Abstract
Objective
In this study, we aimed to expand on our prior research into the relative efficacy of combining parent training, stimulant medication and placebo (Basic) versus parent training, stimulant, and risperidone (Augmented) therapy by examining treatment effects for attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), and conduct disorder (CD) symptoms and peer aggression, symptom-induced—impairment, and informant discrepancy.
Method
Children (6-12 years; N=168) with severe physical aggression, ADHD, and co-occurring ODD/CD received an open trial of parent training and stimulant medication for 3 weeks. Participants failing to show optimal clinical response were randomly assigned to Basic or Augmented therapy for an additional 6 weeks.
Results
Compared with Basic therapy, children receiving Augmented therapy experienced greater reduction in parent-rated ODD severity (p=.02, Cohen's d=0.27) and peer aggression (p=.02, Cohen's d=0.32), but not ADHD or CD symptoms. Fewer children receiving Augmented (16%) than Basic (40%) therapy were rated by their parents as impaired by ODD symptoms at Week 9/endpoint (p=.008). Teacher ratings indicated greater reduction in ADHD severity (p=.02, Cohen's d =0.61) with Augmented therapy, but not for ODD or CD symptoms or peer aggression. Although both interventions were associated with marked symptom reduction, a relatively large percentage of children were rated impaired for at least one targeted disorder at Week 9/endpoint by parents (Basic 47%; Augmented 27%) and teachers (Basic 48%; Augmented 38%).
Conclusion
Augmented was superior to Basic therapy in reducing severity of ADHD and ODD symptoms, peer aggression, and symptom-induced impairment, but clinical improvement was generally context-specific, and effect sizes ranged from small to moderate.
Keywords: ADHD, risperidone, stimulant drug, aggression, multiple drug therapy
Introduction
The use of multiple medications for the clinical management of the same psychiatric disorder or target symptom is generally discouraged in best practices guidelines, particularly drug combinations that involve atypical antipsychotics, owing to concerns about weight gain and metabolic syndrome. Conversely, many clinicians would argue that dual-drug therapy is justifiable in the case of medications with different mechanisms of action or for children with co-morbid conditions that have different pathologies and recommended therapies or who have not responded optimally to conventional mono-drug therapies. Unfortunately, psychiatric symptoms are rarely pathognomonic; boundaries between disorders are often ambiguous; risk factors are generally nonspecific; and etiologies are poorly understood. In the absence of controlled trials demonstrating that multiple drug therapy for the same disorder is harmful, the practice has become commonplace, despite a lack of evidence supporting efficacy for most applications.1-3 A case in point is children with attention-deficit/hyperactivity disorder (ADHD), many of whom have co-occurring oppositional defiant disorder (ODD) or conduct disorder (CD), and in some cases, exhibit clinically severe physical aggression. Not only is this combination of symptoms seriously disabling for the child, but it can have significant consequences for family, school, and community. The clinical management of such patients poses many challenges for clinicians, not the least of which are selecting an appropriate intervention plan and dealing with a host of psychosocial issues.
Stimulant medications are a well-established and highly effective treatment for ADHD symptoms, and there is considerable evidence they suppress ODD symptoms in children with ADHD andODD.4,5 Moreover, direct observation studies conducted in public school settings indicate stimulants are highly effective in suppressing a wide range of aggressive behaviors including physical aggression in children with ADHD.6,7 Parent-training in child behavior management skills is also effective for controlling these same symptoms and behaviors,8 and when used in combination with stimulants, results in even greater therapeutic improvement.9,10 Nevertheless, the extraordinary heterogeneity in the ADHD clinical phenotype is widely appreciated, and there is considerable controversy as to whether every child with ADHD who exhibits serious physical aggression can be adequately managed with these treatments either alone or in combination. One common practitioner strategy for child patients who show some benefit with stimulant medication is to add a second drug, such as risperidone.1 Although research indicates that risperidone is effective for some ADHD symptoms (hyperactivity) in child patients with autism spectrum disorders (ASD)11 and suppresses a wide range of oppositional/aggressive behaviors in children with disruptive behaviors and below-average IQ,12 there is very little research into the efficacy of dual-drug therapy or its effect on ADHD, ODD, or CD symptoms among youth without ASD or subaverage IQ. One notable exception is a study of clonidine added to stimulant medication for children with ADHD and co-occurring ODD or CD and elevated T scores (≥70) on the Child Behavior Checklist (CBCL)13 Aggression subscale.14 A larger percentage of children receiving stimulant and clonidine than stimulant and placebo were treatment responders as assessed with the Conduct subscale of the parent-completed Conners Behavior Checklist15.
To address these concerns, we conducted a multi-site, 9-week clinical trial (Treatment of Severe Childhood Aggression, TOSCA) that compared the relative efficacy of parent-training in child behavior management techniques and stimulant medication and placebo (hereafter referred to as Basic therapy) versus parent training and stimulant medication and risperidone (Augmented therapy). In addition to engaging in serious physical aggression, each child met diagnostic criteria for ADHD with co-occurring ODD/CD. The initial publication describing results for the primary hypotheses16 reported that Augmented treatment was superior to Basic treatment in reducing the severity of disruptive behaviors (Cohen's d=.43) as measured by the primary outcome measure, the parent-completed Nisonger Child Behavior Rating Form Disruptive total (NCBRF D-total).17 Moreover, there was little evidence of increased risk of adverse events (AEs) at the end of the acute treatment phase for children receiving Augmented treatment. The present article expands on our previous report by addressing several new topics including (a) drug effects for DSM-IV-defined ADHD, ODD and CD symptoms and (b) interpersonal peer aggression; (c) caregiver reports of symptom-induced impairment; (d) teachers' ratings of treatment effects to include school functioning; and (e) informant discrepancy (i.e., differences in parents' versus teachers' ratings of drug response). In addressing these issues, we sought to determine if Augmented therapy was more effective for the treatment of the aforementioned disorders and reduction in disorder-specific impairment. In addition, we examined whether symptomatic improvement observed in the home was evident in the school setting. As academic functioning is a major concern in children with ADHD, we also evaluated teachers' perceptions of drug effects on global ratings of test/quiz performance, homework, and classroom participation.
Method
Participants
Participants were 168 children between 6 and 12 years of age (mean=8.9±2.0) recruited at 4 different sites (Columbus, Cleveland, Pittsburgh, Stony Brook). Descriptively, participants were primarily boys (77%) of average IQ (mean=97.1±14.1) and white/Caucasian/European geographic ancestry (53%) and living with working parents (mothers = 52%, fathers = 53%) who had at least some college education (mothers = 66%; fathers = 35%), and relatively low family incomes of ≤ $40,000 per year (57%; see Table S1, available online).
