Abstract
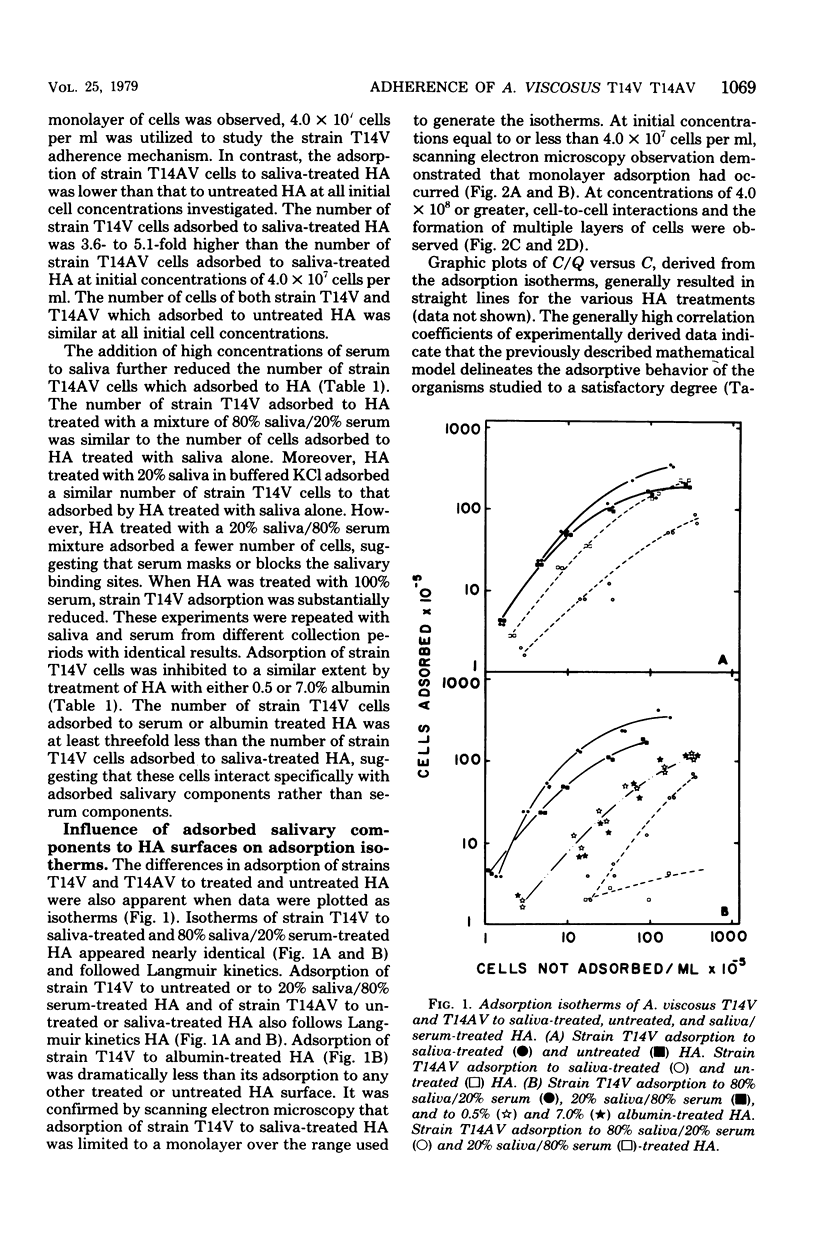
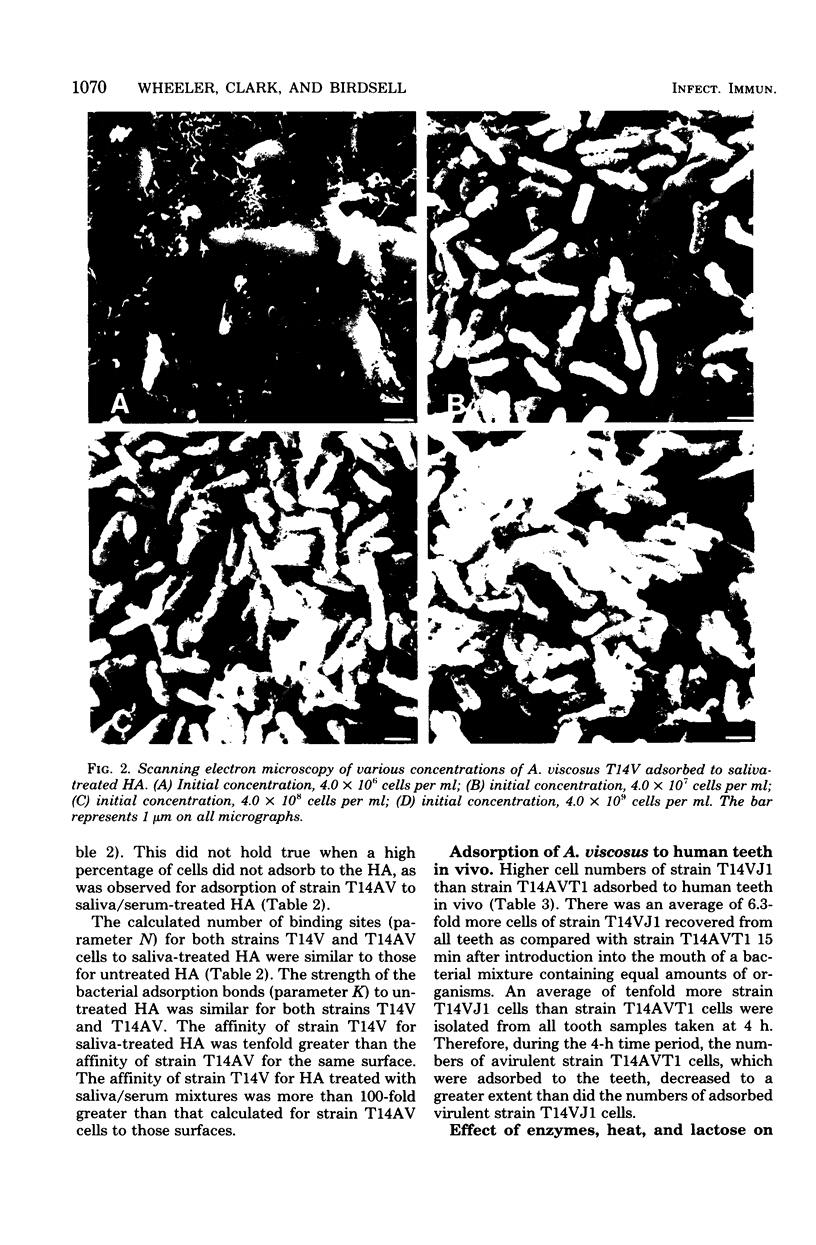
Adsorption of Actinomyces viscosus strains T14V and T14AV to hydroxyapatite (HA) surfaces was studied, using an adsorption model based on the Langmuir adsorption isotherm. Data generally followed the adsorption model as judged by high correlation coefficients obtained for both strains to most of the treated surfaces studied. The number of binding sites for strains T14V and T14AV cells to human saliva-treated HA was similar to that for untreated HA. The affinity of strain T14V for saliva-treated HA was tenfold greater than the affinity of strain T14AV for that surface. To approximate the pellicle of the gingival crevice and margin and to determine whether adherence by strain T14V was to specific saliva or serum receptors, experimental pellicles were formed on HA by saliva/serum mixtures. The number of binding sites on the saliva/serum-treated HA remained the same as for the saliva-treated surface. Although the affinity of strain T14V cells for the saliva/serum HA surface remained generally the same as the affinity for the HA treated with saliva alone, the affinity of strain T14AV cells decreased further as the serum content increased. Strain T14V cell numbers adsorbed to serum-treated HA, and albumin-treated HA were less than those adsorbed to saliva-treated HA, indicating that the adherence by strain T14V was to specific saliva receptors. In vivo results from streptomycin-resistant mutants of both strains T14V and T14AV confirmed in vitro results using saliva-serum pellicles. Pretreatment of strain T14V with proteolytic enzymes and heat inhibited adherence to saliva-treated HA, suggesting that the adherence receptor(s) on the cell surface of strain T14V is protein in nature.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brecher S. M., van Houte J., Hammond B. F. Role of colonization in the virulence of Actinomyces viscosus strains T14-Vi and T14-Av. Infect Immun. 1978 Nov;22(2):603–614. doi: 10.1128/iai.22.2.603-614.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho Martínez F., Armijo Moreno M., Naranjo Sintes R., Serrano Ortega S., de Dulanto F. Hipodermitis nodular subaguda migratriz. Actas Dermosifiliogr. 