hopes contributed to the rise of various scientific projects, including the Human Genome Diversity Project (HGDP) and the international human genome Haplotype Map (HapMap) project. In the wake of technological improvements, large scale studies of genetic variants that influence disease susceptibility and drug response were a near reality by the close of the 20th century.
Genome wide association studies and whole genome sequencing appear to suggest that genomic medicine is gradually opening up a new era in scientific medicine. In fact, it is the field of human population genomics itself that is in the making. A traditional goal of population genetics is the construction of mathematical models that map the effects of inbreeding and the four evolutionary “forces” (mutation, drift, selection, and gene flow). Unfolding the genetic history of human populations and understanding the biomedical implications of population substructure have also become crucial and urgent issues in human population genomics.
Genomic studies of isolated populations and the use of cluster analysis to determine continental ancestry have necessitated a theoretical framework to justify sampling strategies and to account for observed human population differences. Race was the readily available and familiar concept to account for subspecies level differences. But applying the biological subspecies concept to human populations has been a matter of a controversy since the rise of Darwinian biology (Maglo 2011). Researchers resurrected this old debate not merely by using race in their studies but by arguing that race is a valid biological category with biomedical implications (Burchard et al. 2003; Risch et al. 2002).
Genomics has thus reenlisted race in what the sociologist Troy Duster called “the molecular reinscription of race” (Duster 2006). During this process of reenlistment, continental genetic clusters became synonymous with race, and researchers began to suggest that race may be considered a useful proxy for human population substructure in biomedical research (Burchard et al. 2003; Cooper, Kaufman, and Ward 2003; Rosenberg et al. 2002; Royal et al. 2010). Thus, race-based stratification in clinical research finds a justification, at least in part, in genomics.
In the US, the OMB Revised Directive 15 played a significant role in promoting the role of race in biomedical research by mandating the collection of data along racial lines in studies supported by federal grants (Kahn 2013; Maglo and Martin 2012). These kinds of regulations governing the use of race in scientific and medical research appear to be specific to the US (Cooper, Kaufman, and Ward 2003; Kahn 2013). Nonetheless, epidemiological research has persistently demonstrated that disease incidence varies among human populations. Likewise, researchers have become aware that drug response also varies among populations. In fact, they have also became aware that
…many drugs that show therapeutic potential never reach the market because of adverse reactions in some individuals, whereas other drugs in common use are effective for only a fraction of the population in which they are prescribed.(Wilson et al. 2001, 265)
Epidemiological and clinical knowledge also suggest that population substructure may offer some insights into probing beneficial genetic variants in disease surveillance and pharmacogenomics.
Yet it is not the success of race-based pharmacogenomics that brought race into the clinic but rather the emergence of race-based therapy that was not informed by genomic findings. The clinical reengineering of race posits race as a proxy for continental genetic ancestry and assumes that there is a convergence between clinical categorizations of patients and the phylogenomic determination of human population substructure (Burchard et al. 2003; Maglo 2012; Risch et al. 2002; Tang et al. 2005). BiDil has played a major role in this emerging clinical reengineering of race (Bloche 2004; Duster 2007; Graves 2011; Rusert and Royal 2011).
BiDil as a Race-Specific Drug
As mentioned previously, BiDil is a combination of two preexisting generic drugs, hydralazine hydrochloride and isosorbide dinitrate, neither of which has been approved alone for heart failure. As an anti-hypertensive agent, hydralazine relaxes the arteries and decreases the work of the heart. The anti-anginal agent, isosorbide dinitrate, relaxes the veins as well as the arteries. Isosorbide is believed to work by releasing nitric oxide at the blood vessel wall, but its effect usually wears off after half a day. Hydralazine may prevent the loss of this effect (Echols and Yancy 2006; FDA 2005; Franciosa et al. 2002).
BiDil, the race-specific fixed-dose combination, was brought to the bedside rather unconventionally as the result of three different clinical trials, one of which was controversial. The first Vasodilator Heart Failure Trial (V-HeFT I) compared BiDil to placebo and found no difference between them. A second trial tested the efficacy of BiDil against an active agent enalapril, an ACE inhibitor. The findings of the V-HeFT II showed that enalapril is more efficient than BiDil in treating congestive heart failure in the general US population, but that both enalapril and BiDil had equal efficacy in the African American patients (Cohn 1991; Cohn et al. 1986; Exner et al. 2001; Kahn 2013; Levine, Olivari, and Cohn 1986; Nitromed 2012).
There was no Phase III trial comparing a combination of BiDil and standard therapies, including ACE inhibitors (standard therapies plus BiDil), to an add-on placebo (standard therapies plus placebo). Instead, a third trial commonly referred to as the African-American Heart Failure Trial (A-HeFT), which used BiDil and placebo (each in addition to standard therapies), was conducted exclusively with recruited, self-identified black patients. The A-HeFT study was co-sponsored by NitroMed and the Association of Black Cardiologists and became the first study conducted in a heart failure population in which all of the participants identified themselves as African Americans.
The trial began in May 2001 and compared the effects of BiDil against placebo when taken both in addition to standard heart failure therapies in 1,050 self-identified African Americans. Results of the A-HeFT study showed a 33% reduction in first hospitalization and a 43% increase in survival rate, which appeared to suggest that race may be a valid factor in predicting treatment outcomes in clinical cardiology (Taylor et al. 2004). Under the assumption that the factors influencing the efficacy of BiDil, when used in combination with standard therapies, are confined to populations whose members self-identify as “blacks,” this peculiar “phase III” trial design, which purposefully recruited participants along racial lines, and the subsequent FDA race-based approval drastically reconfigured the perception of the relationship between race and drugs.
The Issue of Scientific Validity and Clinical Utility
A-HeFT investigators touted a superior beneficial effect of BiDil in African Americans while the FDA advocated the moral imperative of health equality in approving the drug. Yet critics countered that race is utterly meaningless in genomics and evidence-based medicine, and that race-based therapy is driven by corporate interests in a race niche market (Bloche 2004; Duster 2007; Graves 2011; Roberts, D. E. 2008, 2012; Rusert and Royal 2011). Entangled in this debate are concerns deriving from various perspectives including the societal, cooperate, scientific and patient perspectives (Maglo 2010). From the genomic perspective – and despite the possibility of an accurate partition of humans into continental genetic clusters – racial drugs are problematic for a number of reasons:2
Most genetic alleles are common in our species and are thus shared across continental regions at a high frequency.
Continental genetic clusters are not discrete and individuals from various subpopulations have partial membership in more than one genetic cluster.
Inclusion of admixed populations in clustering analysis increases the rate of individuals sharing partial membership in multiple clusters.
Continental genetic clusters are not diagnostic tools and do not identify the genetic variants of interest in disease causation and drug response.
Even when a genotype of interest is identified, its effect may be modified by environmental factors, a phenomenon known as “phenotypic plasticity.”
The huge individual variation underscores the need for personalized medicine.
Under these circumstances, making inferences from a clinical trial involving African-Americans to a race-based therapy raises not only epistemic concerns about scientific validity but also ethical issues. In brief, race does not have scientific validity in Darwinian classification and human population genomics. Although the success of the A-HeFT trial at reducing mortality and hospitalization rates among self-identified blacks gave the impression that race has at least clinical validity, the scientific design of A-HeFT was controversial. Moreover, as a race-based therapy, BiDil has the potential to impact society either positively or negatively. It is conceivable that the biomedical use of race might even be clinically harmful without necessarily diminishing overall societal well-being.
To resolve this potential conflict between the societal and the patient perspectives, some researchers have suggested the patient standpoint rule:
In case of conflict over unintended societal consequences of a health policy, the obligation to satisfy the need for efficient (and safe) treatment of patients with debilitating or life threatening conditions is prima facie overriding, unless receiving that treatment will directly and severely affect the health conditions of other bodily independent human beings. (Maglo 2012, 151)
The patient perspective distinguishes between the ethical questions about the general societal welfare and the ethical considerations derived from the putative clinical validity and utility of race. It helps to bracket methodologically the moral calculus of society’s general well-being and to focus on epistemic and ethical issues pertaining primarily to patients’ health outcomes in the clinic.
The key question then concerns the theoretical framework that best accounts for the alleged clinical validity of race as demonstrated by A-HeFT. As mentioned above, race seems to have functioned in A-HeFT merely as an instrumental concept that generated the appearance of clinical validity with a controversial study design (Maglo 2012). There is nothing peculiar about the fact that the use of race under these well-controlled clinical conditions might yield useful actionable therapeutic information. However, the clinical outcomes of A-HeFT do not imply that race is a valid biological category in human population genetics (Cooper, Kaufman, and Ward 2003; Serre and Paabo 2004).