Inclusion criteria were evidence of serious physical aggression as defined by parent-report to a blinded clinician of Level 3 or greater Overt Aggression Scale–M (OAS-M)18 rating of assault against objects (broke several things in anger), others (assault resulting in serious physical injury to another), or self (cut, bruised, burned self but only superficially; Table 1) and severe disruptive behavior (≥ 90th percentile NCBRF D-Total); DSM-IV criteria for any subtype of ADHD plus ODD (n=124) or ODD and CD (n=44); and a rating of at least moderately ill by a blinded clinician (severity score ≥4 Clinical Global Impression [CGI]).19 Exclusion criteria included full-scale IQ<70; pregnancy; medical consideration (seizures, abnormal liver function, first degree family history of type 2 diabetes); lifetime history of pervasive developmental disorder (PDD), psychotic disorder, eating disorders, or substance abuse disorder; current major depressive disorder (MDD) or bipolar disorder (BD); attempted suicide; or evidence of child abuse. Participants needed to be free of psychotropic medicines for 2 or 4 weeks for short- and long-acting drugs, respectively. The study was approved by the institutional review board (IRB) of each investigative site and a multisite data safety and monitoring board (DSMB), parents/guardians signed consent forms, and study participants gave assent before enrollment.
Table 1. Number (%) of Children Who Received Level 3 Parental Ratings of Assaults Against Objects, Other People, and Self From the Overt Aggression Scale–M.
| Time | Objects | Others | Self |
|---|---|---|---|
| Screen | 130 (77%) | 155 (92%) | 14 (8%) |
| Baseline | 106 (63%) | 148 (88%) | 15 (9%) |
Procedure
At the completion of the baseline assessment, the primary caregiver started parent training, which continued throughout the 9-week intervention, and all children began an open trial of stimulant monotherapy, usually Osmotic Release Oral System (OROS) methylphenidate. If unable to tolerate medication or unable to swallow pills, an alternative stimulant was offered. During the first 3 weeks, the primary clinician adjusted stimulant to achieve an optimal therapeutic response defined as a CGI-Improvement score of 1 by a blinded clinician and a parent-rated NCBRF D-Total score <15 (within 0.5 SD of the normative mean). If participants did not show a sufficient clinical response at Week 3, or if they showed deterioration at Week 4 through Week 6 (i.e., dropped below a blinded CGI of 1 or had a NCBRF D-Total >15), the second agent (risperidone or placebo) was added to the treatment package. Randomization was determined at baseline (n=84 for each condition), stratified by site, and balanced by ODD and CD. We adopted the strategy of randomizing at baseline owing in part to National Institute of Mental Health (NIMH) review committee concerns about attrition.20 The second medication was adjusted to achieve an optimal therapeutic response, and all participant assessments were conducted by blinded evaluators without knowledge of treatment assignment or adverse events. More details regarding the background, methods, design, patient recruitment and retention, adverse events, responder criteria, and data analysis models are provided by Farmer et al.20 and Aman et al.16
The mean Week 9 dose of methylphenidate was 45±15 mg/day (Basic) and 46±17 mg/day (Augmented) (p=.88). For the second medication, mean doses were 1.9±0.7 mg/day (placebo) and 1.7+0.6 mg/day (risperidone) (p=.07). Stimulant was administered in the morning, and risperidone/stimulant was given morning and evening (supper or bedtime). To monitor adherence, staff conducted pill counts, and caregivers completed daily medication logs that were reviewed by staff on a weekly basis.
Fourteen children (Basic=3; Augmented=11) dropped out before they completed Week 3 and therefore did not receive the second medication. An additional 8 children were classified as clinical responders by the end of Week 3 (Basic=3; Augmented=5) and did not take the second medication.
For various reasons, including difficulties synchronizing the clinical trial with the child's school year, parent-school conflicts, and divergent levels of teacher involvement across sites, teachers' ratings were available for a subsample of children. Teachers' ADHD Symptom Checklist-4 (ADHD-SC4) ratings were available for 117 children; at least half of the teacher data were missing for 50 (43%) of these children. Children who did and did not have teacher ratings were not different in demographic characteristics, rate of ODD or CD, severity of NCBRF ratings, therapy group assignment, or parents' ratings of treatment effects.
Measures
Main outcomes
The ADHD-SC421 is a 40-item treatment response measure that includes DSM-IV-referenced scales for the symptoms of ADHD and ODD as well as the Peer Conflict Scale,21,22 a 10-item measure of interpersonal peer aggression based on the ADHD School Observation Code.23 Individual items are rated on a scale from 0 (never) to 3 (very often). The Symptom Severity score (dimensional) is the sum of all item scores for a given subscale. To facilitate comparison of results across scales, we report the mean item rating for all symptom severity scores. Numerous studies show the ADHD-SC4 has satisfactory psychometric properties and is a sensitive indicator of drug effects in children with ADHD.21,24 The ADHD-SC4 was completed by parents and teachers at baseline and weekly throughout the 9-week trial.
The Child and Adolescent Symptom Inventory-4R (CASI-4R)25 assesses a broad range of DSM-IV-defined disorders and was administered at initial diagnostic evaluation (parents only), baseline, and Week 9. Parent and teacher versions contain 169 and 120 symptom items, respectively, and both include the ADHD-SC4 symptom categories as well as CD. Symptom Severity scores are calculated similarly to the ADHD-SC4, but the CASI-4R has additional scoring algorithms including the Symptom Count Cutoff (categorical), which indicates whether or not the child meets DSM-IV criteria for the prerequisite number of symptoms. Ratings of “often/very often” and “never/sometimes” indicate the presence or absence of a symptom, respectively. The last item in each CASI-4R symptom scale assesses impairment: “How often do the behaviors in [this symptom category] interfere with youth's ability to do schoolwork or get along with others?” For the ADHD subscale, there is one impairment item for all subtypes. Impairment severity is rated on a 4-point scale, and the Impairment Cutoff score (categorical) is a frequency rating of “often” or “very often.” The Clinical Cutoff score (categorical) indicates whether or not a child meets both Symptom Count Cutoff and Impairment Cutoff for a specific disorder. The teacher version of the CASI-4R has a global classroom functioning scale comprising 3 items (tests/quizzes, homework, class participation) each rated from poor (0) to superior (4). Numerous studies indicate that CASI-4R scales demonstrate satisfactory psychometric properties.26
Subsidiary outcomes
Items in the ADHD-SC4 ADHD, ODD, and Peer Conflict scales can be further subdivided into component symptom dimensions. For example, the ADHD scale is composed of three symptoms subscales: inattention (9 items), hyperactivity (5 items), and impulsivity (4 items). Prior research27,28 indicates the ODD scale consists of at least 2 symptom dimensions, anger-irritability (3 items) and noncompliance (4 items). The anger/irritability dimension is very similar to DSM-5 (APA, 2013) disruptive mood dysregulation disorder (DMDD). The Peer Conflict scale generates three indices of aggression: physical (e.g., hits, trips, pushes), nonphysical (threatening gestures, verbal threats), and object (destroys things).
