Abstract
This study established an aged rat model of cognitive dysfunction using anesthesia with 2% isoflurane and 80% oxygen for 2 hours. Twenty-four hours later, Y-maze test results showed that isoflurane significantly impaired cognitive function in aged rats. Gas chromatography-mass spectrometry results showed that isoflurane also significantly increased the levels of N,N-diethylacetamide, n-ethylacetamide, aspartic acid, malic acid and arabinonic acid in the hippocampus of isoflurane-treated rats. Moreover, aspartic acid, N,N-diethylacetamide, n-ethylacetamide and malic acid concentration was positively correlated with the degree of cognitive dysfunction in the isoflurane-treated rats. It is evident that hippocampal metabolite changes are involved in the formation of cognitive dysfunction after isoflurane anesthesia. To further verify these results, this study cultured hippocampal neurons in vitro, which were then treated with aspartic acid (100 μmol/L). Results suggested that aspartic acid concentration in the hippocampus may be a biomarker for predicting the occurrence and disease progress of cognitive dysfunction.
Keywords: nerve regeneration, brain impairment, anesthesia, postoperative cognitive dysfunction, isoflurane, hippocampal metabolites, metabonomics, gas chromatography-mass spectrometry, aspartic acid, NSFC grant, neural regeneration
Introduction
Postoperative cognitive dysfunction is the deterioration of cognitive performance after anesthesia and surgery, and manifests as impairments in short-term memory, concentration, language comprehension, and social integration skills[1,2,3]. Thirty to forty-one percent of adult patients ranging from 18–60 years or older suffered from postoperative cognitive dysfunction at the time of discharge from hospital[4]. In patients older than 60 years, postoperative cognitive dysfunction was present in 25.8% of patients approximately a week after surgery and in 9.9% of patients 3 months after surgery[5]. Previous studies have shown that the occurrence of postoperative cognitive dysfunction is affected by many factors, including advanced age, low educational level, pre-existing cognitive impairment, alcohol abuse, and severity of coexisting illness[6,7,8]. However, the real cause for postoperative cognitive dysfunction is still unclear. Recently, we found that isoflurane (a general anesthetic agent) exposure impaired the cognitive function in aged rats[9]. Similar cognitive function impairment after general anesthesia was also reported in neonate, young, and adult rodents[9,10,11,12,13]. Further investigations showed differential modulation of isoflurane on neurogenesis in young and aged rats. Isoflurane increased early neuronal differentiation and progenitor proliferation of 60-day old rats[11]. However, it had no effects on neurogenesis in 16-month-old rats[14]. The pathophysiological mechanisms leading to the difference in the incidence rate and duration of postoperative cognitive dysfunction are still unclear. Additionally, the lack of biomarkers hinders the development of preventive and therapeutic strategies for postoperative cognitive dysfunction in clinical anesthesiology.
Metabonomics are defined as “the quantitative measurement of the dynamic multiparametric metabolic response of living systems to pathophysiological stimuli or genetic modification”[15] and are of increasing interest in the determination of comprehensive metabolite profiles of biological body (biofluids, cells, tissues). Metabonomics incorporates whole systems biochemistry and thus provides crucial information on disease processes and gene function[16], and reflects the physiological or pathological status of an organism. Metabolite changes were reported in various neurodegenerative diseases, such as multiple sclerosis[17,18,19], Alzheimer's disease[20,21,22,23], Creutzfeldt-Jakob syndrome[24,25] and Huntington's disease[26,27]. Recently, Watanabe et al.[28] found that N-acetylasparate and myo-inositol concentrations in bilateral, especially left hippocampus changed in patients with amnestic mild cognitive impairment and Alzheimer's diseases. These findings suggested that metabolite changes played important roles in cognitive impairment. Gas chromatography-mass spectrometry has been proven to be a potentially useful method for detection of small molecular metabolites based on its high sensitivity, peak resolution and reproducibility. Additionally, availability of the gas chromatography-mass spectrometry electron impact spectral library further facilitates the identification of diagnostic biomarkers and aids in the subsequent mechanistic elucidation of biological or pathological variations[29].
This study investigated the cognitive dysfunction of aged rats following isoflurane anesthesia, and detected the changes in hippocampal metabolites using gas chromatography-mass spectrometry. This study performed correlation analysis between changes in hippocampal metabolites and cognitive dysfunction in aged rats. These data will be useful in predicting the occurrence and development of postoperative cognitive dysfunction and will assist in elucidating the mechanisms underlying this phenomenon.
Results
Quantitative analysis of experimental animals
A total of 30 female Sprague-Dawley rats were randomly divided into two groups: isoflurane-treated group (n = 20) and control group (n = 10). Rats in the control group received 80% oxygen without administration of isoflurane for 2 hours. Rats in the isoflurane-treated group received 2 hours of 2% isoflurane and 80% oxygen. All rats underwent Y-maze testing 24 hours after anesthesia. After the last Y-maze test, rats were sacrificed for gas chromatography-mass spectrometry detection. All rats received similar care and were involved in the final analysis.
Physiological data during anesthesia
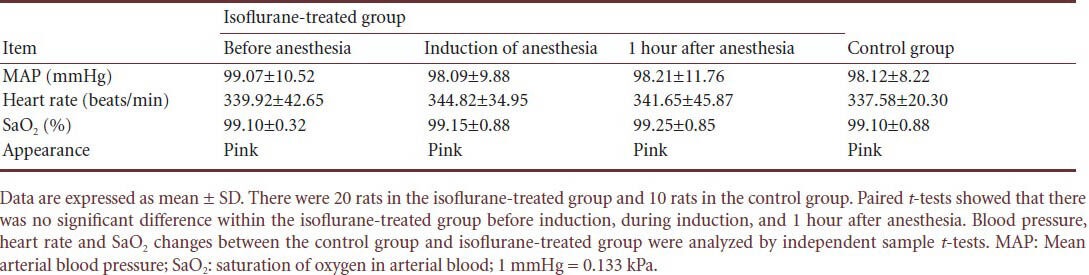
To observe changes in vital signs during anesthesia, heart rate, mean arterial blood pressure and saturation of oxygen in arterial blood (SaO2) of rats in the isoflurane-treated group were monitored. The rats in the isoflurane-treated group showed stable mean arterial blood pressure, SaO2 and heart rate during anesthesia compared with the rats in the control group. There were no signs of cyanosis, hypotension, respiratory depression or hypoxia (Table 1).
