Abstract
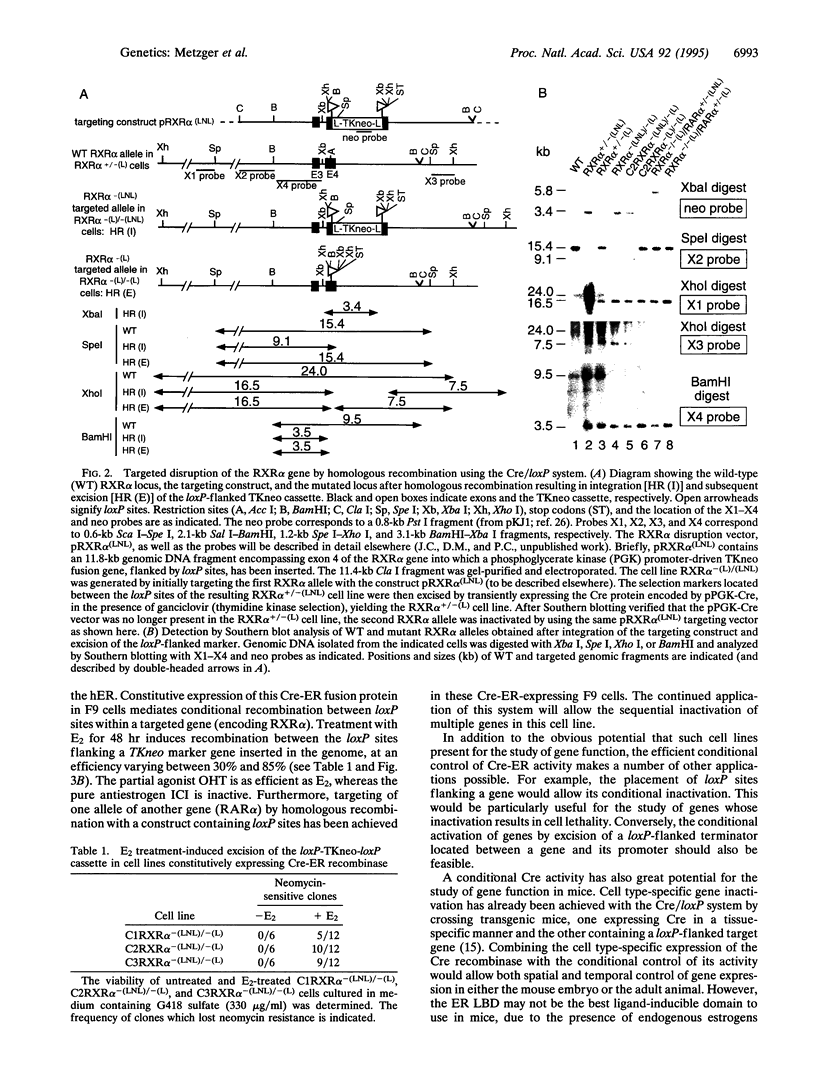
We have developed a strategy to generate mutant genes in mammalian cells in a conditional manner by employing a fusion protein, Cre-ER, consisting of the loxP site-specific Cre recombinase linked to the ligand-binding domain of the human estrogen receptor. We have established homozygous retinoid X receptor alpha-negative (RXR alpha-/-) F9 embryonal carcinoma cells constitutively expressing Cre-ER and have shown that estradiol or the estrogen agonist/antagonist 4-hydroxytamoxifen efficiently induced the recombinase activity, whereas no activity was detected in the absence of ligand or in the presence of the antiestrogen ICI 164,384. Furthermore, using a targeting vector containing a selection marker flanked by loxP sites, we have inactivated one retinoic acid receptor alpha allele in such a line, demonstrating that the presence of the recombinase does not inhibit homologous recombination. Combining this conditional site-specific recombination system with tissue-specific expression of Cre-ER may allow modification of the mammalian genome in vivo in a spatiotemporally regulated manner.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adra C. N., Boer P. H., McBurney M. W. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60(1):65–74. doi: 10.1016/0378-1119(87)90214-9. [DOI] [PubMed] [Google Scholar]
- Ali S., Lutz Y., Bellocq J. P., Chenard-Neu M. P., Rouyer N., Metzger D. Production and characterization of monoclonal antibodies recognising defined regions of the human oestrogen receptor. Hybridoma. 1993 Aug;12(4):391–405. doi: 10.1089/hyb.1993.12.391. [DOI] [PubMed] [Google Scholar]
- Baubonis W., Sauer B. Genomic targeting with purified Cre recombinase. Nucleic Acids Res. 1993 May 11;21(9):2025–2029. doi: 10.1093/nar/21.9.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Transcriptional control by nuclear receptors. FASEB J. 1991 Apr;5(7):2044–2051. doi: 10.1096/fasebj.5.7.2010057. [DOI] [PubMed] [Google Scholar]
- Boylan J. F., Lohnes D., Taneja R., Chambon P., Gudas L. J. Loss of retinoic acid receptor gamma function in F9 cells by gene disruption results in aberrant Hoxa-1 expression and differentiation upon retinoic acid treatment. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9601–9605. doi: 10.1073/pnas.90.20.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan J. F., Lufkin T., Achkar C. C., Taneja R., Chambon P., Gudas L. J. Targeted disruption of retinoic acid receptor alpha (RAR alpha) and RAR gamma results in receptor-specific alterations in retinoic acid-mediated differentiation and retinoic acid metabolism. Mol Cell Biol. 1995 Feb;15(2):843–851. doi: 10.1128/mcb.15.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson S. K., Smithies O. Altering mice by homologous recombination using embryonic stem cells. J Biol Chem. 1994 Nov 4;269(44):27155–27158. [PubMed] [Google Scholar]
- Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994 Apr;5(2):115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Marth J. D., Orban P. C., Mossmann H., Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994 Jul 1;265(5168):103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Krust A., Green S., Argos P., Kumar V., Walter P., Bornert J. M., Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986 May;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Green S., Staub A., Chambon P. Localisation of the oestradiol-binding and putative DNA-binding domains of the human oestrogen receptor. EMBO J. 1986 Sep;5(9):2231–2236. doi: 10.1002/j.1460-2075.1986.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufkin T., Dierich A., LeMeur M., Mark M., Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991 Sep 20;66(6):1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Lohnes D., Mark M., Dierich A., Gorry P., Gaub M. P., LeMeur M., Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D., Berry M., Ali S., Chambon P. Effect of antagonists on DNA binding properties of the human estrogen receptor in vitro and in vivo. Mol Endocrinol. 1995 May;9(5):579–591. doi: 10.1210/mend.9.5.7565805. [DOI] [PubMed] [Google Scholar]
- Picard D. Regulation of protein function through expression of chimaeric proteins. Curr Opin Biotechnol. 1994 Oct;5(5):511–515. doi: 10.1016/0958-1669(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Sauer B., Henderson N. Targeted insertion of exogenous DNA into the eukaryotic genome by the Cre recombinase. New Biol. 1990 May;2(5):441–449. [PubMed] [Google Scholar]
- Schwartz F., Maeda N., Smithies O., Hickey R., Edelmann W., Skoultchi A., Kucherlapati R. A dominant positive and negative selectable gene for use in mammalian cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10416–10420. doi: 10.1073/pnas.88.23.10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Sauer B., Hoess R., Abremski K. Bacteriophage P1 cre gene and its regulatory region. Evidence for multiple promoters and for regulation by DNA methylation. J Mol Biol. 1986 Jan 20;187(2):197–212. doi: 10.1016/0022-2836(86)90228-7. [DOI] [PubMed] [Google Scholar]
- Strickland S. Mouse teratocarcinoma cells: prospects for the study of embryogenesis and neoplasia. Cell. 1981 May;24(2):277–278. doi: 10.1016/0092-8674(81)90313-5. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tora L., Mullick A., Metzger D., Ponglikitmongkol M., Park I., Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J. 1989 Jul;8(7):1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H., Maandag E. R., Clarke A., Hooper M., Berns A. Consecutive inactivation of both alleles of the pim-1 proto-oncogene by homologous recombination in embryonic stem cells. Nature. 1990 Dec 13;348(6302):649–651. doi: 10.1038/348649a0. [DOI] [PubMed] [Google Scholar]