Statistical Analyses
A constrained longitudinal data analysis (cLDA) model,29 in which both baseline and post-baseline values for parent-rated mean ADHD-SC4 symptom severity scores are treated as dependent variables, was used in the intention to treat (ITT) population. This included all 168 randomized participants, and contained children randomized to Augmented therapy but who did not receive it or who dropped out before end of Week 3 (n=16). Fixed effects included those for time, group, time-by-group interaction, site, and disorder type. An unstructured variance covariance matrix was assumed for the repeated measures within each subject. Empirical-based sandwich estimators were obtained to assess the group differences at Week 9 (endpoint) given their robustness against the deviations from model assumptions.30 For the CASI-4R CD subscale, baseline and Week 9 (endpoint) measures with last observation after Week 3 carried forward (LOCF) were analyzed by a cLDA model with fixed effects for time, group, site, and disorder. Owing to concerns about missing data, teachers' ADHD-SC4 ratings were modeled similarly to parents' ratings with the exception that teachers' ratings were analyzed for changes in behavior from baseline to Week 9 (endpoint) with last observation after Week 3 carried forward. There was no treatment group bias in missing teacher data. In these exploratory analyses, a straightforward correction for multiple comparisons would risk excessive Type 2 error; nevertheless, given the possibility of Type 1 error, we adopted a more conservative p value of 0.025 for the 4, primary within-informant outcomes. Analyses of subsidiary outcomes were conducted for the expressed purpose of isolating more homogeneous traits for consideration in future research. Effect sizes (Cohen's d) were calculated comparing the 2 treatment arms by using a 2-sample t-test of change score at Week 9 (endpoint) (i.e., change from baseline to Week 9 for participants with complete LOCF data). Model assumptions were assessed by examination of residuals. Some variables were square root transformed to accommodate the assumption of normality. Fisher's exact test (comparison of treatment groups) and McNemar's test (different informant, same children) were used to calculate significant group differences in categorical variables (cutoff scores). All analyses were conducted in SAS (version 9.2, SAS Institute Inc., Cary, NC).
Results
Primary Outcomes (Dimensional)
According to parents' ADHD-SC4 symptom severity ratings, Augmented therapy was superior to Basic therapy in reducing ODD but not ADHD symptom severity (Table 2). Teachers' ratings indicated Augmented therapy was more effective for reducing ADHD but not ODD symptom severity. Augmented therapy was also associated with a significantly greater reduction in the severity of parent- but not teacher-rated peer aggression at Week 9. For both informants, group differences in CASI-4R CD severity ratings were not significant.
Table 2. Mean (SD) Attention-Deficit/Hyperactivity Disorder-Symptom Checklist (ADHD-SC4) Item Severity Scores for Parent Training, Stimulant, and Placebo (Basic) Versus Parent Training, Stimulant, and Risperidone (Augmented).
| Symptoms | Baseline | Week 3 | Week 9 | p=a | p=b | Estimate (CI)b | ESc | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n | M (SD) | n | M (SD) | n | M (SD) | |||||
| PARENT RATINGS | ||||||||||
| ADHD | .051 | .129 | 0.15 (-0.04, 0.34) | .13 | ||||||
| Basic | 84 | 2.4 (0.5) | 82 | 1.3 (0.8) | 71 | 1.0 (0.7) | ||||
| Augmented | 84 | 2.3 (0.6) | 75 | 1.3 (0.7) | 66 | 0.8 (0.5) | ||||
| ODD | .002 | .014 | 0.27 (0.06, 0.49) | .27 | ||||||
| Basic | 84 | 2.4 (0.5) | 82 | 1.4 (0.9) | 71 | 1.1 (0.8) | ||||
| Augmented | 84 | 2.3 (0.6) | 75 | 1.5 (0.8) | 66 | 0.8 (0.6) | ||||
| CDd | NA | .145* | 0.06 (-0.02, 0.13) | .09 | ||||||
| Basic | 84 | 0.6 (0.4) | NA | NA | 77 | 0.2 (0.2) | ||||
| Augmented | 84 | 0.5 (0.4) | 73 | 0.1 (0.2) | ||||||
| Peer Conflict Scale | .175 | .022* | 0.14 (0.02, 0.26) | .32 | ||||||
| Basic | 84 | 1.5 (0.9) | 82 | 0.8 (0.8) | 71 | 0.6 (0.7) | ||||
| Augmented | 83 | 1.5 (0.9) | 75 | 0.8 (0.8) | 66 | 0.3 (0.4) | ||||
| TEACHER RATINGS | ||||||||||
| ADHD | NA | .021 | 0.35 (0.05, 0.65) | .61 | ||||||
| Basic | 46 | 1.6 (0.6) | 39 | 0.8 (0.7) | 48 | 0.8 (0.6) | ||||
| Augmented | 40 | 1.8 (0.8) | 34 | 0.9 (0.7) | 38 | 0.6 (0.5) | ||||
| ODD | NA | .120* | 0.17 (-0.04, 0.38) | .34 | ||||||
| Basic | 46 | 1.2 (1.0) | 38 | 0.4 (0.6) | 48 | 0.4 (0.6) | ||||
| Augmented | 40 | 1.5 (1.1) | 34 | 0.6 (0.8) | 38 | 0.4 (0.6) | ||||
| CDd | NA | .087* | 0.14 (-0.02, 0.31) | .14 | ||||||
| Basic | 45 | 0.4 (0.4) | NA | NA | 39 | 0.1 (0.2) | ||||
| Augmented | 37 | 0.6 (0.6) | 30 | 0.1 (0.3) | ||||||
| Peer Conflict Scale | NA | .163* | 0.15 (-0.10, 0.40) | .29 | ||||||
| Basic | 46 | 0.7 (0.8) | 38 | 0.2 (0.5) | 48 | 0.2 (0.3) | ||||
| Augmented | 40 | 0.9 (0.9) | 34 | 0.3 (0.6) | 38 | 0.2 (0.4) | ||||
NOTE: CD = conduct disorder; ES=effect size (Cohen's d); ODD=oppositional defiant disorder, NA=not applicable.
P values for treatment multiplied by visit interaction.
P values and estimates (CI) represent results of mixed model for comparison of therapies at Week 9 (parents' ratings) or change from baseline to week 9 (teachers' ratings).
Effects size for baseline to Week 9 change.
From Child and Adolescent Symptom Inventory-4R.
p values, estimate (CI), and effect sizes represent results from square root transformation on specified CASI measures.
Subsidiary Outcomes (Dimensional)
Analyses of ADHD-SC4 ODD component symptom dimensions indicated significant treatment effects (Augmented>Basic) for parents' severity ratings of anger/irritability and noncompliant behavior but not for any of the 3 core symptom domains of ADHD (Table 3). For teachers' ADHD ratings, Augmented therapy was superior in reducing severity of impulsivity symptoms, and findings for inattention symptoms were marginally significant (p=.06; Cohen's d=0.38), but there were no significant differences for teacher ODD subscales.