Table 1.
Vital signs seen in rats during isoflurane anesthesia
Isoflurane impaired cognitive functions of aged rats
In the Y-maze test, we agreed that an animal had mastered and remembered the maze when it entered the non-shock (illuminated) arm in nine out of 10 consecutive trials, thus an increase in the total number of trials required by the rats mastering the Y-maze indicated a decrease in learning and memory ability in the animals[5]. In the present study, the number of trials in the isoflurane-treated group was significantly higher than that in the control group (70.75 ± 15.30, vs. 45.4 ± 11.21; P < 0.05) at 24 hours after isoflurane anesthesia. This result suggested that isoflurane impaired cognitive functions of aged rats.
Isoflurane induced hippocampal metabolite changes of aged rats
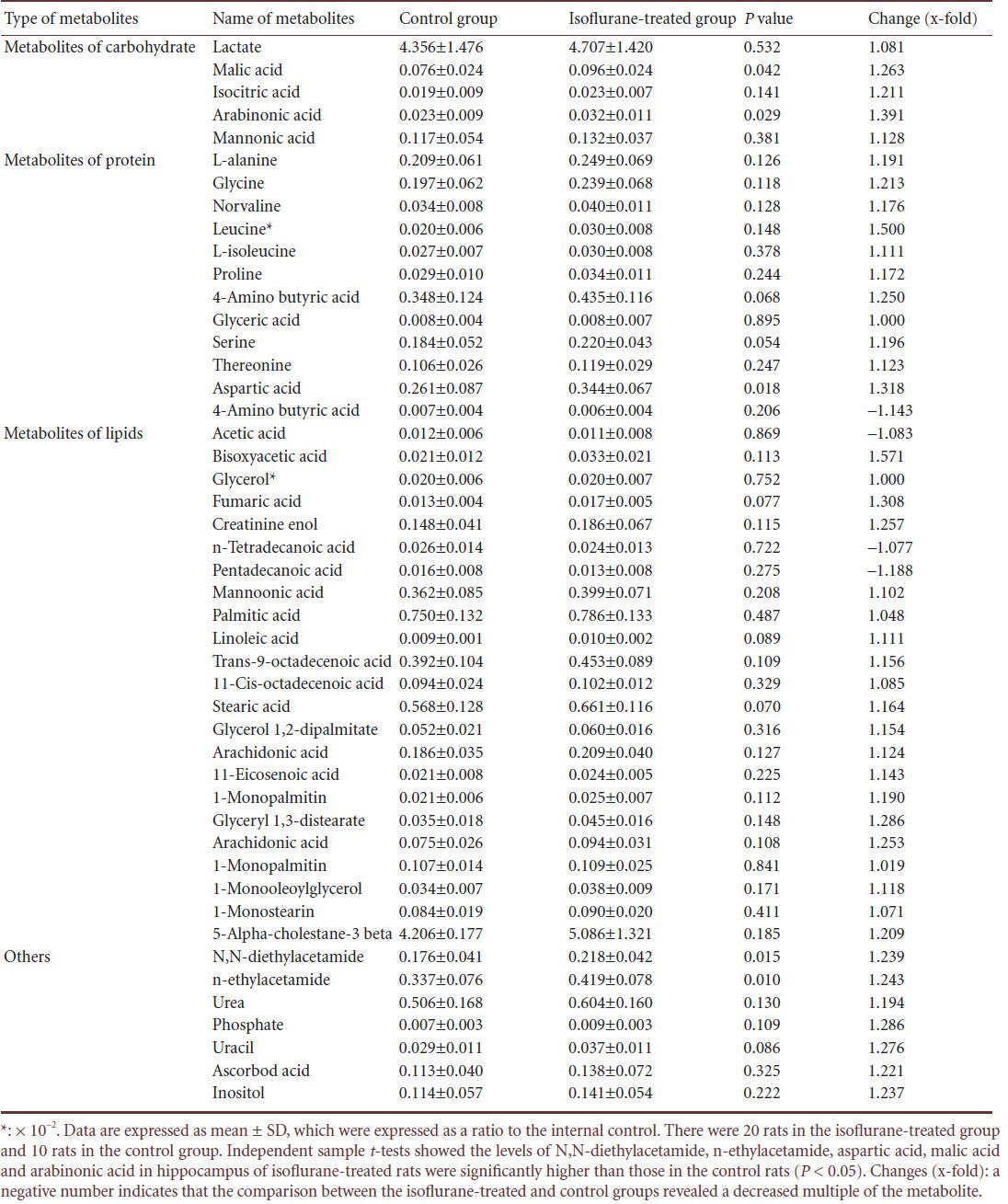
Gas chromatography-mass spectrometry was validated prior to the analysis of the experimental samples, including stability and the reproducibility of sample preparation. A total of 47 interesting peaks determined by gas chromatography-mass spectrometry were qualitatively and quantitatively analyzed in the hippocampus of control and isoflurane-treated rats (Table 2). Compared with the control group, N,N-diethylacetamide levels (which were expressed as a ratio of the level of N,N-diethylacetamide to the level of internal control) in the isoflurane-treated group were 0.218 ± 0.042, which was 1.24 times that of the control group (P = 0.017). N-ethylacetamide was 0.419 ± 0.078, which was 1.24 times that of the control group (P = 0.012). Among the protein metabolites, in the isoflurane-treated group, aspartic acid was 0.344 ± 0.067, 1.32 times that of the control group (P = 0.018), and among the metabolites of carbohydrate, malic acid in the isoflurane-treated group was 0.096 ± 0.024, which was 1.26 times that of the control group (P = 0.042). Arabinonic acid was 0.032 ± 0.011, 1.93 times that of controls (P = 0.029). These results showed that isoflurane had significant effects on hippocampal metabolites in aged rats at 24 hours after anesthesia.
