Abstract
Post-traumatic stress disorder (PTSD) is a serious mental health injury which can manifest after experiencing a traumatic life event. The disorder is characterized by symptoms of re-experiencing, avoidance, emotional numbing and hyper-arousal. Whilst its aetiology and resultant symptomology are better understood, relatively little is known about the underlying cortical pathophysiology, and in particular whether changes in functional connectivity may be linked to the disorder. Here, we used non-invasive neuroimaging with magnetoencephalography to examine functional connectivity in a resting-state protocol in the combat-related PTSD group (n = 23), and a military control group (n = 21). We identify atypical long-range hyperconnectivity in the high-gamma-band resting-state networks in a combat-related PTSD population compared to soldiers who underwent comparable environmental exposure but did not develop PTSD. Using graph analysis, we demonstrate that apparent network connectivity of relevant brain regions is associated with cognitive-behavioural outcomes. We also show that left hippocampal connectivity in the PTSD group correlates with scores on the well-established PTSD Checklist (PCL). These findings indicate that atypical synchronous neural interactions may underlie the psychological symptoms of PTSD, whilst also having utility as a potential biomarker to aid in the diagnosis and monitoring of the disorder.
Keywords: Post-traumatic stress disorder, Magnetoencephalography (MEG), Resting-state, Functional connectivity, Neural network
Graphical abstract
Highlights
-
•
Soldiers with PTSD display increased connectivity in high gamma resting state.
-
•
Left frontal, temporal and hippocampus regions show hyperconnectivity in PTSD.
-
•
Emotionally-salient stimuli induced increased connectivity in soldiers without PTSD.
-
•
Connectivity strength in left hippocampus correlates with PTSD symptom severity.
1. Introduction
Post-traumatic stress disorder (PTSD) is a mental health problem, characterized by anxious and depressive features, which develops after exposure to a traumatic life event. It is placed in the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) trauma and stressor-related disorders category, and PTSD is principally comprised of four symptom clusters: re-experiencing; avoidance; emotional numbing; and hyperarousal (American Psychiatric Association, 2013). Incidence of the disorder in the general population is around 5–10% (Kessler et al., 2005), and is thought to be much higher in some military populations returning from recent combat deployments in hazardous regions (Richardson et al., 2010). Knowledge remains scant, however, regarding the neurobiological basis of PTSD, limiting our understanding of the disorder and hampering the search for reliable biomarkers.
Structural and functional neuroimaging studies of PTSD using magnetic resonance imaging (MRI and fMRI) and positron emission tomography (PET) have reported atypical neuroanatomy and differential activation patterns in a number of cortical and subcortical structures (Hull, 2002). Known to play a role in episodic memory, the hippocampi show both structural and functional abnormalities (Bremner et al., 2003). Additionally, the amygdalae (Etkin and Wager, 2007), ventromedial prefrontal (Gold et al., 2011), and the dorsal anterior cingulate cortices (Shin et al., 2011) have been reported to show atypical function in the disorder (for an extensive biological review of PTSD, see Pitman et al., 2012).
In recent years, advances in the analyses of resting-state networks and the functional connectivity among brain regions have allowed researchers to map ongoing and spontaneous communication required for the temporal coordination of cognitive and sensory processing (Damoiseaux et al., 2006). In particular, changes in functional connectivity defined by ongoing oscillatory synchrony (Wang, 2010) have proven to be useful in mapping cortical pathophysiology, thought to underlie a number of neurophysiological disorders (Tewarie et al., 2013), including magnetoencephalographic (MEG) studies of PTSD. The technique of MEG allows spatiotemporal patterns of brain activity to be mapped with millisecond precision, elucidating functional brain changes on time scales to which fMRI is blind (Hari and Salmelin, 2012); fMRI only images ultra-low frequencies. Furthermore, MEG benefits from being able to directly record ongoing brain activity, as opposed to changes in the haemodynamics associated with neural firing.
MEG studies of PTSD suggest enhanced slow wave generators in left temporal regions, and decreased oscillations in parieto-occipital cortex are related to PTSD (Kolassa et al., 2007). Georgopoulos et al. (2010) suggested that patterns of abnormal synchronous oscillations could differentiate PTSD from control subjects, particularly poor communication between right temporo-parietal areas and other brain regions (Engdahl et al., 2010). This group also linked decorrelations in small networks, most evident in the right superior temporal gyrus, with resilience to lifetime trauma in control veterans, but not those with PTSD (James et al., 2013). The above studies collectively suggest that abnormal coherent brain oscillations might contribute to symptoms of the disorder.
Prior studies, however, have not investigated frequency-specific interactions in source-resolved networks, or their association with exposure to stressful stimuli or symptom severity. Here we used source-analysed MEG and graph theoretical analysis to test the hypotheses that veterans with PTSD would express atypical resting-state network synchrony; that these atypical inter-regional interactions would be exacerbated by exposure to stressful, combat-related imagery; and that an aberrant organization of neurophysiological networks is associated with the severity of PTSD symptoms and associated cognitive-behavioural outcomes. We selected strength and degree as our graph properties of interest, as they most directly correspond to network hyperconnectivity or hypoconnectivity when comparing two populations, and thus correspond most closely with our hypothesis that veterans with PTSD would show alterations in the intensity of network-level neurophysiological interactions. Specifically, node strength represents the weighted magnitude of the relation between a seed node and the network, which is the sum of all connections, and node degree indicates the number of connections between a given node and other nodes in the network (Bullmore and Sporns, 2009), which would capture differential network synchrony between veterans with PTSD and their matched controls.
