Abstract
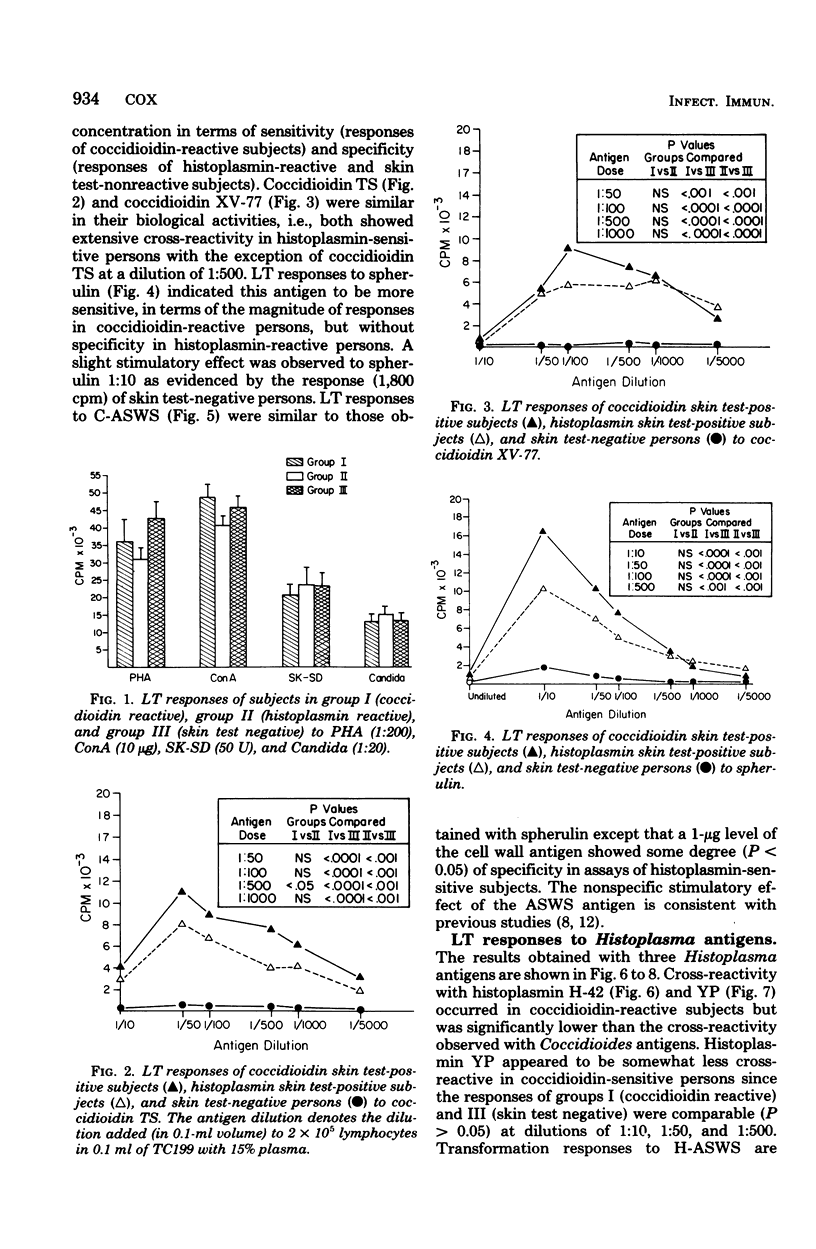
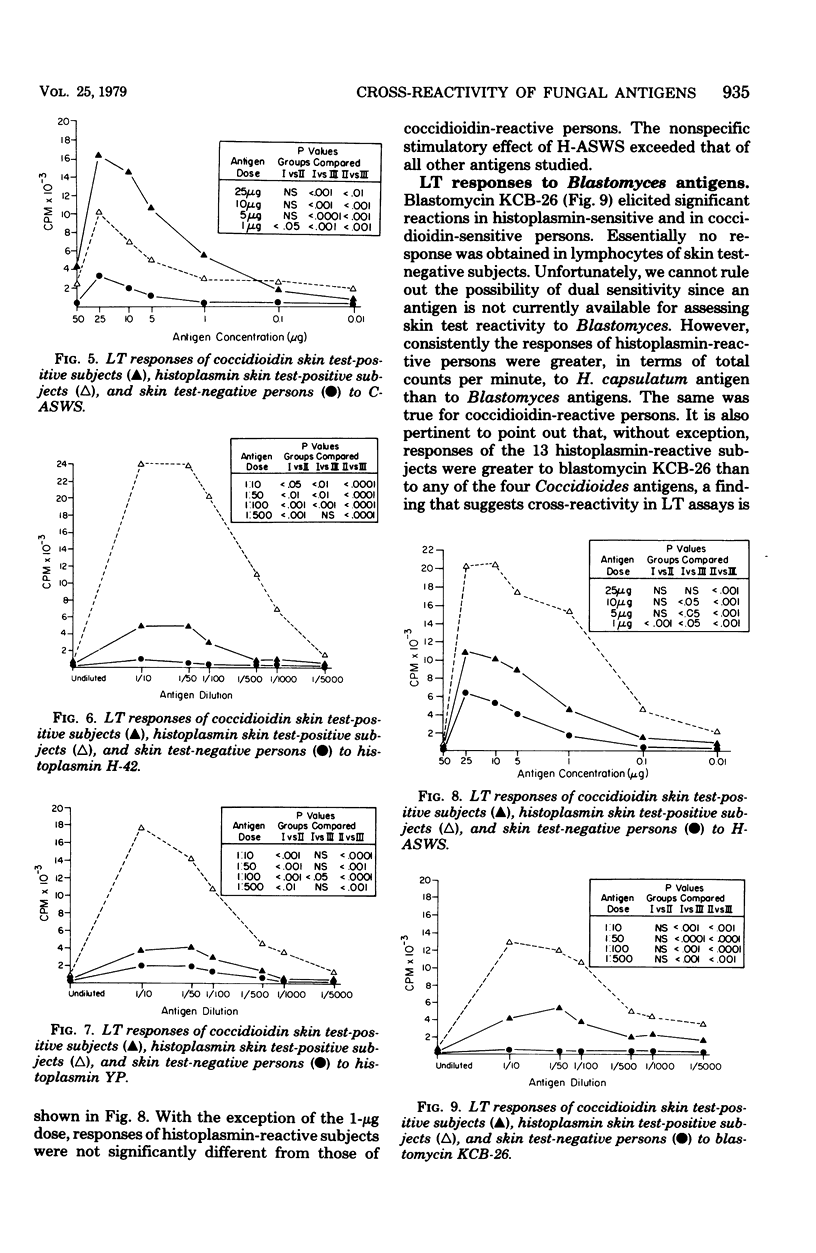
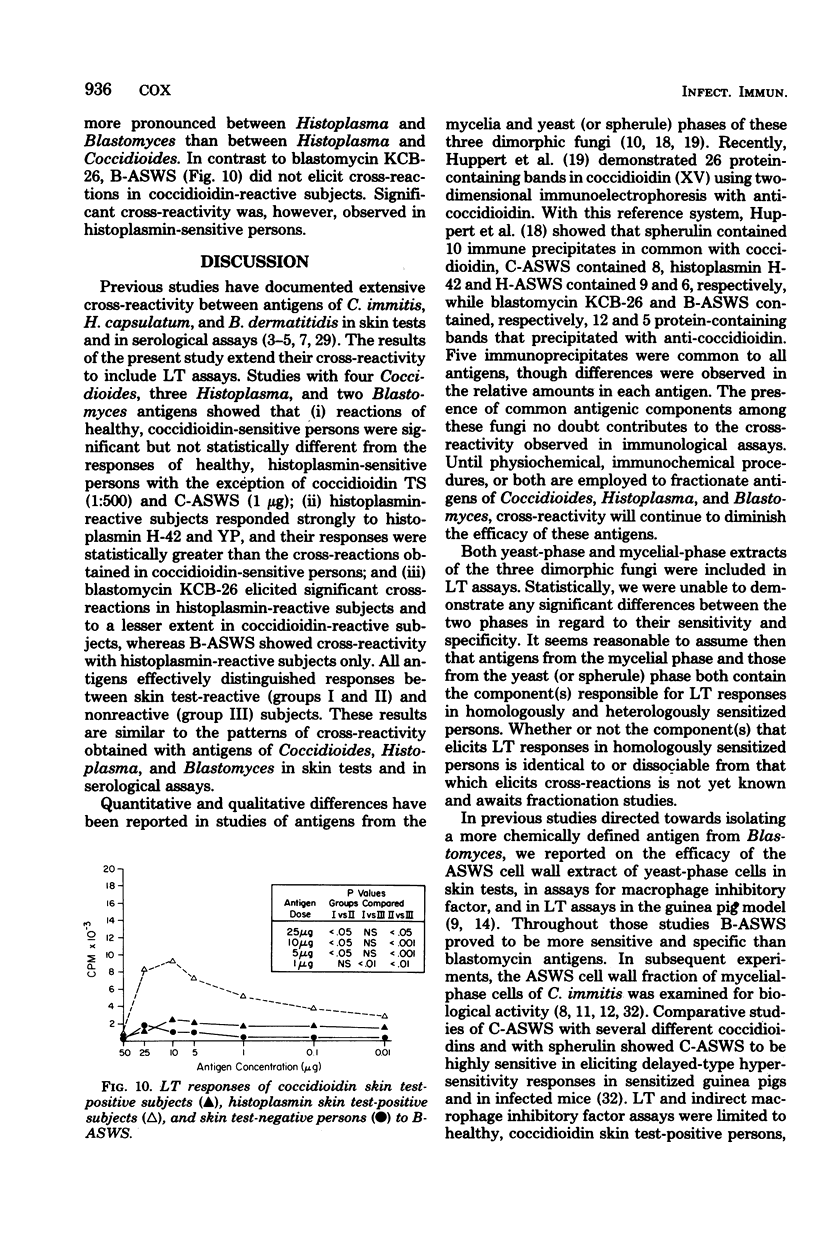
The cross-reactivity of four Coccidioides antigens, three Histoplasma antigens, and two Blastomyces antigens were determined in lymphocyte transformation assays of 11 coccidioidin-reactive, histoplasmin-nonreactive subjects (group I), 13 coccidioidin-nonreactive, histoplasmin-reactive persons (group II), and 13 subjects who were skin test negative to both antigens (group III). Mycelial and yeast (or spherule)-phase antigens of the three fungi were included. Significant cross-reactivity was obtained with both coccidioidins, spherulin, and the alkali-soluble, water-soluble cell wall antigen of C. immitis, to the extent that the responses of histoplasmin-reactive persons were not statistically different (P > 0.05) from those of coccidioidin-reactive persons. In contrast, optimal dilutions of Histoplasma mycelial and yeast-phase lysates effectively distinguished (P < 0.01) responses of histoplasmin- and coccidioidin-reactive persons. The alkali-soluble cell wall antigen of H. capsulatum showed extensive cross-reactivity at most concentrations and was markedly stimulatory to lymphocytes of skin test-negative persons. Blastomycin elicited significant cross-reactions in histoplasmin-sensitive subjects and to a lesser extent in coccidioidin-sensitive subjects. The alkali-soluble cell wall antigen cross-reacted in cultures of histoplasmin-reactive persons but not in those of coccidioidin-reactive persons. All antigens effectively distinguished (P < 0.001) homologous responses of skin test-positive persons (groups I and II) from those of skin test-negative persons (group III). The extensive cross-reactivity in lymphocyte transformation assays in the absence of cross-reactivity in skin tests suggests that these two immune responses may be mediated by different T lymphocyte populations, may be elicited by different antigenic components, or both.