Clinical validity and utility are indeed sufficient to justify the therapeutic use of a treatment. Thus, what is at stake in this debate is not a denial of the accuracy of A-HeFT’s empirical findings about hospitalization and survival rates on computational grounds. The problem is rather the epistemic justification of the peculiar “Phase III” study design. In fact, the A-HeFT study design may have been more justifiable if a genuine cross-population (add-on) placebo-control Phase III trial failed to demonstrate therapeutic efficacy of BiDil, while a subsequent retrospective analysis suggested the potential for sub-population-based benefits.
These background considerations influenced the formulation of our hypothesis that the controversy over the scientific status of race and BiDil (see Appendix C) has an impact on physicians’ therapeutic judgments about prescribing this drug. Thus, the objectives of the empirical components of our study were threefold: first, to identify factors influencing physicians’ decision to prescribe BiDil; second, to determine whether race is the major influential factor of physicians’ decisions; and third, to determine whether the controversy over AHe-FT has an impact on physicians’ prescription patterns.
METHODS
Participants and Procedures
We conducted an online survey with internal medicine residents and physicians at the University of Cincinnati from December 2010 to March 2011. All health care providers (attending physicians and medical residents) from the Department of Internal Medicine at the University of Cincinnati were invited to participate in the study, as internists in general and cardiologists, specifically, (as a subspecialty of Internal Medicine) are the main caregivers of patients with heart failure. Basic scientists affiliated with the University of Cincinnati Department of Internal Medicine were excluded. A total of 280 individuals were contacted and invited to participate in the current study.
The survey was anonymous and administrated via REDCap. The survey was developed by the investigators based on their knowledge of the scientific literature and experience in medical practice. The study was approved by the University of Cincinnati Institutional Review Board (IRB). Documentation of informed consent was waived by the IRB, and the electronic informed consent form stipulated that by taking the survey constituted, an individual consented to participate in the study.
Eligible participants were randomly assigned to one of two groups, Group A (Apologists) and Group B (Eliminativists). While the survey questions were identical for both groups, each group read a different introductory passage. The introductory passage for the Apologists merely restated that the clinical findings supporting BiDil were scientifically proven and sound, while the introductory passage for the Eliminativists presented BiDil as a controversial drug that relied on equivocal scientific claims (Appendices B and C). Apologists received no such information and therefore served as the control group. Group names were chosen because we expected physicians in the Apologist Group to defend the use of BiDil along racial lines and physicians in the Eliminativist Group to be less inclined to prescribe BiDil along racial lines after reading our critical introductory document.
Study participants were first asked to provide information about their demographic characteristics, their prescription patterns of BiDil over the past five years prior to participating in the study, their comfort level in taking race into account in prescribing the drug, and their beliefs about its use in the clinic. The survey then described three hypothetical congestive heart failure conditions and asked the participants to determine the level of indication for prescribing BiDil to a patient given his/her demographic characteristics, including sex, age (35-65 years or 65-90 years), and race (African descent [hereafter, “Black or African American”], European descent [hereafter, “White or European American”], White Hispanic, and Black Hispanic). Thus, the participants were repeatedly asked to determine a justifiable use of BiDil for each of the three hypothetical conditions given a patient’s age, gender, and race/ethnicity.
Racial/ethnic categories were selected for practical purposes and to mimic V-HeFT, which contrasted clinical outcomes for self-identified blacks and whites, and A-HeFT, which focused on blacks only. Response options for each question ranged from low (1) to high (7). Condition 1 was very similar to the patients’ conditions in A-HeFT, Condition 2 represented a patient for whom BiDil should most likely not be prescribed as it may hasten death, and Condition 3 represented characteristics implying an appropriate use of the drug. (All three clinical conditions are described in more detail in Appendix A.)
Data Analysis
Data analysis occurred in three distinct phases: a descriptive phase that reports the characteristics of study participants; a comparison phase in which subjects’ responses to specific questions about BiDil prescription patterns and perspectives are compared; and a modeling phase in which physicians’ willingness to prescribe BiDil was evaluated based on experimental group, exposure to various clinical scenarios, and selected personal characteristics. All analyses were conducted at the nominal α = 0.05 level without adjustments for multiple comparisons. All data were analyzed using SAS v9.3.
Descriptive Phase
Participants’ demographic characteristics, including age, race, gender, and years in professional practice, were assessed for the sample as a whole and by experimental group (Apologist group, Eliminativist group). Participants were also asked about previous awareness of the BiDil controversy.
Sample characteristics were compared to determine the comparability of the two groups for later analysis. The type of statistical test varied depending upon the nature of the data: age (t-test), race (Fisher’s Exact), gender (χ2), and years in medical practice (Kruskal-Wallis).
Comparison Phase
To test our hypothesis about whether awareness of the BiDil controversy produced differences in responses attributable to group assignment, we first asked study participants to relay their own opinions about factors influencing their prescription patterns about BiDil; their comfort level taking race into account when prescribing BiDil; and general beliefs about the drug itself.
Except for the four questions pertaining to comfort using race in the patient-physician relationship, for which two-sample t-tests were used, Fisher’s Exact tests were used for all comparisons due to the small and unbalanced nature of the responses in specific categories.
Modeling Phase
In an effort to further test our hypothesis that one’s willingness to prescribe BiDil to patients depended on multiple factors, including a patient’s age, race, and gender, data were analyzed using a linear mixed effects model. The outcome of interest was patterns of physicians’ willingness to prescribe BiDil under the three hypothetical congestive heart failure conditions; a variation between the Eliminativist and Apologist groups would suggest an association between prescription patterns and awareness of the BiDil controversy. Testable covariates included a series of three patient characteristics (age, race, gender); presenting clinical condition (established cardiomyopathy, newly diagnosed cardiomyopathy, non-ischemic cardiomyopathy); and experimental group (Apologist, Eliminativist).
RESULTS
Descriptive Phase
Of the 280 potential respondents, 70 (25%) elected to complete the on-line survey; 31/70 (44%) were in the Apologist Group and 39/70 (56%) were in the Eliminativist Group. Of the 70 respondents who completed the survey, 15 (21%) provided only demographic information (7 in the Apologist Group and 8 in the Eliminativist Group). The rates of missing data were not significantly different between the two groups.
Respondents were on average 38.6 years of age (standard deviation [SD] = 11.9, n = 62) and averaged 4.5 years of professional practice (range = 1.25 - 18.75 years, n = 68). The sample had slightly more males (54%, n = 37) than females (46%, n = 32), with self-reported race as follows: Asian (9%, n = 6), Asian Indian (17%, n = 12), Black or African American (3%, n = 2), Hispanic (4%, n = 3), Pacific Islander (1%, n = 1), White or European American (64%, n = 44), and Other (1%, n = 1).
When the two groups were compared on the aforementioned variables, no statistically significant differences were observed: age (p = 0.50), years in professional practice (p = 0.63), gender (p = 0.31), or race (p = 0.32) (see Table 1).
Table 1.
Physicians’ Demographic Characteristics
| Variable | Apologist Group | Eliminitavist Group | p-value | ||
|---|---|---|---|---|---|
| Age (n, Mean, SD) | 29 | 37.5 (12.2) | 33 | 39.6 (11.7) | 0.50 |
| Years in Practice | |||||
| n (Median), Q1-Q3 | 30 (3.75) | 1.25-12.75 | 38 (6) | 2.1-8.75 | 0.63 |
| Gender (n, %) | |||||
| Female | 16 | 53.3 | 16 | 41.1 | |
| Male | 14 | 46.7 | 23 | 59.0 | 0.31 |
| Race (n, %) | |||||
| Asian | 4 | 12.9 | 2 | 5.3 | |
| Asian Indian | 5 | 16.1 | 7 | 18.4 | |
| Black or African American | 1 | 3.2 | 1 | 3.2 | |
| Hispanic | 3 | 9.7 | 0 | 0.0 | |
| Pacific Islander | 0 | 0.0 | 1 | 2.6 | |
| White or European American | 18 | 58.1 | 26 | 68.4 | |
| Other | 0 | 0.0 | 1 | 2.6 | 0.32 |
Comparison Phase
Participants in both experimental groups were asked a series of questions relating to their opinions about factors influencing their prescription patterns for BiDil, their comfort level with taking race into account when prescribing BiDil, and general beliefs about the drug itself.
Of the 44 health care providers who prescribed BiDil over the last five years (2005-10), 91% reported that they prescribed the drug to self-identified blacks, while 7% prescribed it to whites. Only one respondent reported having prescribed the drug to black Hispanics.