2. Methods and materials
2.1. Participants
MEG data were recorded from 23 Canadian Armed Forces soldiers, who deployed in support of the Afghan mission and were subsequently diagnosed with PTSD (all male, mean age = 37.4, SD = 6.8, age range 22–48). Twenty-one soldiers (all male, mean age = 33.05, SD = 5.26, age range 18–45) who also participated in the Afghan mission but did not develop PTSD were recruited as a control group.
Participants were included in the PTSD group if they met the following criteria: they have a diagnosis of combat-related PTSD from an operational trauma stress support centre (OTSSC); PTSD symptoms were present from 1 to 4 years prior to participation in the study; they were engaged in regular mental health follow-up; and they had moderate or greater severity on the PTSD Checklist (PCL > 50). The diagnosis was determined by a psychiatrist or psychologist specializing in trauma-related mental health injuries and conducted through a comprehensive, semi-structured interview based upon DSM-IV-TR diagnostic criteria (American Psychiatric Association, 2000), along with Canadian Armed Forces (CAF) standardized psychometric testing. Interview-based clinician diagnosis of mental disorders is considered superior to pen and paper or self-administered screening methods. All participants in the PTSD group were recruited from one of the CAF OTSSCs, which are centres of excellence for the diagnosis and treatment of trauma-related mental health injuries. There were usually more than one DSM-IV-TR ‘A1’ stressor-related criteria (American Psychiatric Association, 2000) identified as a traumatic event contributing to the development of PTSD (direct personal experience of an event that involves actual or threatened death or injury), with diagnosis related to operational exposure. Control soldiers were matched on rank, education level, handedness and military experience.
Additional inclusion criteria applied to both groups included: no history of a traumatic brain injury (TBI), screened by a psychiatrist through a review of their electronic health record, telephone interview, and administration of the Defence and Veteran's Brain Injury Centre (DVBIC) 3 item screening tool; English-speaking and able to understand task instructions and give informed consent. Exclusion criteria included ferrous metal inside the body that might be classified as MRI contraindications or items that might interfere with MEG data acquisition; presence of implanted medical devices; seizures or other neurological disorders, or active substance abuse; certain ongoing medications (anticonvulsants, benzodiazepines, and/or GABA antagonists) known to directly or significantly influence electroencephalographic (EEG) findings. As this was a naturalistic sample, however, all PTSD patients were on evidenced-based psychotropic medication(s), such as selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs) and Prazosin.
All participants underwent cognitive-behavioural testing in addition to the MEG resting-state scan. These assessments included: estimates of IQ from the Wechsler Abbreviated Scale of Intelligence (WASI); the Alcohol Use Disorders Identification Test (AUDIT); Conner's Attention-Deficit Hyperactivity Disorder Test; the Generalized Anxiety Disorder 7 (GAD-7) test; Patient Health Questionnaire (PHQ9); and the Post Traumatic Stress Disorder Checklist (PCL). Within the PTSD group, there were significant rates of co-morbid mental disorders, including major depressive disorder (MDD; 74% of the PTSD group). These findings are consistent with prevalence rates established through large scale studies in military populations (Garber et al., 2012).
2.2. Procedure and MEG data acquisition
Resting-state MEG data were collected in two separate runs. Participants were supine and instructed to rest with eyes open and maintain visual fixation on an x within a circle on the screen. Following the first resting-state run, the participants completed a number of imaging protocols, with a memory-related paradigm containing trigger images, such as scenes of traumatic events (e.g. battlefield casualties) intermixed with neutral images, an emotional faces task, and a verbal task that contained neutral as well as salient trigger words (such as ‘Kandahar’ and ‘grenade’). They then completed a second resting-state run. We expected the affective stimuli to induce arousal and attentional mechanisms, and perhaps differentially activate the PTSD group compared to the controls. We refer to the initial scan as the pre-triggering resting-state run and the subsequent acquisition as the post-triggering resting-state run.
MEG data were collected inside a magnetically-shielded room on a CTF Omega 151 channel system (CTF Systems, Inc., Coquitlam, Canada) at The Hospital for Sick Children, at 600 Hz for 300 s per resting-state run. Throughout the run, head position was continuously recorded by three fiducial coils placed on the nasion, and left and right pre-auricular points.
After the MEG session, anatomical MRI images were acquired using the 3 T MRI Research scanner (Magnetom Tim Trio, Siemens AG, Erlangen, Germany) in a suite adjacent to the MEG. Structural data were obtained as T1-weighted magnetic resonance images using resolution 3D MPRAGE sequences (repetition time [TR] = 2300 ms; echo time [TE] = 2.9 ms; flip angle [FA] = 9°; field-of-view [FOV] = 28.8 × 19.2 cm; 256 × 256 matrix; 192 slices; 1 mm isovoxel) on a 12-channel head coil. MEG data were coregistered to the MRI structural images using the reference fiducial coil placements. A multi-sphere head model was constructed for each individual and their brain space was normalized to a standard Montreal Neurological Institute (MNI) brain using SPM2.
2.3. MEG data processing
2.3.1. Seed definition and virtual electrode recording
MEG data were band-pass filtered offline at 1–150 Hz, a notch filter applied at 60 Hz (8 Hz bandwidth) and a third-order spatial gradient environmental noise-cancellation applied to the recording. A priori sources (seeds) of interest in cortex and sub-cortical regions were identified from the Automated Anatomical Labeling (AAL; Tzourio-Mazoyer et al., 2002) atlas giving 90 locations for time-series to be extracted and analysed. Broadband time-series (‘virtual electrodes’) from these voxels were reconstructed using a vector beamformer on the basis of the 90 AAL coordinates for each subject and filtered into five classical EEG bandwidths for further analysis: Theta (4–7 Hz), Alpha (8–14 Hz), Beta (15–30 Hz), ‘low’ Gamma (30–80 Hz), and ‘high’ Gamma (80–150 Hz).