1977 May-Jun;68(5-6):283–292. [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., McIntire F. C. Identification of the virulence-associated antigen on the surface fibrils of Actinomyces viscosus T14. Infect Immun. 1978 Jan;19(1):312–319. doi: 10.1128/iai.19.1.312-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Walker D. L., Chan K. H. Association of long surface appendages with adherence-related functions of the gram-positive species Actinomyces naeslundii. J Bacteriol. 1978 Jun;134(3):1171–1175. doi: 10.1128/jb.134.3.1171-1175.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson T., Magnusson I. Affinity for hydroxyapatite of salivary substances inducing aggregation of oral streptococci. Caries Res. 1976;10(1):8–18. doi: 10.1159/000260185. [DOI] [PubMed] [Google Scholar]
- Ericson T. Salivary glycoproteins. Composition and adsorption to hydroxylapatite in relation to the formation of dental pellicles and calculus. Acta Odontol Scand. 1968 May;26(1):3–21. doi: 10.3109/00016356809004577. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Hammond B. F., Steel C. F., Peindl K. S. Antigens and surface components associated with virulence of Actinomyces viscosus. J Dent Res. 1976 Jan;55:A19–A25. doi: 10.1177/002203457605500111011. [DOI] [PubMed] [Google Scholar]
- Hay D. I. The adsorption of salivary proteins by hydroxyapatite and enamel. Arch Oral Biol. 1967 Aug;12(8):937–946. doi: 10.1016/0003-9969(67)90088-x. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., KEYES P. H. AEROBIC, GRAM-POSITIVE, FILAMENTOUS BACTERIA AS ETIOLOGIC AGENTS OF EXPERIMENTAL PERIODONTAL DISEASE IN HAMSTERS. Arch Oral Biol. 1964 Jul-Aug;9:401–414. doi: 10.1016/0003-9969(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Fitzgerald R. J., Stanley H. R. Plaque formation and periodontal pathology in gnotobiotic rats infected with an oral actinomycete. Am J Pathol. 1965 Dec;47(6):1157–1167. [PMC free article] [PubMed] [Google Scholar]
- Jordan H. V., Hammond B. F. Filamentous bacteria isolated from human root surface caries. Arch Oral Biol. 1972 Sep;17(9):1333–1342. doi: 10.1016/0003-9969(72)90166-5. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C., PERLMANN G. E. Preparation and properties of type-specific M antigen isolated from a group A, type 1 hemolytic streptococcus. J Exp Med. 1952 Jul;96(1):71–82. doi: 10.1084/jem.96.