With respect to their awareness of the scientific controversy, only 17 of 53 (32%) respondents reported that they were aware of the scientific dispute over BiDil prior to participating in the study, while 36 (68%) reported that they were not aware of the controversy at all (see Table 2).
Table 2.
Physicians’ Prescription Patterns and Major Influential Factors
| Variable | Apologist Group | Eliminitavist Group | |||
|---|---|---|---|---|---|
| n | % | n | % | p-value | |
| Which of the following category of patients have you prescribed BiDil to over the past 5 years? | |||||
| Black or African American | 19 | 100.0 | 23 | 85.2 | |
| Black Hispanic | 0 | 0.0 | 1 | 3.7 | |
| White European American | 0 | 0.0 | 3 | 11.1 | 0.25 |
| What factor most influenced your decision to prescribe BiDil? | |||||
| Inefficacy of standard therapies | 6 | 27.3 | 11 | 39.3 | |
| Self-identified race | 9 | 40.9 | 5 | 17.8 | |
| Severity of disease | 4 | 18.2 | 8 | 28.6 | |
| Other | 3 | 13.6 | 4 | 14.3 | 0.36 |
| Which factor do you believe best explains the differential efficacy of BiDil? | |||||
| Environmental | 1 | 4.6 | 0 | 0.0 | |
| Pharmacogenomic | 7 | 31.8 | 6 | 22.2 | |
| Physiological | 3 | 13.6 | 8 | 29.6 | |
| Unknown | 11 | 50.0 | 13 | 48.2 | 0.37 |
Regarding factors influencing therapeutic behaviors, 33% of respondents indicated that the major factor influencing their judgments about the prescription of BiDil was the inefficacy of standard therapies, 27% reported self-identified race, 25% reported the severity of the disease, and 15% cited other unspecified factors. Among physicians who reported self-identified race as a major influential factor, 62% were in the Apologist Group and 38% were in the Eliminativist Group. However, the group difference was not statistically significant.
With respect to factors such as whether or not a physician had prescribed BiDil in the past 5 years, what factors most influenced the decision to prescribe BiDil, or what factors may best explain differences in efficacy of BiDil, there were no statistically significant differences between physicians in either the Apologist or the Eliminativist group (see Table 2).
With respect to physicians’ comfort using race in the patient-physician relationship, physicians in the Apologist group appeared to be as comfortable as physicians in the Eliminativist group with respect to all dimensions measured, including: taking race into account when treating patients with congestive heart failure (p = 0.10), prescribing BiDil rather than hydralazine and isosorbide dinitrate (p = 0.46), appraisal of their patients’ general concerns about the race-based efficacy of BiDil (p = 0.90), and importance of discussing information related to the race-based efficacy of BiDil with their patients (p = 0.26). In short, no statistically significant differences were observed between the two groups with respect to comfort level discussing race-based, treatment-related components of BiDil (see Table 3).
Table 3.
Comfort and Concern Level in using Race in the Patient-Physician Relationship
| Survey Question | Apologist Group | Eliminitavist Group | p-value | ||||
|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | ||
| How comfortable are you taking race into account when treating patients with congestive heart failure? | 19 | 76.63 | 18.08 | 28 | 65.27 | 25.66 | 0.10 |
| How likely are you to prescribe BiDil rather than hydralazine and isosorbide dinitrate? | 21 | 28.95 | 27.94 | 27 | 35.59 | 32.47 | 0.46 |
| How concerned are your patients about the race-based efficacy of BiDil? | 18 | 32.17 | 20.95 | 19 | 33.05 | 22.36 | 0.90 |
| How important is it for you to discuss information about the race-based efficacy of BiDil? | 21 | 67.47 | 22.41 | 27 | 58.81 | 28.91 | 0.26 |
Note. Response options for each question were scored on a 100 point scale from not at all (0) to very much (100).
Physicians by and large believed that there is no clinical difference between using BiDil for a patient versus using a combination of the two generic drugs (hydralazine and isorbide dinitrate); no statistically significant difference was observed between the two groups of physicians (p = 1.00). Likewise, physicians in both groups considered BiDil to be cost-prohibitive for their patients (p = 1.00). Also of interest is the fact that physicians in both groups reported that based on their experience, insurance companies do not cover use of BiDil for nonblack patients (p = 0.77). (See Table 4)
Table 4.
Physicians’ Beliefs about BiDil
| Variable | Apologist Group | Eliminitavist Group | |||
|---|---|---|---|---|---|
| Response | n | % | n | % | p-value |
| Do you believe that BiDil is contributing to reducing health disparities related to cardiovascular disease? | |||||
| Yes | 6 | 28.6 | 11 | 37.9 | |
| No | 15 | 71.4 | 18 | 62.1 | 0.56 |
| Does a combined administration of hydralazine and isosorbide dinitrate achieve the same result as the fixed dose BiDil? | |||||
| Yes | 20 | 90.9 | 25 | 89.3 | |
| No | 2 | 9.1 | 3 | 10.7 | 1.00 |
| Do you find BiDil cost prohibitive for your patients? | |||||
| Yes | 19 | 82.6 | 25 | 83.3 | |
| No | 4 | 17.4 | 5 | 16.7 | 1.00 |
| Do some patients raise the issue of race-based efficacy of BiDil by themselves? | |||||
| Yes | 4 | 18.2 | 3 | 10.3 | |
| No | 18 | 81.8 | 26 | 89.7 | 0.45 |
| Based on your experience, do insurance companies cover the use of BiDil for nonblack patients? | |||||
| Yes | 8 | 40.0 | 11 | 45.8 | |
| No | 12 | 60.0 | 13 | 54.2 | 0.77 |
| Do you believe that the use of BiDil requires specific ethical guidelines? | |||||
| Yes | 8 | 33.3 | 6 | 20.7 | |
| No | 16 | 66.7 | 23 | 79.3 | 0.36 |
Modeling Phase
The mixed-effects analyses of the three hypothetical health conditions demonstrated a statistically significant difference between the two experimental groups with respect to their willingness to prescribe BiDil, with physicians in the Eliminativist Group less likely to prescribe the drug than physicians in the Apologist Group (p = 0.003). With respect to self-identified race, physicians were more willing to prescribe the drug to self-identified blacks and self-identified black Hispanics than to self-identified whites and self-identified white Hispanics (p < 0.001). However, exposure to the BiDil controversy did not yield a statistically significant difference between the two groups in their willingness to take race into account (i.e., no statistically significant two-way interaction between experimental group and race was observed).
These findings suggest that in addition to health condition (p < 0.001), race is a strong influential factor in prescribing BiDil (p < 0.001), while age (p = 0.06) and gender (p = 0.44) of the patients have no significant effect. While the clinical condition was a strong indicator of prescribing habits, very little difference was noted between Conditions 1 and 3. Model results are summarized by least squares means with associated 95% confident limits, type III F statistics and p-values (See Table 5 and Figures 1-3).
Table 5.
Least Square Means Estimates of Physical Willingness to Prescribe BiDil
| Factor | Estimate (SE) | Confidence Interval 0.95LL, 0.95UL | F-value | Pr > F |
|---|---|---|---|---|
| Experimental Group | 9.95 | 0.003 | ||
| Apologists | 55.99 (0.67) | 54.64, 57.34 | ||
| Eliminativists | 53.18 (0.59) | 51.99, 54.37 | ||
| Race | 123.29 | < 0.001 | ||
| Black or African American | 65.28 (0.86) | 63.59, 66.98 | ||
| Black Hispanic | 60.85 (0.88) | 59.10, 62.60 | ||
| White or European American | 47.08 (0.90) | 45.31, 48.86 | ||
| White Hispanic | 45.12 (0.91) | 43.33, 46.93 | ||
| Age Group | 3.66 | 0.06 | ||
| 35 to 65 years | 55.44 (0.62) | 54.20, 56.68 | ||
| 65 to 90 years | 53.74 (0.64) | 52.44, 55.03 | ||
| Gender | 0.60 | 0.44 | ||
| Female | 54.24 (0.63) | 52.98, 55.51 | ||
| Male | 54.93 (0.63) | 53.67, 56.19 | ||
| Experimental Condition | 239.41 | < 0.001 | ||
| Established cardiomyopathy | 60.15 (0.77) | 58.62, 61.68 | ||
| Newly diagnosed cardiomyopathy | 41.06 (0.78) | 39.55, 42.56 | ||
| Non-ischemic cardiomyopathy | 62.55 (0.78) | 61.01, 64.09 |
Note. LL = lower limit, UL = upper limit for 95% confidence interval.