Beamformers are a type of spatial filter used to suppress signals from unwanted noise sources, whilst being optimally sensitive to activity in a given brain location (in this particular case, the AAL seed locations). Individual weight vectors are applied to each sensor measurement and summated to give estimated source activity to a particular cortical seed location (Quraan and Cheyne, 2010). Additionally, MEG beamformers are effective at suppressing ocular artefacts generated by eye movements, which are a particular problematic source noise in EEG, and non-ocular artefacts, such as cardiac and muscle activity (Muthukumaraswamy, 2013). Thus, beamforming removes artefacts, not requiring the rejection of data.
2.3.2. Assessing functional connectivity: weighted phase lag index
Each of the 5 band-pass filtered waveforms were then submitted to a functional connectivity analysis, using the weighted phase lag index (wPLI; Vinck et al., 2011). The instantaneous phase of each sample from the filtered time-series was calculated using the Hilbert transform. The degree of phase synchronization between all pairwise combinations of the seeds was computed using the wPLI, which is based on the magnitude of the imaginary component of the cross-spectrum (Lau et al., 2012). Ranging between 0 and 1, these values quantify the phase synchrony between two cortical/sub-cortical sources, referred to as functional connectivity.
2.3.3. Statistical analysis
Adjacency matrices with wPLI values acting as edge weights for all sources were constructed, which resulted in a 90 × 90 [×5 frequencies] matrix of weighted undirected graphs for each participant. Adjacency matrices were then divided into groups and inferential statistics investigating group differences for edge weights were implemented using non-parametric permutation testing, which do not require that the data distributions be normal, using the Network Based Statistic (NBS) Toolbox (Zalesky et al., 2010). False positives due to multiple comparisons were controlled for using the false discovery rate (FDR; corrected, p < 0.05), across the whole 90 × 90 matrix. Network measures of node strength were used to assess the importance of a node within the networks and these measures were obtained using Brain Connectivity Toolbox (Rubinov and Sporns, 2010). Brain networks were visualized using BrainNet Viewer (Xia et al., 2013). Further behavioural correlation analyses were conducted using MATLAB Statistics Toolbox (The Mathworks, Inc.).
3. Results
Measures for the cognitive-behavioural test batteries and the characteristics of the soldiers with and without PTSD are presented in Table 1.
Table 1.
Cognitive-behavioural assessment measures for PTSD and control participants.
| PTSD | Control | Test statistic | |
|---|---|---|---|
| n | 23 | 21 | |
| WASI | 108 (14.09) | 116.81 (13.67) | t = 2.10, df = 42, p = 0.0420 |
| AUDIT | 8.70 (7.67) | 6.10 (3.91) | t = −1.40, df = 42, p = 0.170 |
| Conner's | 24.09 (9.57) | 7.19 (4.99) | t = −7.24, df = 42 p < 0.001 |
| GAD-7 | 15.04 (4.41) | 1.95 (1.99) | t = −12.50, df = 42, p < 0.001 |
| PHQ9 | 16.87 (5.09) | 1.86 (2.31) | t = −12.39, df = 42, p < 0.001 |
| PCL | 63.95 (7.81) | NA |
WASI, Wechsler Abbreviated Scale of Intelligence; AUDIT, Alcohol Use Disorders Identification Test; Conner's, Attention-Deficit Hyperactivity Disorder Test; GAD-7, Generalized Anxiety Disorder 7; PHQ9, Patient Health Questionnaire; PCL, Post Traumatic Stress Disorder Checklist.
3.1. Functional connectivity analyses: pre- and post-triggering resting-state networks
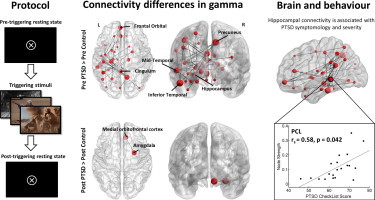
Increased high gamma (80–150 Hz) resting oscillatory network synchrony was observed in the PTSD soldiers compared to control soldiers in the pre-triggering resting-state run (p < 0.05, FDR-corrected; Fig. 1A). This network comprised 34 nodes of varying degree and strength, localized predominantly in the left hemisphere, with connectivity differences in important nodes noted in the left frontal, left temporal, and right parietal regions. For the other frequency bands, the analysis revealed only the occasional significant edge connecting two nodes (p < 0.05), but these patterns are inconclusive in terms of revealing any large-scale network connectivity differences, and were not investigated further.
Fig. 1.