Table 3. Mean (SD) Item Severity Scores for Attention-Deficit/Hyperactivity Disorder-Symptom Checklist (ADHD-SC4) ADHD and Oppositional Defiant Disorder (ODD) Symptom Subscales for Parent Training, Stimulant, and Placebo (Basic) Versus Parent Training, Stimulant, and Risperidone (Augmented).
| Disorder | Baseline | Week 3 | Week 9 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n | M (SD) | n | M (SD) | n | M (SD) | pa | pb | Estimate (CI)b | ESc | |
| PARENT RATINGS | ||||||||||
| ADHD | ||||||||||
| Attention | .034 | .156 | 0.15 (-0.06, 0.36) | 0.11 | ||||||
| Basic | 84 | 2.5 (0.6) | 82 | 1.4 (0.8) | 71 | 1.1 (0.7) | ||||
| Augmented | 84 | 2.4 (0.6) | 75 | 1.4 (0.8) | 66 | 0.9 (0.6) | ||||
| Hyperactivity | .329 | .237 | 0.13 (-0.08, 0.33) | 0.15 | ||||||
| Basic | 84 | 2.3 (0.8) | 82 | 1.2 (0.9) | 71 | 0.8 (0.8) | ||||
| Augmented | 84 | 2.2 (0.8) | 75 | 1.2 (0.9) | 66 | 0.6 (0.6) | ||||
| Impulsivity | .475 | .243 | 0.14 (-0.10, 0.39) | 0.08 | ||||||
| Basic | 84 | 2.3 (0.7) | 82 | 1.4 (0.9) | 71 | 1.1 (0.9) | ||||
| Augmented | 84 | 2.1 (0.9) | 75 | 1.3 (0.8) | 66 | 0.8 (0.7) | ||||
| ODD | ||||||||||
| Anger/irritability | .001 | .026 | 0.27 (0.03, 0.50) | 0.19 | ||||||
| Basic | 84 | 2.3 (0.7) | 82 | 1.4 (0.9) | 71 | 1.1 (0.9) | ||||
| Augmented | 84 | 2.2 (0.7) | 75 | 1.4 (0.8) | 66 | 0.7 (0.6) | ||||
| Noncompliance | .005 | .015 | 0.28 (0.05, 0.50) | 0.30 | ||||||
| Basic | 84 | 2.4 (0.5) | 82 | 1.4 (0.9) | 71 | 1.1 (0.8) | ||||
| Augmented | 84 | 2.3 (0.6) | 75 | 1.5 (0.8) | 66 | 0.8 (0.6) | ||||
| TEACHER RATINGS | ||||||||||
| ADHD | ||||||||||
| Inattention | NA | .060 | 0.33 (-0.01, 0.68) | 0.38 | ||||||
| Basic | 46 | 1.8 (0.8) | 39 | 1.0 (0.8) | 48 | 1.0 (0.7) | ||||
| Augmented | 40 | 2.0 (0.8) | 34 | 1.1 (0.8) | 38 | 0.8 (0.6) | ||||
| Hyperactivity | NA | .218 | 0.24 (-0.15, 0.63) | 0.30 | ||||||
| Basic | 46 | 1.3 (0.8) | 38 | 0.5 (0.7) | 48 | 0.4 (0.5) | ||||
| Augmented | 40 | 1.5 (1.0) | 34 | 0.7 (0.8) | 38 | 0.5 (0.6) | ||||
| Impulsivity | NA | .011* | 0.29 (0.07, 0.51) | 0.75 | ||||||
| Basic | 46 | 1.4 (0.9) | 38 | 0.7 (0.9) | 48 | 0.7 (0.8) | ||||
| Augmented | 40 | 1.7 (1.0) | 34 | 0.9 (0.9) | 38 | 0.5 (0.6) | ||||
| ODD | NA | |||||||||
| Anger/irritability | .317 | 0.19 (-0.19, 0.57) | 0.17 | |||||||
| Basic | 46 | 1.2 (1.0) | 38 | 0.4 (0.7) | 48 | 0.5 (0.7) | ||||
| Augmented | 40 | 1.4 (1.2) | 34 | 0.6 (0.9) | 38 | 0.4 (0.7) | ||||
| Noncompliance | NA | .175 | 0.29 (-0.13, 0.70) | 0.23 | ||||||
| Basic | 46 | 1.2 (1.0) | 38 | 0.4 (0.6) | 48 | 0.4 (0.7) | ||||
| Augmented | 40 | 1.5 (1.2) | 34 | 0.6 (0.8) | 38 | 0.4 (0.6) | ||||
NOTE: ES=effect size (Cohen's d), NA=not applicable.
P values for treatment multiplied by visit interaction.
P values and estimates (CI) represent results of mixed model for comparison of therapies at Week 9 (parent's ratings) or change from baseline to week 9 (teacher's ratings).
Effects size for baseline to Week 9 change.
p values, estimate (CI), and effect sizes represent results from square root transformation on specified CASI measures.
According to parents' ratings, Augmented was superior to Basic therapy for improving physical aggression and object aggression, and nonphysical aggression was marginally significant (p=.053; Cohen's d=0.14, Figure 1). Teachers' ratings evidenced beneficial treatment effects for object aggression (p=0.03; Cohen's d=0.47) (Augmented > Basic).
Figure 1.
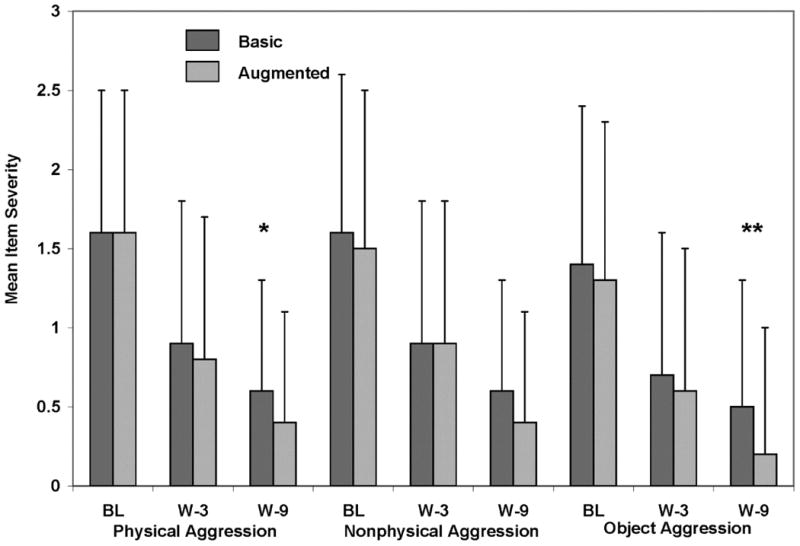
Mean (SD) peer conflict scale severity scores (parents' ratings) for parent training, stimulant and placebo (basic) versus parent training, stimulant, and risperidone (augmented). Note: BL = baseline; W-3 = Week 3; W-9 = Week 9. *p<.05 **p<.01
Symptom-Induced Impairment (Categorical)
Treatment groups were compared for the percentage of children who met CASI-4R Impairment Cutoff criteria at Week 9. Analyses were limited to children who met Impairment Cutoff at baseline, which is indicated in Figure 2. A larger percentage of children receiving Basic (47%) therapy met CASI-4R Impairment Cutoff criteria for at least one targeted disorder at Week 9 than Augmented (27%) therapy according to parents' ratings (ϕ=-.20). With regard to specific disorders, Basic therapy was associated with a higher rate of ODD symptom-induced impairment at Week 9 (Basic=40%, Augmented=16%, ϕ=-0.26). Teachers' ratings did not indicate treatment group differences for Impairment Cutoff, but ADHD was marginally significant (Basic=50%, Augmented= 20%, p=.09, ϕ=-0.31).
Figure 2.