Table 2.
Changes in hippocampal metabolite induced by isoflurane in aged rats at 24 hours after anesthesia
Hippocampal metabolites significantly correlated with cognitive function in isoflurane-treated aged rats
To identify a correlation between metabolites in the hippocampus and cognitive function in isoflurane-treated aged rats, correlation between trail number in the Y-maze tests and the relative concentration of five metabolites with significant changes was detected. Significant positive correlation was found between the Y-maze tests and aspartic acid (r = 0.5, P = 0.005, r2 = 0.25), N,N-diethylacetamide (r = 0.373, P = 0.042, r2 = 0.139), n-ethylacetamide (r = 0.405, P = 0.026, r2 = 0.164), and malic acid (r = 0.139, P < 0.001, r2 = 0.019). However, there was no significant positive correlation between trail number in the Y-maze test and arabinonic acid (r = 0.065, P = 0.732, r2 = 0.004; Figure 1). These results showed that the hippocampal level of aspartic acid, N,N-diethylacetamid, n-ethylacetamide, and malic acid was correlated with cognitive function in isoflurane-treated aged rats.
Figure 1.
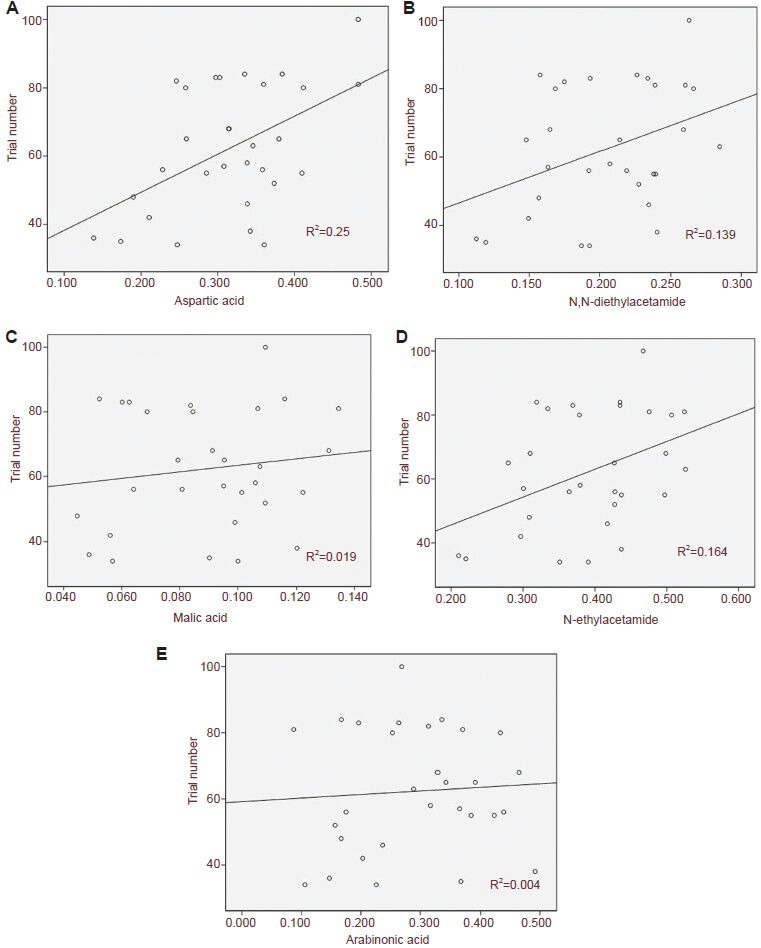
Correlation between trial number in the Y-maze and the relative concentration of hippocampal metabolites in isoflurane-treated aged rats.
The metabolites were expressed as a ratio to the internal control. Significant correlation was found between aspartic acid (A; r = 0.5, P = 0.005, r2 = 0.25), N,N-diethylacetamid (B; r = 0.373, P = 0.042, r2 = 0.139), malic acid (C; r = 0.139, P < 0.001, r2 = 0.019), and n-ethylacetamide (D; r = 0.405, P = 0.026, r2= 0.164) and trial number in the Y-maze. No significant correlation was found between arabinonic acid (E; r = 0.065, P = 0.732, r2 = 0.004) and trial number in the Y-maze using a linear regression model.
Increased aspartic acid impaired cultured hippocampal neurons
To detect the toxic effect of increased aspartic acid on neurons, we first cultured hippocampal neurons for 7 days, which showed typical neuronal morphology and high level of microtubule-associated protein 2 expression (Figure 2A, C). Then we treated the cultured hippocampal neurons with 100 µmol/L aspartic acid. Compared with the control (Figure 2A, C), neurons treated with increased aspartic acid had fewer and shorter processes and smaller cell bodies (Figure 2B, D). Quantitative studies showed significant loss of neurons in the aspartic acid-treated group compared with the neurons in the control group (63.67% vs. 14.29%; P < 0.05).
Figure 2.
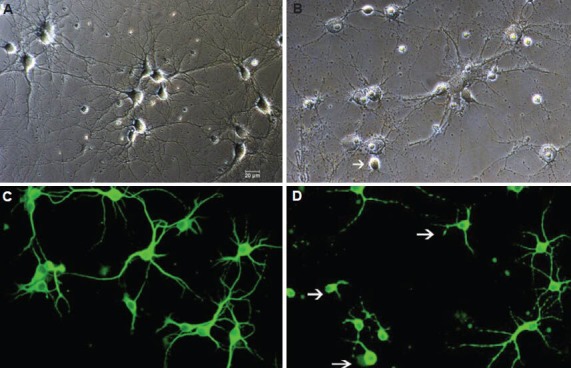
Effect of aspartic acid on cultured hippocampal neuron viability (× 200).