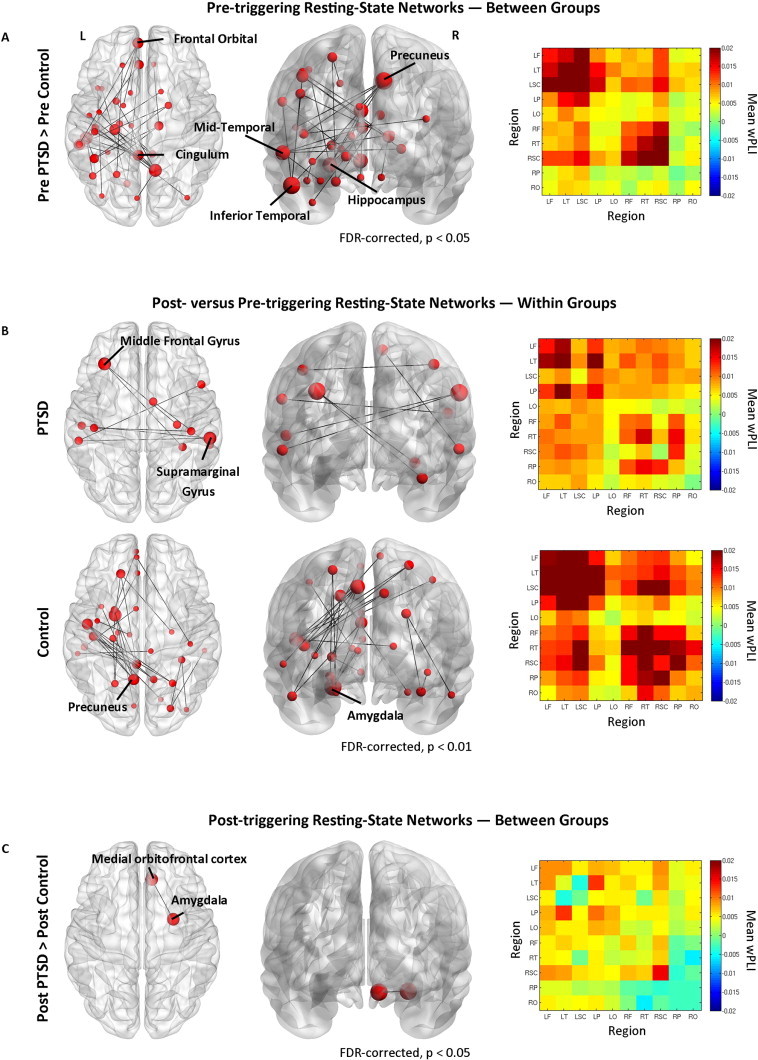
Network connectivity maps in the high gamma band, and inter- and intra-regional adjacency matrices. (A) Contrasts of pre-triggering resting-state networks (RSN) for the PTSD versus control soldier participants in the 80–150 Hz band, showing hyper connectivity in the PTSD group compared to controls (p < 0.05, FDR-corrected) with node size scaled to degree. Mean wPLI region × region adjacency matrix for PTSD minus control group, with each element of the graph showing vertices depicting hemisphere-divided lobes, such that the top left and bottom right quadrants denote intra-hemisphere connectivity (left and right, respectively); the top right and bottom left quadrants show inter-hemispheric lobe connectivity. Regions preceded by L denote left hemisphere, R denotes right hemisphere; F, frontal; T, temporal, SC, sub-cortical; P, parietal; O, occipital. (B)Top row, within-group pre- versus post-triggering RSN for the PTSD participants in the 80–150 Hz band showing increased connectivity in the post-triggering RSN (p < 0.01, FDR-corrected). Bottom row, same as above except for the control soldier participants. (C) Post-triggering RSN for PTSD versus control groups (p < 0.05, FDR-corrected).
All seed regions found to have significantly different connectivity patterns as a result of the permutation analysis (FDR-corrected, p < 0.05) are listed in Fig. 2A, first ordered by hemisphere, and then by lobe. Fig. 2A illustrates the magnitude of the difference between the groups in node degree (in other words, the number of significant connections or edges between nodes). In Fig. 2B individual group-mean measures of node strength are shown (the sum of edge weights for a particular node; PTSD soldiers denoted by the black bars, control soldiers by the white bars). Important, high-strength nodes with a degree difference greater than 3 are highlighted by the asterisk in Fig. 2A. None of the other frequency bands displayed significant atypical network connectivity in the between-groups pre-triggering resting-state analysis.
Fig. 2.
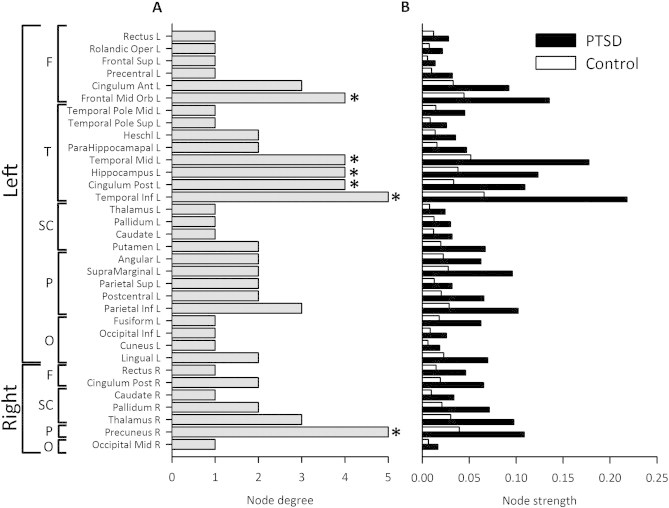
Summary of brain regions identified as being hyperconnected in the PTSD group compared to controls in the pre-triggering resting-state run derived from the NBS analyses (p < 0.05, FDR-corrected). (A) The list of brain regions that were found to be significant is initially divided by hemisphere, then by lobe, and then finally by degree in ascending order. Nodes marked with an asterisk (*) indicate high-strength, critical network nodes (degree ≥ 4) that were used in the cognitive-behavioural correlation analyses. (B) A between-group comparison of the mean within-network node strengths (sum of the wPLI) values.
A within-group comparison of pre- versus post-triggering resting-state networks revealed a similar pattern of network connectivity changes for the control soldiers after viewing trigger images. In the post-resting-state they displayed enhanced high gamma band connectivity compared to the pre-triggering resting-state run, with increases in connectivity confined mostly to the left hemisphere (p < 0.01, FDR-corrected; Fig. 1B, bottom row); the most highly connected nodes were the left precuneus (node degree = 4) and left amygdala (node degree = 4). The PTSD soldiers showed relatively minor increases in high gamma network connectivity between the pre- and post-triggering resting-state sessions (p < 0.01, FDR-corrected; Fig. 1B, top row), with enhanced inter-hemispheric synchrony involving the left middle frontal gyrus (node degree = 2) and the right supramarginal gyrus (node degree = 2).