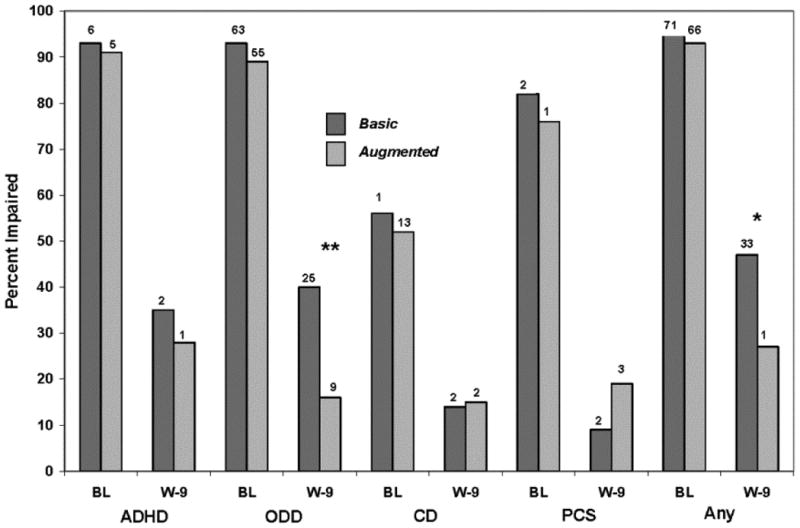
Treatment group differences in percentage of children who received parent Child and Adolescent Symptom Inventory-4R (CASI-4R) impairment cutoff scores for attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), conduct disorder (CD), Peer Conflict Scale (PCS), or any of these types of symptoms (any) at week 9 (endpoint). Note: Analyses limited to children rated impaired at baseline. Basic = receiving parent training, stimulant, and placebo; augmented = parent training, stimulant, and risperidone. BL = baseline; W-9 = Week 9. *p<.05 ** p<.01
Informant Discrepancy in Base Rates (Categorical)
To examine informant discrepancy in base rates, we calculated the percentage of all children who met CASI-4R Impairment Cutoff Criteria for targeted disorders at baseline. Parents (n=166) compared with teachers (n=82) rated a larger percentage of youth in the total sample as impaired by ODD symptoms and peer aggression at baseline (Figure 3). When we limited analyses to children for whom we had both parent and teacher report (n=82), parents' ratings indicated higher baseline Impairment Cutoff rates for ADHD (p=0.033), ODD (p=.0001), and any disorder (p=.05) than teachers' ratings.
Figure 3.
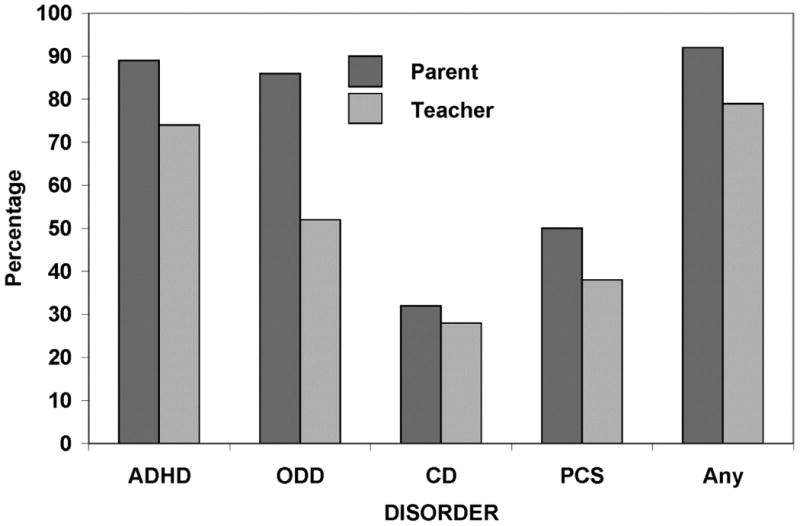
Percentage of all children who received parent (n=156) versus teacher (n=82) Child and Adolescent Symptom Inventory-4R (CASI-4R) impairment cutoff scores for attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), conduct disorder (CD), Peer Conflict Scale (PCS), or any of these types of symptoms (any) at baseline.
Outcome Criterion (Categorical)
The use of different CASI-4R categorical scoring algorithms to assess treatment effects resulted in different inferences about efficacy. For example, the superiority of Augmented over Basic therapy was significant for parent CASI-4R ODD Impairment Cutoff (Figure 2). However, group differences were not significant for Symptom Cutoff (p=.31, ϕ=-0.10), and only marginally significant for Clinical Cutoff (p=.06, ϕ=-0.18).
Classroom Functioning
CASI-4R teacher ratings of classroom functioning indicated marked improvement (higher mean item scores at Week 9 than baseline), but differences between treatments were marginally significant (F=3.33, p=.071, Cohen's d=0.45). Augmented therapy resulted in greater improvement than Basic therapy: baseline (Basic: M=1.5±0.7; Augmented M=1.3±0.7) and Week 9 (Basic: M=1.6±0.8; Augmented M=1.8±0.7).
Discussion
The TOSCA study involved a particularly challenging subgroup of children with ADHD who had co-occurring ODD/CD and clinically serious physical aggression. Participants were randomly assigned (at baseline) to receive Basic versus Augmented therapy if they did not show an optimal response to an initial 3-week open trial of parent training plus stimulant medication. In general, parent training plus stimulant medication was associated with marked reductions in ADHD and ODD symptoms and peer aggression at Week 3 with Augmented therapy, evidencing superior gains in therapeutic improvement by Week 9. Consistent with the extant literature, the magnitude of treatment effects varied as a function of type of symptom and informant (setting), but significant treatment group differences were generally in the low to moderate range.
According to teachers' (but not parents') ratings, children receiving Augmented therapy experienced greater therapeutic improvement in ADHD symptoms (Cohen's d=0.61), particularly impulsivity, which likely reflects heterogeneity in the pathophysiology of ADHD traits. As most children were rated both by parents and teachers as being impaired by ADHD symptoms at baseline, this result supports the efficacy of Augmented therapy for school-based (but not home-based) ADHD; however, as previously noted, findings for teachers' ratings are based on a subgroup of children. There is a voluminous literature indicating greater sensitivity of teachers' versus parents' ratings of drug response for the symptoms of ADHD, ODD, peer aggression, and even chronic tic disorder in children with ADHD and ADHD with ODD,4,5,31 and this was also the case in the present study with regard to ADHD symptoms.
According to parents' (but not teachers') ratings, Augmented therapy was associated with less-severe ODD symptoms at Week 9 compared with those receiving Basic therapy (Cohen's d=0.27). Clinical improvement was not limited to reductions in symptom severity. A smaller percentage of children who were impaired by ODD symptoms at baseline were impaired at Week 9 if they were receiving Augmented (16%) rather than Basic (40%) therapy. As all children in the study met Kiddie-SADS32 criteria for ODD,16 these findings suggest that Augmented therapy is an effective treatment for parent-defined ODD in children with co-occurring ADHD and severe aggression. Augmented therapy was superior in suppressing anger/irritability symptoms of ODD. It is noteworthy that CASI-4R ODD anger and irritability symptoms are very similar to the symptoms of DSM-5-defined DMDD,33 but a diagnosis of DMDD also requires persistent disruption in mood between outbursts and mild to moderate impairment in a second setting. Although TOSCA screening criteria excluded current (but not lifetime) MDD and BD, all other mood-related disorders (or symptoms), including chronic anger and irritability, were not excluded. Moreover, prior research indicates that anger/irritability is associated with anxiety and depression.27,28,34 Regardless, these results suggest that one or more components of Augmented therapy may be relevant for future research involving children with DMDD.