Representative pictures demonstrate the condition of cultured hippocampal neurons under phase-contrast microscope (A) and microtubule-associated protein 2 expression (C; immunofluorescence cytochemistry staining with FITC, epifluorescence microscope) in the control group. Neurons showed typical neuronal morphology. After treating with increased aspartic acid (100 μmol/L), parts of the neurons were lost and surviving neurons displayed shorter and fewer processes (arrows; B and D).
Discussion
The present study evaluated the effects of isoflurane on metabolites in the hippocampus and its correlation with cognitive dysfunction. We demonstrated that isoflurane impaired the cognitive function of aged rats at 1 day after anesthesia with varying degrees. Meanwhile, isoflurane induced metabolites level shifts, especially N,N-diethylacetamide, n-ethylacetamide, malic acid and arabinonic acid, and aspartic acid. Moreover, the hippocampal levels of aspartic acid, N,N-diethylacetamide, n-ethylacetamide and malic acid positively correlated with cognitive function in isoflurane-treated aged rats. Increased level of aspartic acid could damage cultured hippocampal neurons.
Isoflurane is commonly used in clinical inhalation anesthetics and its effects on cognitive function have been well described[11,12,14,30,31,32]. For example, volatile anesthetics can impaire the cognitive function of rats[12,14], but some also find volatile anesthetic neuroprotective effects and improvement of cognitive functions[14,33,34]. Amyloid-beta (Aβ) production and/or accumulation are major pathological hallmarks of Alzheimer's disease[35]. Volatile anesthetics can also induce the Aβ oligomerization in vitro[36]. Isoflurane has been shown to induce caspase activation and apoptosis, interfere amyloid precursor protein processing, increase Aβ generation, aggregation and impair learning and memory[10,37,38,39,40,41,42,43,44,45,46]. However, the mechanism of the Aβ oligomerization is unknown. Aspartic acid being isomerized aggregate in long-lived proteins of the aged people, especially in tooth, bone, cartilage, lens, and brain tissues[47], which is one of the most common post-translational modifications. The isomerization product is isoaspartic acid. Formation of isoaspartic acid is supposed to change protein structure and can change protein function and activity, or trigger aggregation[47,48,49]. Moreover, proteins containing isoaspartic acid may not fully degrade because that the isoaspartate residue hinders proteolytic degradation[50]. Isoaspartic acid is one of the direct mechanism related to the pathology of Alzheimer's disease. In our study, isoflurance induced the increased content of aspartic acid in the hippocampus and perhaps indirectly increased the isoaspartate by the isomerization, which finally led to the cognitive impairment of aged rats.
Although it has been known since the 1960s that aspartic acid has excitatory effects on neurons[51], it took almost 40 years for this amino acid to be considered as a classical neurotransmitter. It occurs in mammalian brain and has been implicated in the regulation of synaptic plasticity[52,53]. Aspartic acid is highly expressed in the whole brain during embryonic and perinatal periods, and strongly decreases during adulthood[54,55,56]. Nevertheless, in this study, aspartic acid in the hippocampus of the aged rats treated with isoflurane increased and was significantly correlated with cognitive impairment. Cui et al[57] also found that the aspartic acid and glutamine content in the rats’ hippocampus increased with early spatial memory impairment, possibly by disrupting glutamate-N-methyl-D-aspartic acid receptor signaling and triggering aberrant tau hyperphosphorylation in the hippocampus.
To further testify our outcomes and observe the toxic effect of aspartic acid, this study cultured the hippocampal neuron in vitro and treated the hippocampal neuron with aspartic acid. A previous research showed that the concentration of d-Asp was 164 nmol/g in the cerebral hemisphere of the newborn rat[58]. Thus, our primary study first tested the dose-effects of aspartic acid (80, 100, and 150 µmol/L) on the cultured hippocampal neurons, and found that aspartic acid of 80 µmol/L was minimally excitotoxic, causing very little damage and death in these neurons. Moreover, aspartic acid of 150 µmol/L caused very serious loss of neurons. Therefore, our study detected the toxic effects of aspartic acid of 100 µmol/L, and demonstrated that the axonal and dendritic processes almost disappeared, even causing the loss of cell bodies in the group treated with aspartic acid. Increased aspartic acid directly impaired the neuron, which may be one of the underlying mechanisms of postoperative cognitive dysfunction induced by isoflurane.
Moreover, alterations in amino acid neurotransmitters in the brain are associated with cognitive impairments, degenerative diseases, and brain injury[59]. Aspartic acid concentrations in the patients with Alzheimer's disease have been found to alter, although these data are conflicting and further studies are warranted to corroborate these results. In 1990s, Fisher et al.[60] found aspartic acid concentration in the cerebrospinal fluid of patients with Alzheimer's disease was significantly higher than in normal cerebrospinal fluid. Integration of excitatory and inhibitory signals is a basic attribute of neuronal communication, and synaptic plasticity correlated with learning and memory requires the balance of excitatory and inhibitory neurotransmitters. This balance will be breakdown because of the increase in excitatory neurotransmitters and might have caused an imbalance between excitatory and inhibitory signals and decreased synaptic plasticity, resulting in cognitive impairment. Increased levels of endogenous aspartic acid induced an enhancement of the N-methyl-D-aspartate receptor-dependent long-term potentiation LTP and spatial memory abilities of mice. They accelerate the age-dependent decay of basal glutamatergic α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid receptor transmission, synaptic plasticity and cognition in aged animals[61].
This study believed that hippocampal levels of aspartic acid could be used as biomarkers to predict the occurrence and progression of postoperative cognitive dysfunction in patients. However, the present study only used the female aged rat, and only detected the changes of cognitive function at the early stage after isoflurane. All the correlations are relatively weak. Thus further clinical study is still necessary for the actual effect size of aspartic acid as biomarkers in predicting the occurrence and progression of postoperative cognitive dysfunction in patients.