Finally, a between-group comparison in the post-triggering resting-state run revealed only minor network differences after viewing the trigger images, with the right orbital-frontal cortex and right amygdala showing enhanced connectivity in the PTSD group compared to the controls (p < 0.05, FDR-corrected; Fig. 1C). This suggests viewing emotionally-salient stimuli brings about network connectivity changes to the control group that resemble the atypical resting-state synchrony shown in the PTSD group, such that these otherwise-similar populations who underwent comparable experiential events show broadly comparable network connectivity patterns after the triggering stimuli, with a small increase in connectivity between the right amygdala and right orbitofrontal gyrus.
3.2. Seed connectivity correlates of cognitive-behavioural outcomes
To test the hypothesis that aberrant resting-state synchrony is associated with cognitive-behavioural outcome and symptom severity, correlations of node strength against test measures were performed (Fig. 3). To understand inter-subject variability in the neuropsychological data, both groups were combined as the participants were experientially-similar individuals, likely to exhibit cognitive-behavioural and affective tendencies along a spectrum.
Fig. 3.
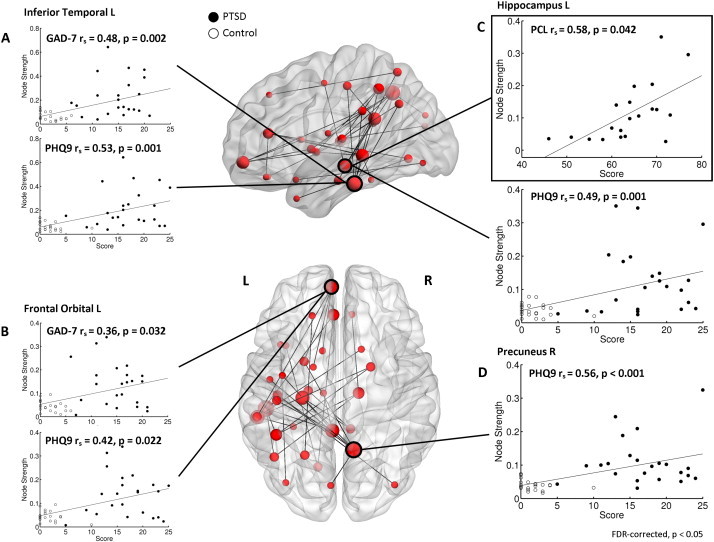
Network connectivity identified in pre-triggering RSN for PTSD versus control groups, and within-network node connectivity (wPLI; undirected weighted strength measures) correlated with cognitive-behavioural assessment outcomes (p < 0.05, FDR-corrected). (A) Mixed/combined PTSD and control group left inferior temporal cortex node strength correlated against GAD-7 (top plot; rs (42) = 0.48, p = 0.002), and PHQ9 (bottom plot; rs (42) = 0.53, p = 0.001). (B) Left frontal orbital strength versus GAD-7 (top plot; rs (42) = 0.36, p = 0.032) and PHQ9 (bottom plot; rs (42) = 0.42, p = 0.022). (C) In the bounded box, left hippocampus PTSD Group-only connectivity strength versus PCL (top plot; rs (18) = 0.58, p = 0.042). Below, node strength versus PHQ9 (bottom plot; rs (42) = 0.49, p = 0.001). (D) Right precuneus strength versus PHQ9 (rs (42) = 0.56, p < 0.001).
Network strength values were not normally distributed and therefore correlation analyses were performed using Spearman's rank order correlation. These analyses were restricted to high degree nodes that were found to be significant following the analysis, which provided 6 seeds/region of interest (ROI) in 3 lobes to explore: left frontal mid-orbital cortex in frontal regions; the left middle temporal gyrus, left posterior cingulum, left hippocampus, and left inferior temporal gyrus in the temporal lobe; and finally the right precuneus in parietal cortex (* in Fig. 2A denotes the nodes used in the correlation analysis). Importantly, these nodes were found in regions that are often implicated in the PTSD literature (Pitman et al., 2012).
Measures of anxiety (based on the GAD-7 score) and depression (from the PHQ9) were found to significantly correlate (p > 0.05, FDR-corrected) with the atypical network gamma connectivity strength in the left inferior temporal (Fig. 3A) and left orbitofrontal regions (Fig. 3B). Furthermore, correlations for depression scores were also found to correlate with connectivity in the left hippocampus (Fig. 3C) and right precuneus (Fig. 3D). Finally, scores from the PTSD Checklist (PCL; characterizes PTSD symptomology) were found to correlate highly with gamma network strength in the left hippocampus in the PTSD Soldiers (Fig. 3C).
4. Discussion
This study provides the first evidence of frequency-specific alterations in source-resolved network synchrony in PTSD. In particular, combat-related PTSD soldiers display long-range hyperconnectivity in the high-frequency gamma band compared to matched controls who did not develop PTSD. We uniquely demonstrate that exposure to stressful combat-related imagery can exacerbate atypical network connectivity associated with PTSD in military veterans. Furthermore, this study is the first to provide evidence that altered network topologies of critical brain regions are associated with cognitive-behavioural outcomes and symptom severity in PTSD, suggesting frequency- and region-specific alterations in neurophysiological synchrony may serve as a biomarker for PTSD.
In contrast to an experientially-similar control soldier population, the PTSD group show significant network connectivity abnormalities in regions often implicated in PTSD, such as the episodic memory-related hippocampal (Bremner et al., 2003; Etkin and Wager, 2007; Pitman et al., 2012) and frontal regions (Gold et al., 2011; Shin et al., 2004). Additionally, we found connectivity differences in temporal (Kolassa et al., 2007; Engdahl et al., 2010; James et al., 2013) and parietal regions (Kolassa et al., 2007) which are largely consistent with those reported in the literature (Pitman et al., 2012).