Many children with ADHD engage in atypically higher rates of peer aggression, an important characteristic of CD, and though less well-appreciated, a common clinical feature of ODD.27,28 In the present study, parents' ratings indicated that Augmented therapy was superior to Basic therapy in suppressing peer aggression (Cohen's d=.32), to include physical (Cohen's d=0.22) and object (Cohen's d=0.16) aggression, and ratings of nonphysical (verbal/symbolic) aggression were marginally significant (p=.053; Cohen's d=0.14). Treatment effects were cross-situational as teachers' ratings were significant for object aggression (Cohen's d=0.47). Collectively, these results are particularly noteworthy as the amelioration of aggressive behavior was a major objective of the TOSCA study. Others have also found divergent drug effects for different types of peer aggression,6,7 which supports the multidimensionality of aggression and heterogeneity in its pathogenic antecedents.
Informant Discrepancy
Several decades of research have shown that ratings of child psychopathology from different informants generally evidence only modest agreement. This is likely explained in part by differences in raters and their perspectives and the settings in which children are assessed, and the child's natural ability to adjust phenotypic traits to changing environmental features (behavioral plasticity), which appears to vary as a function child genotype.28,35-38 Our finding that parents' but not teachers' ratings of ODD symptoms differentiated treatment groups is likely explained by the use of parent report to confirm the ODD/CD inclusion criterion. For example, more children met CASI-4R impairment criteria for ODD at baseline according to parents' (86%) than teachers' (52%) ratings. In community-based and referred samples, rates of ODD are similar for both informants, but overlap is minimal.27,28,39 Therefore, it seems plausible that perceived differences in initial symptom severity may partially explain informant discrepancy in treatment effects, i.e., there was less “room for improvement” in teachers' ratings.
Informant discrepancy poses potential challenges for clinical management as well as nosology. Prior research,28 for example, indicates the associated clinical features of ODD vary depending on whether the child is identified as ODD by parent or teacher, but not both (source-exclusive), and CASI-4R impairment status may have interacted with or moderated clinical response in home versus school setting. Our findings for a group of children with largely source-exclusive ODD indicating significant improvement in parent- (but not teacher-) rated symptoms with Augmented therapy support this interpretation. Equally important, our results appear to strike at the heart of a common bias that youth with non-cross-situational pathology are more likely to have environmentally-induced problems, and therefore are poorer candidates for pharmacotherapy.28
This study has several strengths including the use of multiple sites, rigorous assessment battery, state-of-the-art data monitoring, and a sample large enough to detect even small-to-moderate treatment effects. Nevertheless, the generalization of results to everyday clinical practice is bounded by sample characteristics and methodology. As previously noted,16 this is not a “typical” sample of children with ADHD. They all had ODD and were considered to be very physically aggressive according to their parents, and their families were generally of below-average income. For example, according to parent OAS report, in the 30 days prior to evaluation, 97% of the children assaulted (or would have if not prevented) another person resulting in serious physical injury. Participants who dropped out or who were found to be clinical responders in Weeks 1-3 did not contribute to the treatment signal for those receiving Augmented Treatment. Nor did we control for parental psychopathology, which could have influenced perceptions of behavioral change. To this list we would add (a) the caregivers of all participants had access to parent training throughout the 9-week trial; (b) all treatments were provided free of charge; and (c) families were reimbursed for time and travel expenses and (d) were available for weekly clinic visits for 9 weeks. There were too few females to assess potential sex differences in response to treatment, and our findings may not generalize to less severely impaired children, preschoolers, teenagers, or children with intellectual disability.
Teacher ratings were available for only a subgroup of children, which has implications for statistical power. For example, although effect sizes for significant treatment group differences based on parents' ratings were often comparable to teachers' ratings, the latter were generally nonsignificant. When we limited our analyses of parent ratings to children who also had teachers' ratings, the former remained significant. Many children did not meet teacher-rated symptom or impairment criteria for ODD or peer aggression; therefore, our results do not speak to the efficacy of Augmented therapy for teacher-defined syndromes other than ADHD.
With regard to the broader issue of clinical necessity, we offer the following interpretive caveat. As we and others have noted,16,20,40 had TOSCA adopted a longer initial open trial of parent training plus stimulant medication or a different criterion for improvement, it is possible a smaller percentage of children would have received Augmented therapy. For example, most children receiving Basic therapy were able to complete the 9-week trial (i.e., did not deteriorate to a clinically unacceptable degree). Nevertheless, the Basic therapy group did receive regular dose increments of placebo during Weeks 4-8 and at a level consistent with the Augmented therapy group, suggesting less than optimal functioning. This is consistent with the high rate of perceived impairment in the Basic therapy group at endpoint. It also warrants repeating that the duration of Augmented therapy was a maximum of 6 weeks, and pending analyses of intermediate (3 month) and long-term (12 month) outcomes may lead to different conclusions about clinical utility.
Findings also address two treatment assessment strategies that may warrant consideration in future research as well as everyday clinical practice. Consistent with the results of others,41 a single-item caregiver rating of impairment was as effective in detecting differences between treatment groups as a multi-item symptom severity score. In addition, caregiver-impairment ratings were a more sensitive indicator of clinical improvement than CGIs completed by blinded clinicians.16 Rates of psychiatric conditions based on symptom cutoff (number of symptoms), impairment cutoff (child's ability to do schoolwork or get along with others), or clinical cutoff (symptom cutoff plus impairment cutoff) generally show modest agreement.38 As the results of this study indicate for parents' ratings of ODD, this also applies to conclusions about relative improvements from treatment, which underscores the limitations of relying on a single measure to determine efficacy in clinical trials and routine patient management.
TOSCA results suggest that augmentation with risperidone for pediatric patients with ADHD and co-occurring ODD/CD who are receiving an ongoing regimen of parent training and stimulant medication may result in added therapeutic benefit for some cases, with a seemingly modest increased risk in adverse events,16 at least during the short term. Importantly, as the distinguishing subject selection criterion in this study was clinically significant physical aggression, parents reported reduced physical aggression directed toward peers and objects with Augmented therapy. Teacher ratings indicated less severe ADHD symptoms with Augmented therapy. Though the breadth of clinical improvement was encouraging, effect sizes were generally small for the added benefits of Augmented therapy, probably due to the large improvement already attained by the Basic multimodal treatment. The fact that perceptions of relative efficacy were setting-specific highlights the importance of obtaining treatment response information from multiple informants. The clinical severity of the study sample is underscored by the fact at Week 9 almost half of the children receiving two evidence-based treatments (Basic) and over a fourth of those receiving three treatments (Augmented) continued to exhibit symptoms that their parents perceived as interfering with social or academic functioning. Thus these findings should not be generalized beyond severe cases of irritability and aggression with ADHD. Although they provide new evidence to inform clinical practice, their application still requires thoughtful personalization of the treatment plan for individual patients.