In addition, significant positive correlation was found between the Y-maze tests and N,N-diethylacetamide, n-ethylacetamide, and malic acid. Metabolic NADH shuttles promote the transfer of reducing cytosolic equivalents across the inner mitochondrial membrane into the mitochondria for oxidation to regulate the cellular redox and bioenergetic states. The α-glycerophosphate shuttle and malate-aspartate shuttle are the main parts of NADH shuttles. Here we found the changes of N,N-diethylacetamide, n-ethylacetamide and malic acid in aged rats with postoperative cognitive dysfunction. However, we still do not know the changed metabolites function in the occurrence and progression of postoperative cognitive dysfunction.
Materials and Methods
Time and setting
This experiment was conducted at the Experimental Center of the Third Xiangya Hospital of Central South University, China from January to September in 2010.
Materials
A total of 30 female Sprague-Dawley rats aged 22–24 months and weighing 300–500 g were used in this study for cognitive function evaluation. Five embryonic day 18 Sprague-Dawley rats were used to prepare hippocampal neuronal cultures. All animals were purchased from Hunan Agriculture College in China (license No. SCXK (Xiang) 2006-0001), and were housed under controlled environmental conditions (room temperature 22 ± 2°C, humidity 65 ± 1%) on a 12-hour light/dark cycle with free access to food and water. Experimental procedures were approved by the Animal Ethics Committee of the Central South University in China.
Methods
Establishment of the rat model of postoperative cognitive dysfunction after anesthesia
Anesthesia was given in a chamber flushed with 3% isoflurane (Abbott, Chicago, IL, USA) and 80% oxygen. After the righting reflex was diminished, rats were then anesthetized through endotracheal intubation with 2% isoflurane and 80% oxygen with a respiratory rate of 39.19 ± 3.78 breaths/min. Pilot studies demonstrated that the PaCO2 was 52.38 ± 7.74 mmHg at this time. The anesthesia was maintained at this concentration for 2 hours[12]. The concentration of isoflurane, oxygen, and carbon dioxide (PaCO2) was monitored continuously by Datex equipment (Datex-Ohmeda, Madison, WI, USA). Mean arterial blood pressure was intermittently monitored with a rat tail blood pressure cuff. To objectively evaluate the effects of anesthesia, we measured EctCO2, mean arterial blood pressure and heart rate, and respiratory rate in rats from the control and isoflurane-treated groups before, during and 1 hour after anesthesia induction. Rectal temperature was controlled at 37 ± 0.5°C. Rats in the control group were placed in a chamber flushed with 80% oxygen without isoflurane anesthesia.
Y-maze test
The Y-maze test is widely used to evaluate abilities of spatial learning and memory in rodents[4]. Cognitive functions were detected by an investigator who was blinded to intervention using the modified Y-maze apparatus 24 hours after delivery of isoflurane anesthesia or 80% oxygen. The behavioral tests were carried out in a separate room (temperature was approximately 22°C) and were conducted during the dark phase. The Y-maze was used to assess spatial memory. The apparatus consists of three opaque-black arms connected into a Y shape. The floor of the stem of the Y arm and one of the two branch arms contained electric wires through which an electric shock could be applied. The branch arm lacking wires remained illuminated throughout the test (because rats are typically neophobic, they prefer to avoid an illuminated area). During the test, rats were placed in the stem of the Y-maze. When animals received an electric shock, they entered into one of the two branch arms. Before the next trial, rats were manually returned to the stem of the Y-maze. The same procedure was repeated until the animal entered into the non-shocking arm in nine out of 10 consecutive trials. The total number of trials was counted, indicating cognitive function.
Sample pretreatment of hippocampal tissues
After the Y-maze test, animals were anaesthetized with 10% hydronium. Animals were exsanguinated by cardiac puncture within 15 minutes, and then the brain was removed from the skull, and sectioned midsagittally. Rat hippocampi were removed from the brain immediately and washed in ice-cold physical saline solution. The isolated hippocampi were stored at −80°C until use. Hippocampal tissues (30 mg) were weighed and homogenized with a T10 basic homogenizer (IKA, Staufen, Germany) for 30 seconds at 0°C. Chloroform (100 mL), methanol (200 mL), and water (100 mL) were added to extract metabolites from the hippocampus. Methanol (400 mL) was then added and the sample was vigorously vortexed for 30 seconds. The resulting fluid was transferred to a 2 mL GC sampling vial containing 30 mL internal standards (heptadecanoic acid-methanol 1 mg/mL) and vortexed again for 30 seconds. Methanol (500 mL) was subsequently added, homogenized and centrifuged at 16,000 r/min for 20 minutes at 4°C. The resulting supernatant was dried in a vacuum centrifuge concentrator before the derivatization.
Gas chromatography-mass spectrometry analysis
All gas chromatography-mass spectrometry analyses were double-blinded and performed on a Shimadzu GCMS-QP2010 gas chromatography quadrupole mass spectrometer (Shimadzu, Kyoto, Japan). Helium carrier gas was used at a constant flow rate of 1.0 mL/min. A sample of 1.0 µL was injected into a DB-5 ms capillary column (30 m × 0.25 mm inside diameter film thickness 0.25 µm) at a split ratio of 1:10. The column temperature was initially maintained at 70°C for 4 minutes, and then increased at a rate of 8°C/min from 70 to 300°C and held for 3 minutes. Mass conditions were as follows: ionization voltage, 70 eV; ionsource temperature, 200°C; interface temperature, 250°C; full scan mode in the 35–800 atomic mass unit mass ranges with 0.2 second scan velocity; detector voltage, 0.9 kV.