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A. Bacteriology of human experimental gingivitis: effect of plaque and gingivitis score. Infect Immun. 1978 Sep;21(3):830–839. doi: 10.1128/iai.21.3.830-839.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhall C. W. Amino acid composition of experimental salivary pellicles. J Periodontol. 1977 Feb;48(2):78–91. doi: 10.1902/jop.1977.48.2.78. [DOI] [PubMed] [Google Scholar]
- Mayhall C. W., Butler W. T. The carbohydrate composition of experimental salivary pellicles. J Oral Pathol. 1976 Nov;5(6):358–370. doi: 10.1111/j.1600-0714.1976.tb01787.x. [DOI] [PubMed] [Google Scholar]
- Mayhall C. W. Concerning the composition and source of the acquired enamel pellicle of human teeth. Arch Oral Biol. 1970 Dec;15(12):1327–1341. doi: 10.1016/0003-9969(70)90021-x. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. H., Palenik C. J., Stamper K. E. Factors affecting the aggregation of Actinomyces naeslundii during growth and in washed cell suspensions. Infect Immun. 1978 Sep;21(3):1003–1009. doi: 10.1128/iai.21.3.1003-1009.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSSERMAN E. F. A modified technique of immunoelectrophoresis facilitating the identification of specific precipitin arcs. J Immunol. 1960 Jan;84:93–97. [PubMed] [Google Scholar]
- Olsson J., Krasse B. A method for studying adherence of oral streptococci to solid surfaces. Scand J Dent Res. 1976 Jan;84(1):20–28. doi: 10.1111/j.1600-0722.1976.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Fischlschweiger W., Birdsell D. C. Modification of surface composition of Actinomyces viscosus T14V and T14AV. Infect Immun. 1978 Dec;22(3):934–944. doi: 10.1128/iai.22.3.934-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978 Sep;21(3):821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönju T., Rölla G. Chemical analysis of the acquired pellicle formed in two hours on cleaned human teeth in vivo. Rate of formation and amino acid analysis. Caries Res. 1973;7(1):30–38. doi: 10.1159/000259822. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Green D. B. Relationship between the concentration of bacteria in saliva and the colonization of teeth in humans. Infect Immun. 1974 Apr;9(4):624–630. doi: 10.1128/iai.9.4.624-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