Supplementary Material
Table S1: Demographic Characteristics of Children Receiving Parent Training, Stimulant, and Placebo (Basic) Versus Parent Training, Stimulant, and Risperidone (Augmented)
Acknowledgments
This study was supported by grants from National Institute of Mental Health (NIMH) to the Ohio State University (R01 MH077907), Case Western Reserve University (R01 MH077750), University of Pittsburgh (R01 MH077676), and SUNY Stony Brook (R01 MH 077997). The project was also supported by a National Institutes of Health (NIH) General Clinical Research Center grant M01RR10710 (SUNY Stony Brook) and Clinical and Translational Science Awards (CTSA) from the National Center for Advancing Translational Sciences grants 8UL1TR000090-05 (the Ohio State University) and UL1 RR024153 and UL1TR000005 (University of Pittsburgh).
Dr. Li and Ms. Brown served as the statistical experts for this research.
The authors gratefully acknowledge guidance and supervision of the Data and Safety and Monitoring Board, comprising Drs. Daniel Connor (University of Connecticut Medical School), Walter Meyer, III (University of Texas Medical Branch, Galveston), Carson Reider (the Ohio State University), and Wesley Thompson (University of California, San Diego).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the respective National Centers for Advancing Translational Sciences or NIH.
This article is discussed in an editorial by Dr. Peter Jensen on page xxx.
Supplemental material cited in this article is available online.
Disclosure: Dr. Gadow has been a shareholder in Checkmate Plus, publisher of the Child and Adolescent Symptom Inventory-4R. Dr. Arnold has received research funding from CureMark, Forest, Eli Lilly and Co., and Shire; advisory board honoraria from Biomarin, Novartis, Noven, Roche, Seaside Therapeutics, and Shire; consulting fees from Tris Pharma; and travel support from Noven. Dr. Findling has received research support, acted as a consultant and/or served on a speaker's bureau for Alexza Pharmaceuticals, American Academy of Child and Adolescent Psychiatry, American Physician Institute, American Psychiatric Press, AstraZeneca, Bracket, Bristol-Myers Squibb, Clinsys, Cognition Group, Coronado Biosciences, Dana Foundation, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson and Johnson, KemPharm, Eli Lilly and Co., Lundbeck, Merck, NIH, Novartis, Noven, Otsuka, Oxford University Press, Pfizer, Physicians Postgraduate Press, Rhodes Pharmaceuticals, Roche, Sage, Seaside Pharmaceuticals, Shire, Stanley Medical Research Institute, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, and WebMD. Dr. Bukstein has received royalties from Routledge Press and acted as a consultant for Ezra Innovations and PRIME CME. Dr. Rundberg-Rivera had research support from GlaxoSmithKline, Merck/Schering Plough, NIMH, Covance/Otsuka, and Pfizer. Dr. Sprafkin has been a shareholder in Checkmate Plus, publisher of the Child and Adolescent Symptom Inventory-4R. Dr. Bangalore has received research support from Supernus Pharmaceutica. Dr. Hurt has received research support from Bristol-Myers Squibb. Dr. Aman has received research contracts, consulted with, or served on advisory boards of Biomarin Pharmaceuticals, Bristol-Myers Squibb, CogState, Confluence Pharmaceutica, Coronado Bioscience, Forest Research, Hoffman LaRoche, Johnson and Johnson, Neuren Pharmaceuticals, Novartis, ProPhase LLC, and Supernus Pharmaceutica. Ms. Kipp has received research support from Supernus Pharmaceutica. Ms. Baker has received research support from Amicus, BioMarin, Enobia, Genzyme, GlaxoSmithKline, Hyperion, Shire, Supernus, and Ultragenyx. Drs. Molina, Li, Schneider, Farmer, Rice Jr., Butter, and Mss. Brown, Austin, Buchan-Page, and Grondhuis report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Kenneth D. Gadow, State University of New York (SUNY) at Stony Brook.
Dr. L. Eugene Arnold, Ohio State University, Columbus, OH.
Dr. Brooke S.G. Molina, University of Pittsburgh School of Medicine.
Dr. Robert L. Findling, Johns Hopkins University and the Kennedy Krieger Institute, Baltimore.
Dr. Oscar G. Bukstein, University of Texas-Houston Medical School.
Mss. Nicole V. Brown, Ohio State University, Columbus, OH.
Dr. Nora K. McNamara, Case Western Reserve University, Cleveland.
Dr. E. Victoria Rundberg-Rivera, State University of New York (SUNY) at Stony Brook.
Dr. Xiaobai Li, Ohio State University, Columbus, OH.
Ms. Heidi Kipp, University of Pittsburgh School of Medicine.
Dr. Jayne Schneider, State University of New York (SUNY) at Stony Brook.
Dr. Cristan A. Farmer, Ohio State University, Columbus, OH.
Ms. Jennifer Baker, Children's Hospital of Pittsburgh, University of Pittsburgh Medical Center.
Dr. Joyce Sprafkin, State University of New York (SUNY) at Stony Brook.
Dr. Robert R. Rice, Jr., Ohio State University, Columbus, OH.
Dr. Srihari S. Bangalore, University of Pittsburgh School of Medicine.
Dr. Eric M. Butter, Nationwide Children's Hospital of Columbus.
Mss. Kristin A. Buchan-Page, Ohio State University, Columbus, OH.
Dr. Elizabeth A. Hurt, Ohio State University, Columbus, OH.
Mss. Adrienne B. Austin, Ohio State University, Columbus, OH.
Mss. Sabrina N. Grondhuis, Ohio State University, Columbus, OH.
Dr. Michael G. Aman, Ohio State University, Columbus, OH.