One striking result of this study shows that following tasks involving emotionally-salient trigger images and words, the triggering brings the brain oscillations of veterans, who never developed PTSD, in line with those with the disorder. In other words, the difference between pre- and post-triggering resting-states in the control soldier group is similar to the connectivity differences between the groups in the pre-triggering resting-state. This suggests that trauma events affect connectivity similarly in both groups, but that individuals with PTSD are perhaps unable to return to a baseline state of ongoing, inter-regional oscillatory synchrony.
We speculate that this hyperconnectivity involving the left hippocampus, temporal and frontal regions reflects some of the primary positive symptoms of PTSD, principally comprised of disturbing mental imagery and chronic hyper-arousal, possibly caused by re-experiencing and re-imagining of traumatic events, as well as heightened vigilance to aversive stimuli. This view is supported by findings from human intracranial recordings indicating that hippocampal–cortical gamma synchronization is associated with the formation of episodic memories (Fell et al., 2001) and in states of vigilance (Llinás and Steriade, 2006), both of which are heavily implicated in PTSD symptomology. This interpretation is further supported by an fMRI study that showed hippocampal activation in a fear response study correlated with re-experiencing and hyperarousal symptom cluster scores (Sripada et al., 2013) on a subscale from the Clinician-Administered PTSD Scale (CAPS). Moreover, fMRI resting-state studies of combat veterans have reported increased connectivity between the amygdala and insula in those with PTSD (Rabinak et al., 2011). These changes were interpreted to be related to threat perception and modulated fear responses, cognitive states that are thought to be atypical in PTSD (Rabinak et al., 2011). Similarly, we also demonstrated increases in connectivity between the right amygdala and orbitofrontal cortex in the post-triggering resting-state results, which we believe are associated with an abnormal conditioned fear response ubiquitous in PTSD (Shin et al., 2004), which is also consistent with prior MEG reports of increased gamma event-related synchronization of the right amygdala during perception of emotional stimuli (Luo et al., 2009). We consider that this particular observation may be related to the chronic heightened state of arousal that is symptomatic of the disorder.
Despite similarities between our findings and the results of prior fMRI investigations, there are also discrepancies between the current study and other fMRI resting-state results in PTSD. A number of groups report simultaneous increases and decreases in fMRI functional connectivity in distinct networks in PTSD patients (Yin et al., 2011; Jin et al., 2013). Yin et al. (2011) found increased connectivity between the thalamus and bilateral inferior frontal and left middle frontal gyri, as well as the left inferior parietal and right superior parietal regions. These regions have also been shown to be preferentially activated in PTSD patients during a memory encoding task. The left middle frontal gyrus, in particular, (Bremner et al., 2003), is an area implicated in memory recollection. As well, the inferior parietal lobule, has been shown to be differentially active during presentation of emotionally-salient cues (Pagani et al., 2010). Jin and colleagues report atypical connectivity between the right PCC and insula, which they interpreted as underlying altered coupling between memory and perception, subserving the re-experiencing of traumatic episodic events (Jin et al., 2013). Both groups reached similar conclusions regarding the nature of these atypical network interactions, speculating that this enhanced connectivity may be related to positive symptom components of PTSD (such as chronic hyperarousal and traumatic memories), which corroborate the interpretation of our results.
Yin and colleagues, however, also reported decreased connectivity between the thalamus and right medial frontal gyrus and left ACC, as well as a negative relation between CAPS scores and connectivity between the thalamus and right precuneus (Yin et al., 2011). Similarly, Jin et al. reported decreased connectivity between the right amygdala and left middle frontal gyrus in PTSD which was negatively associated with CAPS scores (Jin et al., 2013); other studies also report reduced connectivity in resting-state networks, particularly the default mode network, as well as in the amygdala and hippocampal gyri (Bluhm et al., 2009). Taken together, these effects are more difficult to reconcile with the results of the present study, although it should be noted that these relations existed in different neural systems, measured using distinct graph theoretical metrics. Moreover, these previous resting-state results were obtained using different imaging modalities with dissimilar mechanisms of signal generation with differing spatial and temporal resolutions (see Hall et al., 2013 for a review of the relationship between MEG and fMRI).
One important point to note is that our ability to interpret similarities and differences between the results of the present studies and prior findings with fMRI relies on our emerging understanding of the neurophysiological basis of the BOLD signal. It is well known that neuronal firing rate, high-frequency LFP power changes and the BOLD response are tightly coupled (Mukamel et al., 2005; Nir et al., 2007). Nonetheless, the relation between functional connectivity defined by BOLD amplitude covariations and high-frequency oscillatory dynamics are less well understood. Some report that BOLD amplitude covariations are positively correlated with gamma amplitude envelope changes (Tagliazucchi et al., 2012) and that ongoing BOLD fluctuations represent changes in cortical excitability and neural firing (Hiltunen et al., 2014). Conversely, others suggest only low-frequency oscillatory components influence BOLD cross-correlations (Wang et al., 2012), with a negligible contribution from ongoing gamma activity.
Some researchers have highlighted the potential mechanistic and functional differences between ‘intrinsic coupling modes’ (ICMs; Engel et al., 2013) — two distinct modes of operation in oscillatory brain networks. The first, ‘envelope ICMs’, arise from fluctuations in the amplitude envelopes (or power) of disparate neuronal populations, and their signal cross-correlations which reveal the degree to which they are communicating. A second, distinct type of ICM is the ‘phase ICM’, which is defined by the phase synchronization between the band-limited oscillatory signals emanating from different neural populations, in which communication is purported to be enabled through coherence (Fries, 2012). Here, we examined phase ICMs.