Figure 1.
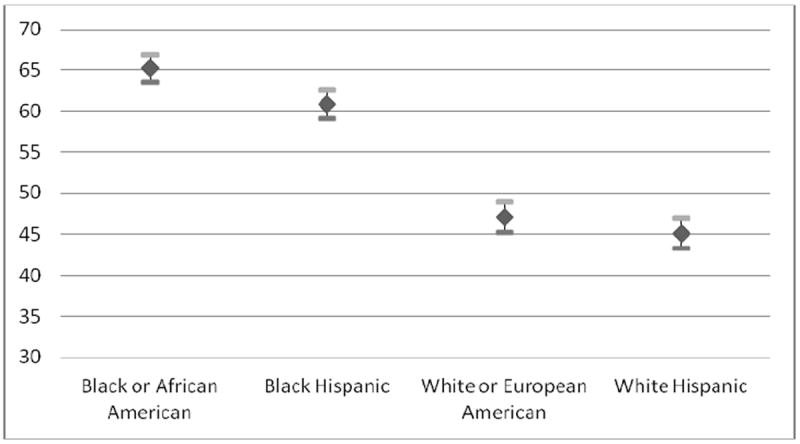
Least Square Means of Physician’s Willingness to Prescribe BiDil by Race
Figure 3.
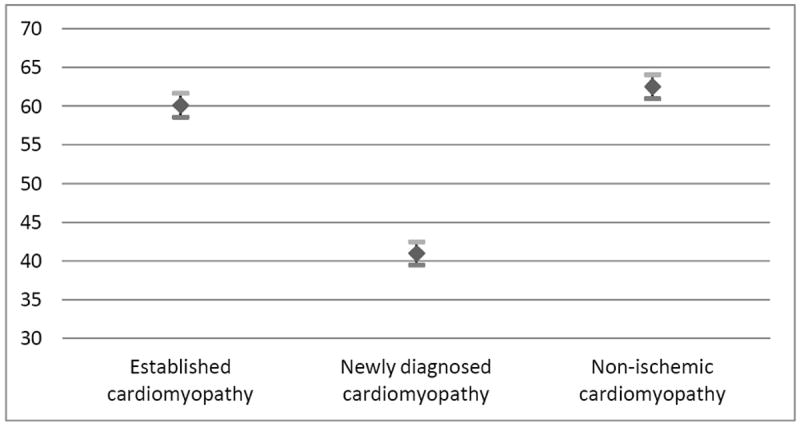
Least Square Means of Physician’s Willingness to Prescribe BiDil by Health Conditions
DISCUSSION
Interpreting the Empirical Findings
The results of our study show that physicians, by and large, prescribe BiDil to blacks more than they prescribe it to whites. Physicians’ prescription patterns prior to participating in the study as well as observed prescriptions patterns under the experimental conditions described in the study suggest that physicians view the drug as indicated more often for blacks than for whites.
While physicians who were unaware of the controversy over BiDil were more likely to use race as a major determinant in selecting BiDil for patients (Table 2), as a whole, physicians were not willing to prescribe BiDil to all black patients suffering from congestive heart failure who checked into the clinic. Rather, they believed that BiDil was indicated for health conditions mimicking NYHA Class II and III patient conditions; they were willing to prescribe BiDil to black patients only when the disease state was severe and life threatening. But even so, they were not willing to prescribe it to black patients under such a severe disease state if BiDil might very likely worsen the patient health condition and hasten death (Figure 3). When the available evidence about the V-HeFT and A-HeFT was presented to physicians as scientifically controversial (Appendix C), their comfort level in taking race into account and their willingness to prescribe BiDil to black patients slightly declined, though the difference was not statistically significant (Table 3).
Physicians reported considering a combination of factors to balance potential benefits against potential harms in prescribing BiDil to black patients. Only 27% of the respondents in our study reported using self-identified race as a major factor in prescribing BiDil. Inefficacy of standard therapy (33%) and health condition (25%) were some of the determining factors (see Table 2). This is consistent with previous findings that physicians use various criteria, including medical history, to determine whether BiDil is indicated for a patient (Akinniyi and Payne 2011; Bonham et al. 2009). For the vast majority of the physicians in our study, the prescription strategy included an instrumental use of race as one of several variables used to determine clinical utility.
The approach seems morally defensible because “no two patients are alike, and a physician should be able to select and modify the course of therapy, as required by the patient’s best interests” (Beauchamp and Childress 2009, 320). The fiduciary responsibility of a health professional requires that s/he give priority to the interest of the patient over other considerations, while non-maleficence demands that the physician exercise due care in determining treatment appropriateness. This is the idea embodied in the patient standpoint rule discussed previously. Yet from the fact that health professionals are morally required to make the best of a situation involving poor evidence in order to serve the best interest of patients, it does not follow that patients’ best interests are served when this duty is discharged. Accordingly, the question remains as to whether the instrumental use of race to engineer clinical validity is in the best interest of the patient qua patient.
Our study highlights both the strengths and weaknesses of the academic debate over race-based therapy. There is a statistically significant difference between the Eliminativist Group and the Apologist Group with respect to their willingness to prescribe BiDil for specific health conditions (Figure 2). On the one hand, awareness about the A-HeFT controversy may have an impact on physicians’ attitudes towards BiDil as a drug. On the other hand, the debate does not appear to have penetrated the medical community to a degree such that it affects physicians’ prescribing behaviors.
Figure 2.
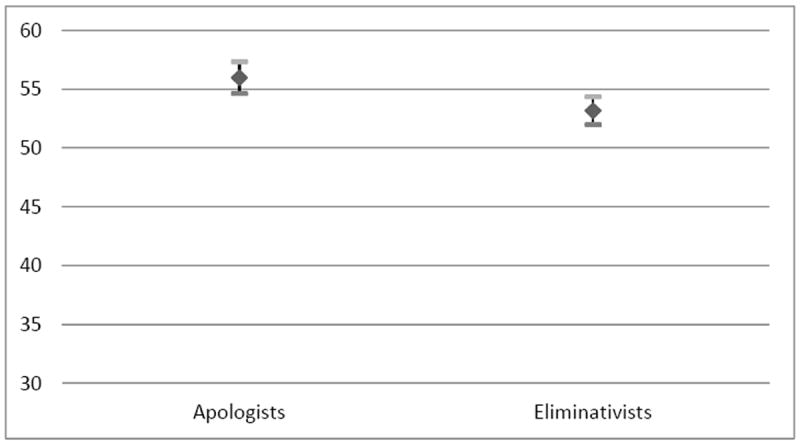
Least Square Means of Physician’s Willingness to Prescribe BiDil by Controversy Awareness
Out of the 17 physicians who reported previous awareness of the debate, 5 were in the Apologist Group and 12 in the Eliminativist Group (which received information about the controversy from our survey). Physicians in the Apologist Group were less likely to report awareness about the debate over BiDil as a race-based therapy. This suggests that many physicians in the Eliminativist group may have learned about the controversy from our study.
If the findings of our study are replicated, then perhaps the manner in which information about race-based therapy is presented to physicians will need to be revisited. In this pilot study, however, we do not have any evidence-based explanation of why the controversy did not seem to have penetrated the medical community. One possible explanation may be the relatively short duration of professional practice reported by many clinicians (average of 4.5 years), and the realization that some clinicians were not even in medical school at the height of the controversy (i.e., when the FDA approved BiDil in 2005).
This possible explanation highlights the fact that the physician desk references and drug labels may not always be the proper vehicles for conveying information about controversies surrounding drugs. Other venues, including the student’s curriculum, continued medical education, professional meetings and conferences may offer additional opportunities for informing clinicians about the quality of the scientific evidence behind this therapy and about the theoretical dispute surrounding it.
Awareness of the theoretical tenets of the debate over race is needed to ensure that physicians develop critical judgment that informs their prescribing attitudes and behaviors in clinical situations. Such awareness is crucial because race very likely will remain a conundrum that challenges the basic assumptions of both opponents and proponents of BiDil as a race-specific drug. For instance, while the vast majority of physicians in our sample have prescribed BiDil to black patients, some physicians (7%) reported having prescribed the drug to white patients. In fact, Jay Cohn, the “father” of BiDil, maintained that some white patients may benefit from the drug as well (Graves 2011; Kahn 2004).