Hippocampal neuronal culture and aspartic acid intervention
Cell cultures were prepared from embryonic day 18 rats and cultured as described previously[62]. The pregnant dam was sacrificed using an approved method of euthanasia, and the uterus was dissected out and placed in a sterile Petri dish. Working in a laminar flow hood, the fetuses were removed from the uterus, and their brains were dissected out and placed in a dish containing Neurobasal Medium (Gibco, Carlsbad, CA, USA). The tissue needs to remain submerged at all times. Using a dissecting microscope (Olympus, Tokyo, Japan), the meninges was removed from the medial aspect of the cerebral hemispheres, and then the hippocampus was dissected out. The hippocampi were collected in a dish containing Neurobasal Medium. When all of the hippocampi were removed, they were placed in a 15-mL conical centrifuge tube, and a volume of 4.5 mL was made up with CMF-HBSS (Invitrogen, NewYork, CA, USA). Trypsin (0.5 mL of 2.5%) was added to this tube and incubated for 15 minutes in a water bath at 37°C. Trypsin solution was gently removed, with the hippocampi remaining at the bottom of the tube. CMF-HBSS (5 mL) was added to the hippocampi, and allowed to stand for 5 minutes at room temperature. This was repeated twice to allow residual trypsin to diffuse from the tissue. Final volume was made up to 2–3 mL with CMF-HBSS (Invitrogen). The hippocampi were then triturated five to 10 times through a regular pipette, and then five to 10 passes through a Pasteur pipette that had been flame-polished so that the tip diameter was narrowed by half. The suspension was forcefully expelled against the wall of the tube to minimize foaming. By the end of this process, no chunks of tissue were visible. The dissociated neurons were plated at a concentration of 150,000 cells per 60-mm dish in plating media [Neurobasal Medium (Gibco) + GlutaMAX-I supplement (Sigma, St. Louis, MO, USA) + B27 serum-free supplement (Gibco)]. Using a micropipette, add the desired number of cells to each of the dishes containing the polylysine-treated coverslips in Neuronal Plating Medium. Approximately 1 week before the animal reaches the correct gestational day, start the glial feeder cultures by seeding about 1 × 105 cells per 60-mm dish. Replace the medium in 60-mm dishes with fresh Glial Medium every 3–4 days. Cultures are suitable for coculturing with neurons when they reach 40–70% confluence. After 3–4 hours, the dishes were examined to ensure that most of the cells have attached, then coverslips were transferred to dishes containing a glial feeder layer in N2 or Neurobasal/B27 medium. The coverslips were inverted so that the paraffin feet were resting on the bottom of the dish. At 3 days after plating, cytosine arabinoside (1-β-D-arabinofuranosylcytosine) was added to a final concentration of 5 µmol/L to curb glial proliferation. In this medium, glial cell growth at 5 days dropped to less than 1.0%, resulting in a nearly pure neuronal population. The medium was changed every 2 days and cells were maintained until day 7.
Aspartic acid (100 µmol/L, BBI, Brescia, Italy) was applied to hippocampal neurons cultured for 7 days. After incubating for 25 minutes, the medium was removed, and followed with three washes with Neurobasal Medium and further incubation for 24 hours.
Immunofluorescence cytochemistry and detection of neuronal viability
For immunostaining, cultured neurons were fixed at indicated times in PBS containing 4% paraformaldehyde/4% sucrose for 20 minutes at 37°C. The neurons were then permeabilized with 0.1% Triton X-100 (Sigma)/PBS for 5 minutes and blocked with 0.5% fish skin gelatin (Sigma)/PBS for 1 hour, both at 27°C. The cells were then stained for somatodendritic markers using polyclonal rabbit anti-microtubule-associated protein 2 antibody (1:150; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight, followed by incubation with fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG (1:200; Jackson Immuno Research Labs, Inc., West Grove, PA, USA) at 37°C for 2 hours. The samples were washed with PBS, dewaxed, and mounted on slide glasses. The stained samples were imaged under an epifluorescence microscope (Zeiss, Oberkochen, Germany) equipped with a 20 × objective lens. The images were obtained with Axiocam MRm CCD camera (Zeiss) and AxioVision software (Zeiss). The toxic effects of aspartic acid on the neurons were evaluated by Live/Dead Assay kit (L-3224; Molecular Probe, Eugene, OR, USA) and the percent of damaged neuron number/total neuron numbers was calculated[63]. Neuronal viability was evaluated and compared with cultured neurons not exposed to aspartic acid.
Statistical analysis
All analyses were performed using SPSS 17.0 software for Windows (SPSS, Chicago, IL, USA). Quantitative data were expressed as mean ± SD. Paired t-tests were used to examine the changes in blood pressure, heart rate and SaO2 within the isoflurane-treated group before induction, during induction and 1 hour after anesthesia. Blood pressure, heart rate, SaO2 and metabolite changes between the control group and isoflurane-treated group were analyzed by independent sample t-tests. Correlation studies between metabolites and cognitive impairment were analyzed through a linear regression model. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30871306.
Conflicts of interest: None declared.
Peer review: This study is the first attempt at elucidating the mechanism behind postoperative cognitive dysfunction using metabonomics. We found that aspartic acid is correlated with cognitive dysfunctions and may be used to predict the occurrence and development of postoperative cognitive dysfunction.