Despite a lack of research examining the interplay between these two modes of action, it nevertheless has been proposed that these network-mediating mechanisms operate largely independently of one another (Engel et al., 2013). Moreover, changes in the degree to which coherently oscillating, or phase synchronized, neuronal groups couple might not necessarily incur increased metabolic demands, which would otherwise result in increases in both the ongoing ultra-slow BOLD fluctuations and/or amplitude envelope covariations seen in other studies. Accordingly, although experimental evidence indicates that BOLD correlations may bear some correspondence to inter-regional covariance of envelope fluctuations, the relation between BOLD correlations and inter-regional phase synchrony, such as that measured in the present study, remains more poorly understood.
Finally, we show a significant correlation between left hippocampal node strength within the aberrant functional network and PTSD symptom severity on the PTSD Checklist in the soldiers with PTSD. This novel finding significantly extends previous research on ongoing local neural synchrony/cortical oscillations (Kolassa et al., 2007) by exploring the network interactions with behavioural function. We have gone beyond studies that have shown sensor-space (non-source resolved) measures of neural interactions in right temporal regions might be linked to PTSD and measures of resilience (James et al., 2013), and shown that source-resolved activity in an important episodic-memory related structure correlates significantly with symptom severity in PTSD.
4.1. Conclusions
This study provides the first evidence that military veterans who underwent similar environmental exposure but are differentiated by a post-deployment PTSD diagnosis, show differences in regional connectivity in high-frequency oscillatory synchrony. Additionally, we uniquely demonstrate that ‘high-level’ areas associated with executive function and memory (the frontal and temporal lobes in particular), and the hippocampus, which is associated with episodic memory, show significant correlations between high gamma neural networks and behavioural outcomes and symptom severity in PTSD.
Financial disclosures and competing interests
The authors declare no competing financial interests or potential conflicts of interest.
Acknowledgements
The authors would like to thank Amanda Robertson and Marc Lalancette for help in the data collection, and Daniel Cassel for help with the data analysis pipeline. This work was supported by funding from Defence Research and Development Canada (DRDC) (contract # W7719-135182/001/TOR) and the Canadian Forces Health Services to MJT and EWP.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth edition, text rev. American Psychiatric Association; Washington, DC: 2000. 2000. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fifth edition. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E.A., Frewen P.A., Stevens T.K., Boksman K., Neufeld R.W.J., Théberge J., Lanius R.A. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of Psychiatry and Neuroscience. 2009;34(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Vythilingam M., Vermetten E., Southwick S.M., McGlashan T., Nazeer A. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. American Journal of Psychiatry. 2003;160(5):924–932. doi: 10.1176/appi.ajp.160.5.924. 12727697 [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews. Neuroscience. 2009;10:186–198. doi: 10.1038/nrn2575. 19190637 [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M. Consistent resting-state networks across healthy subjectsProceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. 16945915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. 17898336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl B., Leuthold A.C., Tan H.-R.M., Lewis S.M., Winskowski A.M., Dikel T.N. Post-traumatic stress disorder: a right temporal lobe syndrome? Journal of Neural Engineering. 2010;7(6):066005. doi: 10.1088/1741-2560/7/6/066005. 20980718 [DOI] [PubMed] [Google Scholar]
- Engel A.K., Gerloff C., Hilgetag C.C., Nolte G. Intrinsic coupling modes: multiscale interactions in ongoing brain activity. Neuron. 2013;80:867–886. doi: 10.1016/j.neuron.2013.09.038. 24267648 [DOI] [PubMed] [Google Scholar]
- Fell J., Klaver P., Lehnertz K., Grunwald T., Schaller C. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nature Neuroscience. 2001;4(12):1259–1264. doi: 10.1038/nn759. 11694886 [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cognitive Science. 2012;9(10):356–362. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Garber B.G., Zamorski M.A., Jetly R. Mental health of Canadian forces members while on deployment to Afghanistan. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 2012;57(12):736–744. doi: 10.1177/070674371205701205. 23228232 [DOI] [PubMed] [Google Scholar]
- Georgopoulos A.P., Tan H.-R.M., Lewis S.M., Leuthold A.C., Winskowski A.M., Lynch J.K. The synchronous neural interactions test as a functional neuromarker for post-traumatic stress disorder (PTSD): a robust classification method based on the bootstrap. Journal of Neural Engineering. 2010;7(1):16011. doi: 10.1088/1741-2560/7/1/016011. 20086271 [DOI] [PubMed] [Google Scholar]
- Gold A.L., Shin L.M., Orr S.P., Carson M.A., Rauch S.L., Macklin M.L. Decreased regional cerebral blood flow in medial prefrontal cortex during trauma-unrelated stressful imagery in Vietnam veterans with post-traumatic stress disorder. Psychological Medicine. 2011;41(12):2563–2572. doi: 10.1017/S0033291711000730. 21733221 [DOI] [PubMed] [Google Scholar]
- Hall E.L., Robson S.E., Morris P.G., Brookes M.J. The relationship between MEG and fMRI. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.11.005. 24239589 [DOI] [PubMed] [Google Scholar]
- Hari R., Salmelin R. Magnetoencephalography: from SQUIDs to neuroscience. Neuroimage 20th anniversary special edition. Neuroimage. 2012;61:386–396. doi: 10.1016/j.neuroimage.2011.11.074. 22166794 [DOI] [PubMed] [Google Scholar]
- Hiltunen T., Kantola J., Elseoud A.A., Lepola P., Souminen K., Starck T., Nikkinen J., Remes J., Tervonen O., Palva S., Kiviniemi V., Palva J.M. Infra-slow EEG fluctuations are correlated with resting-state network dynamics in fMRI. Journal of Neuroscience. 2014;34(2):356–362. doi: 10.1523/JNEUROSCI.0276-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull A.M. Neuroimaging findings in post-traumatic stress disorder. Systematic review. British Journal of Psychiatry: the Journal of Mental Science. 2002;181:102–110. 12151279 [PubMed] [Google Scholar]