However, our results do not imply that racial assumptions do not impact the judgment of the majority of the physicians. Race seems to influence their deliberations in subtle and indirect ways. For instance, the race-based differential efficacy of standard therapies such as ACE inhibitors has generated its own set of controversies. While the V-HeFT II study showed that ACE inhibitors were more effective in whites compared to BiDil, there is no unequivocal evidence that ACE inhibitors do not work in blacks. That is, physicians’ judgments about the inefficacy of standard therapies may carry implicit racial assumptions. As some researchers have pointed out:
…the main health care-related racial disparity in cardiovascular disease is underutilization of standard therapies and procedures (…). Current evidence suggests that we should do more of the same, not more differently. Reframing health disparities as a pharmacologic phenomenon distorts existing evidence and may lead to less evidence-based care. (Bibbins-Domingo and Fernandez 2007, 55)
To be sure, the FDA advocated health equality considerations in approving BiDil, making this issue an important matter for studies assessing the use of BiDil in the clinic. Thirty-three percent of respondents in our study reported that based on their personal prescribing experiences, BiDil is contributing to the reduction of health disparities between racial groups, while 67% of respondents reported observing no positive impact of BiDil in this respect. Other researchers also reported no major impact of BiDil on the health equality issue (Ferdinand and Ferdinand 2009). A confirmation of these observations by larger studies would undercut one of the FDA’s key rationales for approving this race-based therapy.
Furthermore, the FDA maintains that it has not found a bioequivalent to BiDil. Yet physicians in our study appear to have found one in the combination of the two generic components recommended by the American Heart Association since 1990. Indeed, 90% of physicians declared that a combined administration of the generic drugs hydralazine and isosorbide dinitrate achieves the same results as the fixed-dose drug BiDil. No statistically significant difference was observed between the Apologist Group and the Eliminativist Group on this issue (p = 1.00).
Further studies will be needed to determine physicians’ perceptions on the crucial issue of bioequivalence. These studies should also assess the extent to which physicians’ perceptions and prescription behaviors affect the financial status of BiDil. Our findings strongly suggest that the clinic, and not only regulative authorities, may decide the commercial fate of race-based therapies. Eighty-two percent of physicians in our study stated that the cost of BiDil is prohibitive for their patients. The median wholesale price for a BiDil tablet (at the institution where this study was performed) is $2.56/tab, while the costs of the equivalent doses of hydralazine and isosorbide dinitrate are $0.50/tab and $1.19/tab, respectively. An approximately equivalent amount of the agents for a one-month supply would be $230.40 for BiDil versus $152.10 for hydralazine and isosorbide dinitrate. The price difference between the generic option and BiDil was noted by the pharmaceutical company (which offered a Patient Assistance Program to eligible patients: http://www.nitromed.com/pnt/programs.php).
The commercial fate of BiDil is currently a matter of controversy. Krimsky, for one, claimed that, BiDil was a short-lived racial drug (Krimsky 2012b). But for Downey, the corporate officer for Arbor Pharmaceuticals, BiDil is fully “alive” and actually “kicking” (Downey 2012). While Krimsky conceded that the title of his paper (“The Short Life of a Race Drug”) might be misleading about the current availability and use of the drug, he maintained that data from U.S. Securities and Exchange Commission (SEC) indicate that the financial prospects of the drug are bleak (Krimsky 2012a). According to Kahn, although BiDil was a commercial failure for Nitromed, its future commercial prospects depend on the ability of Deerfield Capital (which acquired Nitromed in 2009) to develop “an extended-release version of the drug” (Kahn 2013, 123). Whatever may be BiDil’s commercial fate, it is clear that it has implications for the patient-physician relationship.
Reengineering the Patient Perspective
Our study focused on physicians’ judgments and behaviors rather than on patients’ beliefs and attitudes towards BiDil. Although we did not restrict the study to physicians who have prescribed BiDil in the past, the vast majority of them reported having prescribed the drug prior to participating in the study (Table 2). In fact, when asked about the level of concern of their patients with respect to race-based therapies, physicians in both the Apologist Group and Eliminativist Group affirmed that patients who are aware of the issue show little to no concern. On a scale of 0 to 100, the mean value of patients’ level of concern about the race-based prescription of BiDil reported by their physicians was about the same for both groups. The mean scores were 32.82 for the Apologist Group and 33.05 for the Eliminativist Group with 95% Confidence Interval (CI) of [22, 44] for both groups.
Nonetheless, because we did not directly collect information from patients in this preliminary investigation, we cannot make any generalized claims about their preferences and perceptions of the drug. Although some researchers claim that “BiDil failed because African American patients for the most part did not desire a ‘race-tailored’ drug” (Graves 2011, 144), more systematic investigations of patients’ opinions are needed before one can draw firm conclusions. The evidence available about African American patients’ preferences and perceptions of this race-based therapy are either indirect inferences from marketing and sales data (which do not necessary reflect patients’ preferences and perceptions) or anecdotal tales from a comic episode in the television series House (See the summary of the evidence in [Kahn 2013, 121]).
Because more than 1,000 NYA class III and IV congestive heart failure African American patients reportedly signed informed consent forms to participate in A-HeFT, we believe that congestive heart failure patients as such deserve a voice in this debate. Rather than dismissing claims about African American patients’ putative worries about BiDil and race-based therapies (Graves 2011, De Marco 2010), our study underscores the need to take seriously the patient perspective in this thorny debate by empirically documenting their preferences and perceptions. This is by no means to suggest that the patient perspective provides overriding absolute principles in this debate.
Population-based factors that influence health outcomes must also be considered. These factors may be genetic, epigenetic, or environmental. Yet because breeding populations are all unique, continental genetic clusters as well as regulative authorities’ categories of race are likely to have a limited predictive value in biomedicine. For instance, a study of drug-metabolized enzymes has shown that some African populations cluster with European populations and Ashkenazi Jews rather than with other African populations (Wilson et al. 2001). The within-group variability of clinically relevant alleles of Cytochrome P450 3A5 (CYP3A5) suggests multiple pharmacogenomic profiles among African populations (Bains et al. 2013).
Thus, the emerging therapeutic concept of “each race, its dose,” similar to the old concept of “one dose fits all,” may include too much. That is, it may include non-beneficiary racial members (false positives) and expose them to the risk of mistreatment or toxicity. Yet unlike the decried old concept, it may at the same time exclude too much. It may exclude potential beneficiaries outside the racial group (false negatives), and deprive them of the benefit of treatment that may be indicated for them. A race-based therapy may not be justified, regardless of patients’ preferences, if there is no objectively reasonable and serious effort at resolving the false beneficiary versus non-beneficiary problem.
Genomic research offers the opportunity to identify genetic risk factors that may cause disease or toxicity as well as beneficial alleles that may either protect against disease or influence treatment efficacy. For instance, just like BiDil, Gencaro (Bucindolol hydrochloride), a drug manufactured by ARCA Biopharma for the treatment of chronic heart failure, was reportedly more effective in some racial groups than in others (Bibbins-Domingo and Fernandez 2007). Analyses of DNA samples collected from patients in a clinical trial have shown that the survival effect occurs in patients carrying the β1-adrenergic receptor gene and alpha-2C-adrenergic receptor genetic biomarkers. It is noteworthy that these biomarkers are not confined to one racial group (Maglo 2012). Unlike BiDil, Gencaro is bypassing the race-based therapeutic paradigm to serve as a genetically targeted therapy that moves us closer towards personalized medicine (Maglo 2012). The picture emerging from genomic medicine is far more complex than the BiDil race-based framework suggests.
There are attempts to understand the underlying mechanisms of racial differences in cardiovascular disease and drug responses attributable to genetic differences (Johnson 2008; McNamara et al. 2009). But most physicians in our study did not believe that the putative racial efficacy of BiDil is due to pharmacogenomic factors (see Table 2). Yet the genomics-based approach has the capacity to help resolve the dual problem of false beneficiary inclusion and false non-beneficiaries exclusion. The FDA increasingly recommends taking into account genetic information in disease management when the information is scientifically sound. In the case of present-day warfarin, for example, both genetic and ethnic considerations are taken into account in prescribing it (Kahn 2013, 157-192).
Though we are entering the age of personalized medicine, the utility of genotypic information in predicting health outcome appears more limited than most researchers acknowledge. In fact, recent estimates of the predictive capacity of whole genome sequencing of monozygotic twin pairs show that individuals sharing the same “genometype” often have different health profiles. For instance, out of 24 diseases studied by Roberts and his colleagues (2012), the vast majority of identical twins are likely to receive negative results for 23 genetic tests while at the same time classified as high risk for developing 19 of these diseases due to the effects of non-genetic modifiers of human health. The risk of developing these 19 diseases from [non-genetic factors] was estimated to be about 50-80% minimum of the total risk in the general population (Roberts et al. 2012). This phenomenon is known as phenotypic variability (Maglo and Martin 2012).