Copyedited by Apricò K, Frenchman B, Cai WJ, Li Y, Wang LM, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M
References
- [1].Rasmussen LS, Christiansen M, Hansen PB, et al. Do blood levels of neuron-specific enolase and S-100 protein reflect cognitive dysfunction after coronary artery bypass? Acta Anaesthesiol Scand. 1999;43(5):495–500. doi: 10.1034/j.1399-6576.1999.430502.x. [DOI] [PubMed] [Google Scholar]
- [2].Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- [3].Hudetz JA, Gandhi SD, Iqbal Z, et al. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J Anesth. 2011;25(1):1–9. doi: 10.1007/s00540-010-1042-y. [DOI] [PubMed] [Google Scholar]
- [4].Bilotta F, Doronzio A, Stazi E, et al. Postoperative cognitive dysfunction: toward the Alzheimer's disease pathomechanism hypothesis. J Alzheimers Dis. 2010;22(Suppl 3):81–89. doi: 10.3233/JAD-2010-100825. [DOI] [PubMed] [Google Scholar]
- [5].Wan Y, Xu J, Ma D, et al. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106(3):436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- [6].Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- [7].Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000;20(10):2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- [8].Vanhanen M, Koivisto K, Moilanen L, et al. Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology. 2006;67(5):843–847. doi: 10.1212/01.wnl.0000234037.91185.99. [DOI] [PubMed] [Google Scholar]
- [9].Yan XB, Ouyang W, Li G, et al. Involvement of neuronal nitric oxide synthase in cognitive impairment in isoflurane-treated rats. Involvement of neuronal nitric oxide synthase in cognitive impairment in isoflurane-treated rats. Neurosci Lett. 2012;506(2):240–244. doi: 10.1016/j.neulet.2011.11.015. [DOI] [PubMed] [Google Scholar]
- [10].Ancelin ML, de Roquefeuil G, Scali J, et al. Long-term post-operative cognitive decline in the elderly: the effects of anesthesia type, apolipoprotein E genotype, and clinical antecedents. J Alzheimers Dis. 2010;22(Suppl 3):105–113. doi: 10.3233/JAD-2010-100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Culley DJ, Baxter MG, Yukhananov R, et al. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100(2):309–314. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- [12].Culley DJ, Baxter MG, Crosby CA, et al. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth Analg. 2004;99(5):1393–1397. doi: 10.1213/01.ANE.0000135408.14319.CC. [DOI] [PubMed] [Google Scholar]
- [13].Kelley JB, Balda MA, Anderson KL, et al. Impairments in fear conditioning in mice lacking the nNOS gene. Learn Mem. 2009;16(6):371–378. doi: 10.1101/lm.1329209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Culley DJ, Baxter M, Yukhananov R, et al. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg. 2003;96(4):1004–1009. doi: 10.1213/01.ANE.0000052712.67573.12. [DOI] [PubMed] [Google Scholar]
- [15].Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29(11):1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- [16].Nicholson JK, Connelly J, Lindon JC, et al. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov. 2002;1(2):153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- [17].Mellergård J, Tisell A, Dahlqvist Leinhard O, et al. Association between change in normal appearing white matter metabolites and intrathecal inflammation in natalizumab-treated multiple sclerosis. PLoS One. 2012;7(9):e44739. doi: 10.1371/journal.pone.0044739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Noga MJ, Dane A, Shi S, et al. Metabolomics of cerebrospinal fluid reveals changes in the central nervous system metabolism in a rat model of multiple sclerosis. Metabolomics. 2012;8(2):253–263. doi: 10.1007/s11306-011-0306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brief EE, Vavasour IM, Laule C, et al. Proton MRS of large multiple sclerosis lesions reveals subtle changes in metabolite T(1) and area. NMR Biomed. 2010;23(9):1033–1037. doi: 10.1002/nbm.1527. [DOI] [PubMed] [Google Scholar]
- [20].Frisoni GB, Filippi M. Multiple sclerosis and Alzheimer disease through the looking glass of MR imaging. AJNR Am J Neuroradiol. 2005;26(10):2488–2491. [PMC free article] [PubMed] [Google Scholar]
- [21].Lim TS, Hong YH, Lee HY, et al. Metabolite investigation in both anterior and posterior cingulate gyri in Alzheimer's disease spectrum using 3-tesla MR spectroscopy. Dement Geriatr Cogn Disord. 2012;33(2-3):149–155. doi: 10.1159/000338177. [DOI] [PubMed] [Google Scholar]
- [22].Ibáñez C, Simó C, Martín-Álvarez PJ, et al. Toward a predictive model of Alzheimer's disease progression using capillary electrophoresis-mass spectrometry metabolomics. Anal Chem. 2012;84(20):8532–8540. doi: 10.1021/ac301243k. [DOI] [PubMed] [Google Scholar]
- [23].Czech C, Berndt P, Busch K, et al. Metabolite profiling of Alzheimer's disease cerebrospinal fluid. PLoS One. 2012;7(2):e31501. doi: 10.1371/journal.pone.0031501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kleiner-Fisman G, Bergeron C, Lang AE. Presentation of Creutzfeldt-Jakob disease as acute corticobasal degeneration syndrome. Mov Disord. 2004;19(8):948–949. doi: 10.1002/mds.20140. [DOI] [PubMed] [Google Scholar]
- [25].Waldman AD, Cordery RJ, MacManus DG, et al. Regional brain metabolite abnormalities in inherited prion disease and asymptomatic gene carriers demonstrated in vivo by quantitative proton magnetic resonance spectroscopy. Neuroradiology. 2006;48(6):428–433. doi: 10.1007/s00234-006-0068-1. [DOI] [PubMed] [Google Scholar]
- [26].Unschuld PG, Edden RA, Carass A, et al. Brain metabolite alterations and cognitive dysfunction in early Huntington's disease. Mov Disord. 2012;27(7):895–902. doi: 10.1002/mds.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sturrock A, Laule C, Decolongon J, et al. Magnetic resonance spectroscopy biomarkers in premanifest and early Huntington disease. Neurology. 2010;75(19):1702–1710. doi: 10.1212/WNL.0b013e3181fc27e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Watanabe T, Shiino A, Akiguchi I. Hippocampal metabolites and memory performances in patients with amnestic mild cognitive impairment and Alzheimer's disease. Neurobiol Learn Mem. 2012;97(3):289–293. doi: 10.1016/j.nlm.2012.01.006. [DOI] [PubMed] [Google Scholar]