- James L.M., Engdahl B.E., Leuthold A.C., Lewis S.M., Van Kampen E., Georgopoulos A.P. Neural network modulation by trauma as a marker of resilience: Differences between veterans with posttraumatic stress disorder and resilient controls. JAMA Psychiatry. 2013;70(4):410–418. doi: 10.1001/jamapsychiatry.2013.878. 23426853 [DOI] [PubMed] [Google Scholar]
- Jin C., Qi R., Yin Y., Hu X., Duan L., Xu Q., Zhang Z., Zhong Y., Feng B., Xiang H., Gong Q., Liu Y., Lu G., Li L. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychological Medicine. 2013;44(9):1–10. doi: 10.1017/S003329171300250X. 24168716 [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. 15939837 [DOI] [PubMed] [Google Scholar]
- Kolassa I.T., Wienbruch C., Neuner F., Schauer M., Ruf M., Odenwald M. Altered oscillatory brain dynamics after repeated traumatic stress. BMC Psychiatry. 2007;7:56. doi: 10.1186/1471-244X-7-56. 17941996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau T.M., Gwin J.T., McDowell K.G., Ferris D.P. Weighted phase lag index stability as an artifact resistant measure to detect cognitive EEG activity during locomotion. Journal of Neuroengineering and Rehabilitation. 2012;9:47. doi: 10.1186/1743-0003-9-47. 22828128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R.R., Steriade M. Bursting of thalamic neurons and states of vigilance. Journal of Neurophysiology. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. 16554502 [DOI] [PubMed] [Google Scholar]
- Luo Q., Mitchell D., Cheng X., Mondillo K., Mccaffrey D. Visual awareness, emotion, and gamma band synchronization. Cerebral Cortex (New York, N.Y.: 1991) 2009;19:1896–1904. doi: 10.1093/cercor/bhn216. 19047574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel R., Gelbard H., Arieli A., Hasson U., Fried I., Malach R. Coupling between neuronal firing, field potentials, and fMRI in human auditory cortex. Science (New York, N.Y.) 2005;309:951–954. doi: 10.1126/science.1110913. 16081741 [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D. High-frequency brain activity and muscle artifacts in MEG/EEG: A review and recommendations. Frontiers in Human Neuroscience. 2013;7:138. doi: 10.3389/fnhum.2013.00138. 23596409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y., Fisch L., Mukamel R., Gelbard-Sagiv H., Arieli A., Fried I., Malach R. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Current Biology: CB. 2007;17:1275–1285. doi: 10.1016/j.cub.2007.06.066. 17686438 [DOI] [PubMed] [Google Scholar]
- Pagani M., Nardo D., Högberg G., Jacobsson H., Larsson S.A. FC01-05 - Self-rating scales assessing subjective well-being and distress correlate with CBF in PTSD-sensitive regions. European Psychiatry. 2010;25:179. doi: 10.1017/S0033291711000912. [DOI] [PubMed] [Google Scholar]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W. Biological studies of post-traumatic stress disorder. Nature Reviews. Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339. 23047775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraan M.A., Cheyne D. Reconstruction of correlated brain activity with adaptive spatial filters in MEG. Neuroimage. 2010;49:2387–2400. doi: 10.1016/j.neuroimage.2009.10.012. 19850135 [DOI] [PubMed] [Google Scholar]
- Rabinak C.A., Angstadt M., Welsh R.C., Kenndy A.E. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Frontiers in Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. 22102841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson L.K., Frueh B.C., Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Australian and New Zealand Journal of Psychiatry. 2010;44(1):4–19. doi: 10.3109/00048670903393597. 20073563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. 19819337 [DOI] [PubMed] [Google Scholar]
- Shin L.M., Orr S.P., Carson M.A., Rauch S.L., Macklin M.L., Lasko N.B. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. 14757593 [DOI] [PubMed] [Google Scholar]
- Shin L.M., Bush G., Milad M.R., Lasko N.B., Brohawn K.H., Hughes K.C. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. American Journal of Psychiatry. 2011;168(9):979–985. doi: 10.1176/appi.ajp.2011.09121812. 21724666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., Garfinkel S.N., Liberzon I. Avoidant symptoms in PTSD predict fear circuit activation during multimodal fear extinction. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E., von Wegner F., Morzelewski A., Brodbeck V., Laufs H. Dynamic BOLD functional connectivity in humans and its electrophysiological correlates. Frontiers in Human Neuroscience. 2012;6:339. doi: 10.3389/fnhum.2012.00339. 23293596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewarie P., Schoonheim M.M., Stam C.J., van der Meer M.L., van Dijk B.W., Barkhof F. Cognitive and clinical dysfunction, altered MEG resting-state networks and thalamic atrophy in multiple sclerosis. PloS One. 2013;8(7):e69318. doi: 10.1371/journal.pone.0069318. 23935983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. 11771995 [DOI] [PubMed] [Google Scholar]
- Wang L., Saalmann Y.B., Pinsk M.A., Arcaro M.J., Kastner S. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron. 2012;76:1010–1020. doi: 10.1016/j.neuron.2012.09.033. 23217748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiological Reviews. 2010;90(3):1195–1268. doi: 10.1152/physrev.00035.2008. 20664082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M., Oostenveld R., van Wingerden M., Battaglia F., Pennartz C.M. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage. 2011;55(4):1548–1565. doi: 10.1016/j.neuroimage.2011.01.055. 21276857 [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PloS One. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. 23861951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Jin C., Hu X., Duan L., Li Z., Song M., Chen H., Feng B., Jiang T., Jin H., Wong C., Gong Q., Li L. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: a functional magnetic resonance imaging studyBrain Research. 2011;1411:98–107. doi: 10.1016/j.brainres.2011.07.016. 21813114 [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. 20600983 [DOI] [PubMed] [Google Scholar]