Nevertheless, identical twins are not always genomically “identical” and studies have shown copy number variation (CNVs) among some identical twins (Bruder et al. 2008; Maiti et al. 2011). Genetic mutations that occur during one’s lifetime may also affect human health. Thus, epidemiological and therapeutic phenotypes do not simply vary between subpopulations sharing common genetic ancestry but also differ significantly even among individuals sharing the same genome-type (as in the case of monozygotic twins).
The uniqueness of each individual and of each breeding population poses the most serious challenges to race-based therapy. No subpopulation within a given continent constitutes a biologically representative subpopulation for the whole continental population or racial group (Tishkoff et al. 2009; Tishkoff and Verrelli 2003). As one human population geneticist put it recently:
Ancestry varies from population to population, and from individual to individual. Any attempt to characterize the genetic history of all African-Americans (or any human population, for that matter) by a single number is futile. (Relethford 2012, 255)
Thus A-HeFT results may not be generalizable to subpopulations collectively labeled as “blacks” in US racial categorizations or in genetic clustering studies as “Africans”. While there is no evidence to support the idea that the concept of race has currently only positive consequences in the clinic, it cannot be denied that in the current system of trial and error medicine, genetic ancestry may sometimes be helpful in achieving beneficial biomedical outcomes. However, the potential for ambiguous applications of the race concept underscores the dilemma over the moral permissibility of race-based therapy.
Limitations
As with all studies, this study has a number of limitations that must be acknowledged. First, the present study is a pilot and feasibility study limited to a single medical specialty (internal medicine) at a single institution in a large midwestern city in the US. A second limitation lies in the relatively small sample size. Similar to other pilot studies, findings reported here will need to be corroborated with larger samples across multiple medical specialties to strengthen the knowledge base. In addition, the sample used for the current investigation was primarily white. Future studies will need to recruit participants with more diverse racial backgrounds to understand the various ways in which race may impact physicians’ beliefs and prescribing behaviors. Finally, non-random sampling resulting from selection bias may also impact our findings.
Despite these limitations, however, our study has yielded important information about physicians’ awareness of the scientific controversy surrounding BiDil and their prescription behaviors that demand further and more systematic examination. Additional inquiries into patients’ preferences within the broader landscape of race-based medicines and interventions will continue to facilitate, document, and hopefully strengthen patients’ perspectives in this important area of clinical medicine.
Conclusion
Our study shows that although physicians prescribe BiDil primarily to black patients, their deliberation processes about the appropriateness of the drug appear to conform to prescription patterns consistent with standard of care. Race influenced their prescription patterns in subtle ways as they by and large prescribed the drug to “blacks.” However, physicians’ lack of awareness about the controversy surrounding A-Heft and BiDil suggests the need for new venues to convey information about this drug to health professionals as this information may influence their deliberative processes in selecting BiDil as a therapy.
Acknowledgments
We would very much like to thank Ms. Alison Kissling and Ms. Diana Hechavarría for their invaluable work gathering data and preparing the manuscript. Without their help and support this research would not have been possible. Many thanks also to the anonymous reviewers and the editors for their useful comments on the earlier version of this paper.
Funding: This project was supported by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number UL1RR026314. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Appendix A: Three Hypothetical Congestive Heart Failure Conditions
-
Condition 1
A patient with known ischemic cardiomyopathy and an EF of 25% presents for follow up as an outpatient. The patient is asymptomatic and clinically euvolemic on a stable dose of loop diuretic, ACE-I, betablocker, aspirin, and digoxin. The patient’s BP is 160/75, HR is 75 BPM, RR is 18, and O2 sat is 97% on room air.
-
Condition 2
A patient with newly diagnosed ischemic cardiomyopathy with 3-vessel disease not amiable to revascularization and an EF of 20% presents. The patient has been hospitalized for 2 days and is slowly diuresing but still hypervolemic. The patient is mildly short of breath and slightly hypervolemic. The patient is on a high dose diuretic and has been loaded with digoxin. The patient’s BP is 90/55, HR is 115 BPM, RR is 26, and O2 sat is 91% on 4 L NC.
-
Condition 3
A patient with non-ischemic cardiomyopathy and an EF of 40% presents for follow up as an outpatient. The patient is mildly short of breath and slightly hypervolemic on a stable dose of loop diuretic, ACE-I, betablocker, aspirin, and digoxin. The patient’s BP is 170/85, HR is 85 BPM, RR is 18, and O2 sat is 97% on room air.
Appendix B: Introductory Document for the Apologist Group
The Food and Drug Administration (FDA) approved BiDil (bye-DILL), a drug for the treatment of heart failure in self-identified black patients in 2005.
The Road to BiDil as a Racial Drug
In 1997 the U.S. Food and Drug Administration (FDA) determined that the prospectively defined results of the Veteran’s Affairs Vasodilator Heart Failure Trials (V-HeFT I and II) were not adequate to support approval of BiDil for the treatment of heart failure at that time because those studies did not show convincing evidence of a survival benefit. However, when NitroMed presented retrospective analyses of V-HeFT I and II to the FDA in 2000, indicating a positive survival signal in African Americans, the FDA indicated that a clearly positive trial in African Americans could provide a basis for approval of BiDil for this particular heart failure population.
The African American Heart Failure Trial (A-HeFT) – co-sponsored by NitroMed and the Association of Black Cardiologists, Inc. – was the first study conducted in a heart failure population in which all of the participants identified themselves as African American. This Phase III trial commenced in May 2001 and evaluated the effects of BiDil, a fixed-dose combination of isosorbide dinitrate and hydralazine hydrochloride, in 1,050 self-identified African Americans when taken in addition to standard heart failure therapies. Patients on BiDil experienced a 43% reduction in death and a 33% decrease in hospitalization for heart failure compared to placebo, and a decrease of their symptoms of heart failure.
The Science behind BiDil and its Limitations
BiDil is a combination of two older drugs, neither approved for heart failure – hydralazine and isosorbide dinitrate. As an anti-hypertensive agent, hydralazine relaxes the arteries, and decreases the work of the heart. The anti-anginal agent, isosorbide dinitrate, relaxes the veins as well as the arteries. Isosorbide seems to work by releasing nitric oxide at the blood vessel wall, but its effect usually wears off after half a day. Hydralazine may prevent this loss of effect. But how the two drugs work together is not fully known.
BiDil is indicated for the treatment of heart failure as an adjunct to standard therapy in self-identified black patients to improve survival, to prolong time to hospitalization for heart failure, and to improve patient-reported functional status. There is little experience in patients with NYHA class IV heart failure. Most patients in the clinical trial supporting effectiveness (A-HeFT) received a loop diuretic, an angiotensin converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker, and a beta blocker, and many also received a cardiac glycoside or an aldosterone antagonist.
Appendix C: Introductory Document for the Eliminativist Group
The Birth of a Controversial Drug
The Food and Drug Administration (FDA) approved BiDil (bye-DILL), a drug for the treatment of heart failure in self-identified black patients in 2005. This sparked a storm of controversy of the value of race in science and medicine. Critics charged that the approval of the drug for race specific prescription is motivated more by market incentives rather than by any race-based differential efficacy of the drug.
The Road to BiDil as a Racial Drug
In fact, prior to the approval of BiDil for race specific prescription, the FDA determined in 1997 that the prospectively defined results of the Veteran’s Affairs Vasodilator Heart Failure Trials (V-HeFT I and II) were not adequate to support approval of BiDil for the treatment of heart failure at that time because those studies did not show convincing evidence of a survival benefit. However, when NitroMed presented retrospective analyses of V-HeFT I and II to the FDA in 2000, indicating a positive survival signal in African Americans, the FDA indicated that a clearly positive trial in African Americans could provide a basis for approval of BiDil for this particular heart failure population.
The African American Heart Failure Trial (A-HeFT) – co-sponsored by NitroMed and the Association of Black Cardiologists, Inc. – was the first study conducted in a heart failure population in which all of the participants identified themselves as African American. This Phase III trial commenced in May 2001 and evaluated the effects of BiDil, a fixed-dose combination of isosorbide dinitrate and hydralazine hydrochloride, in 1,050 self-identified African Americans when taken in addition to standard heart failure therapies. Patients on BiDil experienced a 43% reduction in death and a 33% decrease in hospitalization for heart failure compared to placebo, and a decrease of their symptoms of heart failure.
The Science behind BiDil and its Limitations
BiDil is a combination of two older drugs, neither approved for heart failure--hydralazine and isosorbide dinitrate. As an anti-hypertensive agent, hydralazine relaxes the arteries, and decreases the work of the heart. The anti-anginal agent, isosorbide dinitrate, relaxes the veins as well as the arteries. Isosorbide seems to work by releasing nitric oxide at the blood vessel wall, but its effect usually wears off after half a day. Hydralazine may prevent this loss of effect. But how the two drugs work together is not fully known.