References
- 1.Bussing R, Winterstein AG. Polypharmacy in attention deficit hyperactivity disorder treatment: Current status, challenges and next steps. Curr Psychiatry Rep. 2012;14:447–449. doi: 10.1007/s11920-012-0295-6. [DOI] [PubMed] [Google Scholar]
- 2.Comer JS, Olfson M, Mojtabai R. National trends in child and adolescent psychotropic polypharmacy in office-based practice, 1996-2007. J Am Acad Child Adolesc Psychiarty. 2010;49:1001–1010. doi: 10.1016/j.jaac.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy FF, Narrow WE, Rae DS, et al. Concomitant pharmacotherapy among youths treated in routine psychiatric practice. J Child Adolesc Psychopharmacol. 2005;15:12–25. doi: 10.1089/cap.2005.15.12. [DOI] [PubMed] [Google Scholar]
- 4.Connor DF, Glatt SJ, Lopez ID, Jackson D, Melloni RH., Jr Psychopharmacology and aggression. I: A meta-analysis of stimulant effects on overt/covert aggression-related behaviors in ADHD. J Am Acad Child Adolesc Psychiatry. 2002;41:253–261. doi: 10.1097/00004583-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Gadow KD, Nolan EE, Sverd J, Sprafkin J, Schneider J. Methylphenidate in children with oppositional defiant disorder and both co-morbid chronic multiple tic disorder and ADHD. J Child Neurol. 2008;23:981–990. doi: 10.1177/0883073808315412. [DOI] [PubMed] [Google Scholar]
- 6.Gadow KD, Nolan EE, Sprafkin J, Sverd J. School observations of children with attention-deficit hyperactivity disorder and comorbid tic disorder: Effects of methylphenidate treatment. J Dev Behav Pediatr. 1995;16:167–176. [PubMed] [Google Scholar]
- 7.Gadow KD, Nolan EE, Sverd J, Sprafkin J, Paolicelli L. Methylphenidate in aggressive-hyperactive boys: I. Effects on peer aggression in public school settings. J Am Acad Child Adolesc Psychiatry. 1990;29:710–718. doi: 10.1097/00004583-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Chorpita BF, Daleiden EL, Ebesutani C, et al. Evidence-based treatments for children and adolescents: An updated review of indicators of efficacy and effectiveness. Clin Psychol: Sci Pract. 2011;18:154–172. [Google Scholar]
- 9.Conners CK, Epstein JN, March JS, et al. Multimodal treatment of ADHD in the MTA: An alternative outcome analysis. J Am Acad Child Adolesc Psychiatry. 2001;40:159–167. doi: 10.1097/00004583-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Swanson JM, Kraemer HC, Hinshaw SP, et al. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 11.RUPP Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 12.Aman MG, De Smedt G, Derivan A, Lyons B, Findling RL The Risperidone Disruptive Behavior Study Group. Risperidone treatment of children with disruptive behavior disorders and subaverage IQ: A double-blind, placebo-controlled study. Am J Psychiatry. 2002;159:1337–1346. doi: 10.1176/appi.ajp.159.8.1337. [DOI] [PubMed] [Google Scholar]
- 13.Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 14.Hazell PL, Stuart JE. A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. J Am Acad Child Adolesc Psychiatry. 2003;42:886–894. doi: 10.1097/01.CHI.0000046908.27264.00. [DOI] [PubMed] [Google Scholar]
- 15.Goyette CH, Conners CK, Ulrich RF. Normative data on the revised Conners Parent and Teacher Rating Scales. J Abnorm Child Psychol. 1978;6:221–236. doi: 10.1007/BF00919127. [DOI] [PubMed] [Google Scholar]
- 16.Aman MG, Bukstein OG, Gadow KD, et al. What does risperidone add to parent training and stimulant medication for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53:47–60. doi: 10.1016/j.jaac.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aman MG, Leone S, Lecavalier L, Park L, Buican B, Coury D. The Nisonger Child Behavior Rating Form-Typical IQ version, for children with typical IQ. Int Clin Psychopharmacol. 2008;23:232–242. doi: 10.1097/YIC.0b013e3282f94ad0. [DOI] [PubMed] [Google Scholar]
- 18.Coccaro EF, Harvey PH, Kupshaw-Lawrence E, Herbert JL, Bernstein DP. Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. J Neuropsychiatry Clin Neurosci. 1991;3(suppl 2):44–51. [PubMed] [Google Scholar]
- 19.Rapoport J, Conners CK, Reatig N, editors. CGI (Clinical Global Impression) scale – NIMH. Psychopharmacol Bull. 1985;21(4):839–843. Special Issue. [Google Scholar]
- 20.Farmer C, Arnold L, Bukstein O, et al. The treatment of severe child aggression (TOSCA) study: Trial design challenges. Child Adolesc Psychiatry Ment Health. 2011;5(36):1–11. doi: 10.1186/1753-2000-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadow KD, Sprafkin J. ADHD Symptom Checklist-4 2008 Manual. Stony Brook, NY: Checkmate Plus; 2008. [Google Scholar]
- 22.Gadow KD. Peer Conflict Scale. Stony Brook, NY: State University of New York, Department of Psychiatry; 1986. [Google Scholar]
- 23.Gadow KD, Sprafkin J, Nolan EE. ADHD School Observation Code. Stony Brook, NY: Checkmate Plus; 1996. [Google Scholar]
- 24.Sprafkin J, Gadow KD, Nolan EE. The utility of a DSM-IV-referenced screening instrument for attention-deficit/hyperactivity disorder. J Emotional Behav Disord. 2001;9:182–191. [Google Scholar]
- 25.Gadow KD, Sprafkin J. Child and Adolescent Symptom Inventory-4R. Stony Brook, NY: Checkmate Plus; 2005. [Google Scholar]
- 26.Gadow KD, Sprafkin J. The Symptom Inventories: An Annotated Bibliography. Stony Brook, NY: Checkmate Plus; 2013. On-line. Available: www.checkmateplus.com. [Google Scholar]
- 27.Drabick DAG, Gadow KD. Deconstructing oppositional defiant disorder: Clinic-based evidence for an anger/irritability phenotype. J Am Acad Child Adolesc Psychiatry. 2012;51:384–393. doi: 10.1016/j.jaac.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadow KD, Drabick DAG. Anger and irritability symptoms among youth with ODD: Cross-informant versus source-exclusive syndromes. J Abnorm Child Psychol. 2012;40:1073–1085. doi: 10.1007/s10802-012-9637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu K. On efficiency of constrained longitudinal data analysis versus longitudinal analysis of covariance. Biometrics. 2010;66(3):891–896. doi: 10.1111/j.1541-0420.2009.01332.x. [DOI] [PubMed] [Google Scholar]
- 30.Gurka M, Edwards LJ, Muller KE. Avoiding bias in mixed model inference for fixed effects. Stat Med. 2011;30(22):2696–2707. doi: 10.1002/sim.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gadow KD, Sverd J, Nolan EE, Sprafkin J, Schneider J. Immediate-release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:840–848. doi: 10.1097/chi.0b013e31805c0860. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school age children: Present and lifetime version (KSADS—PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5) Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 34.Stringaris A, Goodman R. Three dimensions of oppositionality in youth. Journal of Child Psychology and Psychiatry. 2009;50:216–223. doi: 10.1111/j.1469-7610.2008.01989.x. [DOI] [PubMed] [Google Scholar]
- 35.Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psych Bull. 1987;101(2):213. [PubMed] [Google Scholar]
- 36.Gadow KD, DeVincent CJ, Siegal VI, et al. Allele-specific associations of 5-HTTLPR/rs25531 with ADHD and autism spectrum disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;40:292–297. doi: 10.1016/j.pnpbp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gadow KD, Drabick DAG, Loney J, et al. Comparison of ADHD symptom subtypes as source-specific syndromes. J Child Psychol Psychiatry. 2004;45:1135–1149. doi: 10.1111/j.1469-7610.2004.00306.x. [DOI] [PubMed] [Google Scholar]
- 38.Gadow KD, Kaat AJ, Lecavalier L. Relation of symptom-induced impairment with other illness parameters in clinic-referred youth. J Child Psychol Psychiatry. 2013;54:1198–1207. doi: 10.1111/jcpp.12077. [DOI] [PubMed] [Google Scholar]
- 39.Gadow KD, Sprafkin J. Child Symptom Inventory-4 Screening and Norms Manual. Stony Brook, NY: Checkmate Plus; 2002. [Google Scholar]
- 40.Blader JC. Not just another antipsychotic-for-conduct problems trial. J Am Acad Child Adolesc Psychiatry. 2014;53:17–20. doi: 10.1016/j.jaac.2013.10.007. Editorial. [DOI] [PubMed] [Google Scholar]
- 41.Sleator EK, von Neumann A. Methylphenidate in the treatment of hyperkinetic children. Clin Pediatr. 1974;13:19–24. doi: 10.1177/000992287401300103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Demographic Characteristics of Children Receiving Parent Training, Stimulant, and Placebo (Basic) Versus Parent Training, Stimulant, and Risperidone (Augmented)