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H., Goodwin R. A. Patterns of immune response in chronic pulmonary histoplasmosis. J Infect Dis. 1972 Mar;125(3):269–275. doi: 10.1093/infdis/125.3.269. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CAMPBELL C. C., BINKLEY G. E. Serologic diagnosis with respect to histoplasmosis, coccidioidomycosis, and blastomycosis and the problem of cross reactions. J Lab Clin Med. 1953 Dec;42(6):896–906. [PubMed] [Google Scholar]
- CAMPBELL C. C. The accuracy of serologic methods in diagnosis. Ann N Y Acad Sci. 1960 Aug 27;89:163–177. doi: 10.1111/j.1749-6632.1960.tb20139.x. [DOI] [PubMed] [Google Scholar]
- Catanzaro A., Spitler L. E., Moser K. M. Cellular immune response in coccidioidomycosis. Cell Immunol. 1975 Feb;15(2):360–371. doi: 10.1016/0008-8749(75)90014-3. [DOI] [PubMed] [Google Scholar]
- Chick E. W., Baum G. L., Furcolow M. L., Huppert M., Kaufman L., Pappagianis R. Scientific Assembly statement. The use of skin tests and serologic tests in histoplasmosis, coccidioidomycosis, and blastomycosis, 1973. Am Rev Respir Dis. 1973 Jul;108(1):156–159. doi: 10.1164/arrd.1973.108.1.156. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Brummer E., Lecara G. In vitro lymphocyte responses of coccidioidin skin test-positive and -negative persons to coccidioidin, spherulin, and a coccidioides cell wall antigen. Infect Immun. 1977 Mar;15(3):751–755. doi: 10.1128/iai.15.3.751-755.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Larsh H. W. Yeast- and mycelial-phase antigens of Blastomyces dermatitidis: comparison using disc gel electrophoresis. Infect Immun. 1974 Jul;10(1):48–53. doi: 10.1128/iai.10.1.48-53.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Vivas J. R., Gross A., Lecara G., Miller E., Brummer E. In vivo and in vitro cell-mediated responses in coccidioidomycosis. I. Immumologic responses of persons with primary, asymptomatic infections. Am Rev Respir Dis. 1976 Nov;114(5):937–943. doi: 10.1164/arrd.1976.114.5.937. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Vivas J. R. Spectrum of in vivo and in vitro cell-mediated immune responses in coccidioidomycosis. Cell Immunol. 1977 Jun 1;31(1):130–141. doi: 10.1016/0008-8749(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Davis S. K., Oels H. C. Fractionation of antigenic components of histoplasmin by disc electrophoresis. Am Rev Respir Dis. 1973 Sep;108(3):708–711. doi: 10.1164/arrd.1973.108.3.708. [DOI] [PubMed] [Google Scholar]
- Deighton F., Cox R. A., Hall N. K., Larsh H. W. In vivo and in vitro cell-mediated immune responses to a cell wall antigen of Blastomyces dermatitidis. Infect Immun. 1977 Feb;15(2):429–435. doi: 10.1128/iai.15.2.429-435.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J Infect Dis. 1973 Jun;127(6):694–697. doi: 10.1093/infdis/127.6.694. [DOI] [PubMed] [Google Scholar]
- Huppert M., Adler J. P., Rice E. H., Sun S. H. Common antigens among systemic disease fungi analyzed by two-dimensional immunoelectrophoresis. Infect Immun. 1979 Feb;23(2):479–485. doi: 10.1128/iai.23.2.479-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Spratt N. S., Vukovich K. R., Sun S. H., Rice E. H. Antigenic analysis of coccidioidin and spherulin determined by two-dimensional immunoelectrophoresis. Infect Immun. 1978 May;20(2):541–551. doi: 10.1128/iai.20.2.541-551.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lancaster M. V., Sprouse R. F. Preparative isotachophoretic separation of skin test antigens from blastomycin purified derivative. Infect Immun. 1976 Mar;13(3):758–762. doi: 10.1128/iai.13.3.758-762.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landay M. E., Wheat R. W., Conant N. F., Lowe E. P. Serological comparison of the three morphological phases of Coccidioides immitis by the agar gel diffusion method. J Bacteriol. 1967 Jan;93(1):1–6. doi: 10.1128/jb.93.1.1-6.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatti C. C., Rezkallah M. T., Mendes E., Mendes N. F. In vivo and in vitro evaluation of cell-mediated immunity in patients with paracoccidiodomycosis. Cell Immunol. 1976 Jun 15;24(2):365–378. doi: 10.1016/0008-8749(76)90220-3. [DOI] [PubMed] [Google Scholar]