BiDil is indicated for the treatment of heart failure as an adjunct to standard therapy in self-identified black patients to improve survival, to prolong time to hospitalization for heart failure, and to improve patient-reported functional status. There is little experience in patients with NYHA class IV heart failure.
No Race-Based Efficacy Tested
Most patients in the A-HeFT clinical trial supporting the alleged race-based effectiveness of BiDil received a loop diuretic, an angiotensin converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker, and a beta blocker, and many also received a cardiac glycoside or an aldosterone antagonist.
But Vasodilator Heart Failure Trials (V-HeFT I and II) did not include ACE inhibitors (as standard therapy). Thus on strict scientific grounds, V-HeFT I and II did not clear the road for A-HeFT race-based trial. As some researchers stated about the A-HeFT study, “[…] it might have made clinical and scientific sense to add isosorbide dinitrate and hydralazine to conventional therapy (which by now typically included an ACE inhibitor) and compare this combination to conventional therapy alone — for all patients with heart failure, regardless of race. Such a trial had not been performed, since the standard therapies used in earlier trials did not include ACE inhibitors. But race consciousness offered a faster way through the FDA’s regulatory maze” (Bloche 2004, 2035).
Conflict over the Use of BiDil
In a word, while even proponents of the race-based prescription of BiDil appear uncomfortable with the idea of race being “a descriptor of drug efficacy,” critics charged that: “For health care providers, who focus on the role of health care in influencing health disparities, the implicit message to focus on race-targeted medications conflicts with ample evidence documenting that the main health care–related racial disparity in cardiovascular disease is underutilization of standard therapies and procedures…” (Bibbins-Domingo and Fernandez 2007).
Footnotes
Some more recent estimates put this value at 99.5% (Rotimi and Jorde 2010).
For more details, see e.g., Bamshad et al. 2004; Krieger 2005; Maglo 2011; Maglo and Martin 2012; Rosenberg et al. 2002; Serre and Paabo 2004; and Wilson et al. 2001.
Competing Interests: None declared.
Ethical approval: This study was approved by the institutional review board at the University of Cincinnati.
AUTHOR CONTRIBUTIONS: Koffi Maglo conceived and designed the project, oversaw the recruitment of study participants and data collection, drafted the manuscript, and was involved in the revision of all aspects of the paper together with the other collaborators. Jack Rubinstein assisted Koffi Maglo in the conception and designing of the study, the recruitment of study participants, and the drafting and revision of the manuscript. Bin Huang performed the statistical analyses and reviewed the manuscript. Richard Ittenbach provided input on the study design, conceptualization and integration of study components, and manuscript writing and revisions.
Contributor Information
Koffi N. Maglo, Department of Philosophy, 206 McMicken Hall, PO Box 210374, University of Cincinnati, Cincinnati, OH 45221-0374, Tel (513) 556-6337, maglokn@ucmail.uc.edu.
Jack Rubinstein, University of Cincinnati College of Medicine.
Bin Huang, University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
Richard F. Ittenbach, University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
References
- Akinniyi D, Payne P. BiDil lessons: cardiologists views of a race-based personalized medicine. Journal of the American College of Cardiology. 2011;57(14s1):E1926–E1926. [Google Scholar]
- Bains RK, Kovacevic M, Plaster CA, et al. Molecular diversity and population structure at the Cytochrome P450 3A5 gene in Africa. BMC Genetics. 2013;14:34. doi: 10.1186/1471-2156-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Wooding S, Salisbury BA, Stephens JC. Deconstructing the relationship between genetics and race. Nature Reviews: Genetics. 2004;5(8):598–609. doi: 10.1038/nrg1401. [DOI] [PubMed] [Google Scholar]
- Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 6. New York: Oxford University Press; 2009. [Google Scholar]
- Bibbins-Domingo K, Fernandez A. BiDil for heart failure in black patients: implications of the U.S. Food and Drug Administration approval. Annals of Internal Medicine. 2007;146(1):52–56. doi: 10.7326/0003-4819-146-1-200701020-00009. [DOI] [PubMed] [Google Scholar]
- Bloche MG. Race-based therapeutics. New England Journal of Medicine. 2004;351(20):2035–2037. doi: 10.1056/NEJMp048271. [DOI] [PubMed] [Google Scholar]
- Bonham VL, Sellers SL, Gallagher TH, et al. Physicians’ attitudes toward race, genetics and clinical medicine. Genetics in Medicine. 2009;(13):279–286. doi: 10.1097/GIM.0b013e318195aaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder CE, Piotrowski A, Gijsbers AA, et al. Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. American Journal of Human Genetics. 2008;82(3):763–771. doi: 10.1016/j.ajhg.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard EG, Ziv E, Coyle N, et al. The importance of race and ethnic background in biomedical research and clinical practice. New England Journal of Medicine. 2003;348(12):1170–1175. doi: 10.1056/NEJMsb025007. [DOI] [PubMed] [Google Scholar]
- Cohn JN. Lessons from V-HeFT: questions for V-HeFT II and the future therapy of heart failure. Herz. 1991;16(Spec No 1):267–271. [PubMed] [Google Scholar]
- Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. New England Journal of Medicine. 1986;314(24):1547–1552. doi: 10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- Collins FS. What we do and don’t know about ‘race’, ‘ethnicity’, genetics and health at the dawn of the genome era. Nature Genetics. 2004;36(11 Suppl):S13–15. doi: 10.1038/ng1436. [DOI] [PubMed] [Google Scholar]
- Cooper RS, Kaufman JS, Ward R. Race and genomics. New England Journal of Medicine. 2003;348(12):1166–1170. doi: 10.1056/NEJMsb022863. [DOI] [PubMed] [Google Scholar]
- De Marco M. Views on Personalized Medicine: Do the Attitudes of African American and White Prescription Drug Consumers Differ? Public Health Genomics. 2010;(13):276–283. doi: 10.1159/000242199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey LJ. BiDil: alive and kicking. Lancet. 2012;379(9829):1876. doi: 10.1016/S0140-6736(12)60807-1. [DOI] [PubMed] [Google Scholar]
- Duster T. The molecular reinscription of race: unanticipated issues in biotechnology and forensic science. Patterns of Prejudice. 2006;40(4/5):427–441. [Google Scholar]
- Duster T. Medicalisation of race. Lancet. 2007;369(9562):702–704. doi: 10.1016/S0140-6736(07)60320-1. [DOI] [PubMed] [Google Scholar]
- Echols MR, Yancy CW. Isosorbide dinitrate-hydralazine combination therapy in African Americans with heart failure. Vascular Health and Risk Management. 2006;2(4):423–431. doi: 10.2147/vhrm.2006.2.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. New England Journal of Medicine. 2001;344(18):1351–1357. doi: 10.1056/NEJM200105033441802. [DOI] [PubMed] [Google Scholar]
- FDA. BiDil Package Insert. 2005 Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2005/020727lbl.pdf.
- Ferdinand K, Ferdinand D. Cardiovascular disease disparities: Racial/Ethnic factors and potential solutions. Current Cardiovascular Risk Reports. 2009;3:187–193. [Google Scholar]
- Franciosa JA, Taylor AL, Cohn JN, et al. African-American Heart Failure Trial (A-HeFT): rationale, design, and methodology. Journal of Cardiac Failure. 2002;8(3):128–135. doi: 10.1054/jcaf.2002.124730. [DOI] [PubMed] [Google Scholar]
- Frank D, Gallagher TH, Sellers SL, et al. Primary care physicians’ attitudes regarding race-based therapies. Journal of General Internal Medicine. 2010;25(5):384–389. doi: 10.1007/s11606-009-1190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves JL. and the Council for Responsible Genetics. Evolutionary versus racial medicine. In: Krimsky S, Sloan K, editors. Race and the Genetic Revolution: Science Myth and Culture. New York: Columbia University Press; 2011. pp. 142–172. [Google Scholar]
- Grens K. Race-based medicine? African-American heart drug study raises questions about benefits of racially targeted trials. The Scientist. 2007 Nov 19; Available at: http://www.the-scientist.com/?articles.view/articleNo/25688/title/Race-based-medicine-/
- Handley LJ, Manica A, Goudet J, Balloux F. Going the distance: human population genetics in a clinal world. Trends in Genetics. 2007;23(9):432–439. doi: 10.1016/j.tig.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Johnson JA. Ethnic differences in cardiovascular drug response: potential contribution of pharmacogenetics. Circulation. 2008;118(13):1383–1393. doi: 10.1161/CIRCULATIONAHA.107.704023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorde LB, Watkins WS, Bamshad MJ. Population genomics: a bridge from evolutionary history to genetic medicine. Human Molecular Genetics. 2001;10(20):2199–2207. doi: 10.1093/hmg/10.20.2199. [DOI] [PubMed] [Google Scholar]
- Kahn J. How a drug becomes “ethnic”: law, commerce, and the production of racial categories in medicine. Yale Journal of Health Policy Law and Ethics. 2004;4(1):1–46. [PubMed] [Google Scholar]
- Kahn J. Race in a Bottle: The Story of BiDil and Racialized Medicine in a Post-Genomic Age. New York: Columbia University Press; 2013. [Google Scholar]
- Kitcher Philip. Does ‘race’ have a future? Philosophy & Public Affairs. 2007;35(4):293–317. [Google Scholar]
- Krieger N. Stormy weather: race, gene expression, and the science of health disparities. American Journal of Public Health. 2005;95(12):2155–2160. doi: 10.2105/AJPH.2005.067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N. Methods for the scientific study of discrimination and health: an ecosocial approach. American Journal of Public Health. 2012a;102(5):936–944. doi: 10.2105/AJPH.2011.300544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N. Who and what is a “population”? Historical debates, current controversies, and implications for understanding “population health” and rectifying health inequities. Milbank Quarterly. 2012b;90(4):634–681. doi: 10.1111/j.1468-0009.2012.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Kosheleva A, Beckfiel J. History, haldanes and health inequities: exploring phenotypic changes in body size by generation and income level in the US-born White and Black non-Hispanic populations 1959-1962 to 2005-2008. International Journal of Epidemiology. 2013;42(1):281–295. doi: 10.1093/ije/dys206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimsky S. BiDil: alive and kicking – author’s reply. Lancet. 2012a;379(9829):1876–1877. doi: 10.1016/S0140-6736(12)60807-1. [DOI] [PubMed] [Google Scholar]
- Krimsky S. The short life of a race drug. Lancet. 2012b;379(9811):114–115. doi: 10.1016/s0140-6736(12)60052-x. [DOI] [PubMed] [Google Scholar]
- Levine TB, Olivari MT, Cohn JN. Angiotensin converting enzyme inhibitors in congestive heart failure. Overview in comparison of captopril and enalapril. American Journal of Medicine. 1986;81(4C):36–39. doi: 10.1016/0002-9343(86)90943-5. [DOI] [PubMed] [Google Scholar]
- Long JC, Li J, Healy ME. Human DNA sequences: more variation and less race. American Journal of Physical Anthropology. 2009;139(1):23–34. doi: 10.1002/ajpa.21011. [DOI] [PubMed] [Google Scholar]
- Maglo KN. Genomics and the conundrum of race: some epistemic and ethical considerations. Perspectives in Biology and Medicine. 2010;53(3):357–372. doi: 10.1353/pbm.0.0171. [DOI] [PubMed] [Google Scholar]
- Maglo KN. The case against biological realism about race: from Darwin to the post-genomic era. Perspectives on Science. 2011;19(4):361–390. [Google Scholar]
- Maglo KN. Group-based and personalized care in an age of genomic and evidence-based medicine: a reappraisal. Perspectives in Biology and Medicine. 2012;55(1):137–154. doi: 10.1353/pbm.2012.0006. [DOI] [PubMed] [Google Scholar]
- Maglo KN, Martin LJ. Researching vs. reifying race: the case of obesity research. Humana Mente: Journal of Philosophical Studies. 2012;22:111–143. [Google Scholar]
- Maiti S, Kumar KH, Castellani CA, O’Reilly R, Singh SM. Ontogenetic de novo copy number variations (CNVs) as a source of genetic individuality: studies on two families with MZD twins for schizophrenia. PloS One. 2011;6(3):e17125. doi: 10.1371/journal.pone.0017125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara DM, Tam SW, Sabolinski ML, et al. Endothelial nitric oxide synthase (NOS3) polymorphisms in African Americans with heart failure: results from the A-HeFT trial. Journal of Cardiac Failure. 2009;15(3):191–198. doi: 10.1016/j.cardfail.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Nitromed. BiDil. 2012 Available at: http://www.nitromed.com/PI.pdf.
- Ossorio P, Duster T. Race and genetics: controversies in biomedical, behavioral, and forensic sciences. American Psychologist. 2005;60(1):115–128. doi: 10.1037/0003-066X.60.1.115. [DOI] [PubMed] [Google Scholar]
- Relethford J. Human Population Genetics. Hoboken, N.J.: Wiley-Blackwell; 2012. [Google Scholar]
- Risch N, Burchard E, Ziv E, Tang H. Categorization of humans in biomedical research: genes, race and disease. Genome Biology. 2002;3(7) doi: 10.1186/gb-2002-3-7-comment2007. comment2007.1–2007.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DE. Is race-based medicine good for us? African American approaches to race, biomedicine, and equality. Journal of Law Medicine and Ethics. 2008;36(3):537–545. doi: 10.1111/j.1748-720X.2008.302.x. [DOI] [PubMed] [Google Scholar]
- Roberts DE. Fatal Invention: How Science Politics and Big Business Re-create Race in the Twenty-first Century. New York/London: The New Press; 2012. [Google Scholar]
- Roberts NJ, Vogelstein JT, Parmigiani G, Kinzler KW, Vogelstein B, Velculescu VE. The predictive capacity of personal genome sequencing. Science Translational Medicine. 2012;4(133):133ra158. doi: 10.1126/scitranslmed.3003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root M. Stratifying a population by race. Journal of Social Philosophy. 2010;41(3):260–271. [Google Scholar]
- Rosenberg NA, Pritchard JK, Weber JL, et al. Genetic structure of human populations. Science. 2002;298(5602):2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- Rotimi CN, Jorde LB. Ancestry and disease in the age of genomic medicine. New England Journal of Medicine. 2010;363(16):1551–1558. doi: 10.1056/NEJMra0911564. [DOI] [PubMed] [Google Scholar]
- Royal CD, Novembre J, Fullerton SM, et al. Inferring genetic ancestry: opportunities, challenges, and implications. American Journal of Human Genetics. 2010;86(5):661–673. doi: 10.1016/j.ajhg.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusert BM, Royal CD. Grassroots marketing in a global era: more lessons from BiDil. Journal of Law Medicine and Ethics. 2011;39(1):79–90. doi: 10.1111/j.1748-720X.2011.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar P, Kahn J. BiDil: race medicine or race marketing? Health Affairs. 2005;(Suppl Web Exclusives):W5-455–463. doi: 10.1377/hlthaff.w5.455. [DOI] [PubMed] [Google Scholar]
- Satel SL. I am a racially profiling doctor. New York Times; 2002. May 5, Available at: http://www.nytimes.com/2002/05/05/magazine/i-am-a-racially-profiling-doctor.html. [Google Scholar]
- Satel SL. Erratum: I am a racially profiling doctor. 2006 Apr; Available at: http://www.sallysatelmd.com/html/a-nytimes3.html.
- Serre D, Paabo S. Evidence for gradients of human genetic diversity within and among continents. Genome Research. 2004;14(9):1679–1685. doi: 10.1101/gr.2529604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Quertermous T, Rodriguez B, et al. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. American Journal of Human Genetics. 2005;76(2):268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate SK, Goldstein DB. Will tomorrow’s medicines work for everyone? Nature Genetics. 2004;36(11 Suppl):S34–42. doi: 10.1038/ng1437. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. New England Journal of Medicine. 2004;351(20):2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- Tishkoff SA, Reed FA, Friedlaende FR, et al. The genetic structure and history of Africans and African Americans. Science. 2009;324(5930):1035–1044. doi: 10.1126/science.1172257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff SA, Verrelli BC. Patterns of human genetic diversity: implications for human evolutionary history and disease. Annual Review of Genomics and Human Genetics. 2003;4:293–340. doi: 10.1146/annurev.genom.4.070802.110226. [DOI] [PubMed] [Google Scholar]
- Wilson JF, Weale ME, Smith AC, et al. Population genetic structure of variable drug response. Nature Genetics. 2001;29(3):265–269. doi: 10.1038/ng761. [DOI] [PubMed] [Google Scholar]
- Wolinsky H. Genomes, race and health. Racial profiling in medicine might just be a stepping stone towards personalized health care. EMBO Reports. 2011;12(2):107–109. doi: 10.1038/embor.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]