- Newberry W. M., Jr, Chandler J. W., Jr, Chin T. D., Kirkpatrick C. H. Immunology of the mycoses. I. Depressed lymphocyte transformation in chronic histoplasmosis. J Immunol. 1968 Feb;100(2):436–443. [PubMed] [Google Scholar]
- PAPPAGIANIS D., SMITH C. E., KOBAYASHI G. S., SAITO M. T. Studies of antigens from young mycelia of Coccidioides immitis. J Infect Dis. 1961 Jan-Feb;108:35–44. doi: 10.1093/infdis/108.1.35. [DOI] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T. Histoplasmin sensitivity and coccidioidal infection; occurrence of cross-reaction. Am J Public Health Nations Health. 1949 Jun;39(6):722–736. doi: 10.2105/ajph.39.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse R. F., Goodman N. L., Larsh H. W. Fractionation, isolation and chemical characterization of skin test active components of histoplasmin. Sabouraudia. 1969 Feb;7(1):1–11. doi: 10.1080/00362177085190021. [DOI] [PubMed] [Google Scholar]
- Sweet G. H. Antigens of Histoplasma capsulatum. II. Separation and characterization of yeast-phase precipitinogens. Am Rev Respir Dis. 1971 Sep;104(3):401–407. doi: 10.1164/arrd.1971.104.3.401. [DOI] [PubMed] [Google Scholar]
- The current status of serologic, immunologic and skin tests in the diagnosis of pulmonary mycoses. Report of the Committee on Fungus Diseases and Subcommittee on Criteria for Clinical Diagnosis--American College of Chest Physicians. Chest. 1973 Feb;63(2):259–270. [PubMed] [Google Scholar]
- Ward E. R., Jr, Cox R. A., Schmitt J. A., Jr, Huppert M., Sun S. H. Delayed-type hypersensitivity responses to a cell wall fraction of the mycelial phase of Coccidioides immitis. Infect Immun. 1975 Nov;12(5):1093–1097. doi: 10.1128/iai.12.5.1093-1097.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