- [29].Want EJ, Nordström A, Morita H, et al. From exogenous to endogenous: the inevitable imprint of mass spectrometry in metabolomics. J Proteome Res. 2007;6(2):459–468. doi: 10.1021/pr060505+. [DOI] [PubMed] [Google Scholar]
- [30].Bekker A, Lee C, de Santi S, et al. Does mild cognitive impairment increase the risk of developing postoperative cognitive dysfunction? Am J Surg. 2010;199(6):782–788. doi: 10.1016/j.amjsurg.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stratmann G, Sall JW, May LD, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110(4):834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- [32].Zhu C, Gao J, Karlsson N, et al. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab. 2010;30(5):1017–1030. doi: 10.1038/jcbfm.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Komatsu H, Nogaya J, Anabuki D, et al. Memory facilitation by posttraining exposure to halothane, enflurane, and isoflurane in ddN mice. Anesth Analg. 1993;76(3):609–612. doi: 10.1213/00000539-199303000-00028. [DOI] [PubMed] [Google Scholar]
- [34].Li L, Zuo Z. Isoflurane preconditioning improves short- term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164(2):497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Glenner GG, Wong CW. Alzheime's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- [36].Xie Z, Dong Y, Maeda U, et al. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27(6):1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101(3):703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- [38].Kvolik S, Glavas-Obrovac L, Bares V, et al. Effects of inhalation anesthetics halothane, sevoflurane, and isoflurane on human cell lines. Life Sci. 2005;77(19):2369–2383. doi: 10.1016/j.lfs.2004.12.052. [DOI] [PubMed] [Google Scholar]
- [39].Loop T, Dovi-Akue D, Frick M, et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102(6):1147–1157. doi: 10.1097/00000542-200506000-00014. [DOI] [PubMed] [Google Scholar]
- [40].Wei H, Kang B, Wei W, et al. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037(1-2):139–147. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- [41].Matsuoka H, Kurosawa S, Horinouchi T, et al. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology. 2001;95(6):1467–1472. doi: 10.1097/00000542-200112000-00028. [DOI] [PubMed] [Google Scholar]
- [42].Xie Z, Dong Y, Maeda U, et al. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104(5):988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- [43].Xie Z, Dong Y, Maeda U, et al. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61(12):1300–1306. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- [44].Zhen Y, Dong Y, Wu X, et al. Nitrous oxide plus isoflurane induces apoptosis and increases beta-amyloid protein levels. Anesthesiology. 2009;111(4):741–752. doi: 10.1097/ALN.0b013e3181b27fd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112(4):834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bianchi SL, Tran T, Liu C, et al. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29(7):1002–1010. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ritz-Timme S, Collins MJ. Racemization of aspartic acid in human proteins. Ageing Res Rev. 2002;1(1):43–59. doi: 10.1016/s0047-6374(01)00363-3. [DOI] [PubMed] [Google Scholar]
- [48].Roher AE, Lowenson JD, Clarke S, et al. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer's disease. J Biol Chem. 1993;268(5):3072–3083. [PubMed] [Google Scholar]
- [49].Shimizu T, Watanabe A, Ogawara M, et al. Isoaspartate formation and neurodegeneration in Alzheimer's disease. Arch Biochem Biophys. 2000;381(2):225–234. doi: 10.1006/abbi.2000.1955. [DOI] [PubMed] [Google Scholar]
- [50].Bohme L, Bar JW, Hoffmann T, et al. Proceedings of the 5th General Meeting of the International Proteolysis Society. October 20-24, 2007. Patras, Greece. Biol Chem. 2008;389(8):967–1142. [PubMed] [Google Scholar]
- [51].Curtis DR, Phillis JW, Watkins JC. The chemical excitation of spinal neurones by certain acidic amino acids. J Physiol. 1960;150:656–682. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Errico F, Nisticò R, Palma G, et al. Increased levels of d-aspartate in the hippocampus enhance LTP but do not facilitate cognitive flexibility. Mol Cell Neurosci. 2008;37(2):236–246. doi: 10.1016/j.mcn.2007.09.012. [DOI] [PubMed] [Google Scholar]
- [53].Errico F, Rossi S, Napolitano F, et al. D-aspartate prevents corticostriatal long-term depression and attenuates schizophrenia-like symptoms induced by amphetamine and MK-801. J Neurosci. 2008;28(41):10404–10414. doi: 10.1523/JNEUROSCI.1618-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schell MJ, Cooper OB, Snyder SH. D-aspartate localizations imply neuronal and neuroendocrine roles. Proc Natl Acad Sci U S A. 1997;94(5):2013–2018. doi: 10.1073/pnas.94.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wolosker H, D’Aniello A, Snyder SH. D-aspartate disposition in neuronal and endocrine tissues: ontogeny, biosynthesis and release. Neuroscience. 2000;100(1):183–189. doi: 10.1016/s0306-4522(00)00321-3. [DOI] [PubMed] [Google Scholar]
- [56].Sakai K, Homma H, Lee JA, et al. Emergence of D-aspartic acid in the differentiating neurons of the rat central nervous system. Brain Res. 1998;808(1):65–71. doi: 10.1016/s0006-8993(98)00599-x. [DOI] [PubMed] [Google Scholar]
- [57].Cui B, Wu M, She X, et al. Impulse noise exposure in rats causes cognitive deficits and changes in hippocampal neurotransmitter signaling and tau phosphorylation. Brain Res. 2012;1427:35–43. doi: 10.1016/j.brainres.2011.08.035. [DOI] [PubMed] [Google Scholar]
- [58].Dunlop DS, Neidle A, McHale D, et al. The presence of free D-aspartic acid in rodents and man. Biochem Biophys Res Commun. 1986;141(1):27–32. doi: 10.1016/s0006-291x(86)80329-1. [DOI] [PubMed] [Google Scholar]
- [59].Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- [60].Fisher GH, Petrucelli L, Gardner C, et al. Free D-amino acids in human cerebrospinal fluid of Alzheimer disease, multiple sclerosis, and healthy control subjects. Mol Chem Neuropathol. 1994;23(2-3):115–124. doi: 10.1007/BF02815405. [DOI] [PubMed] [Google Scholar]
- [61].Errico F, Nisticò R, Napolitano F, et al. Persistent increase of D-aspartate in D-aspartate oxidase mutant mice induces a precocious hippocampal age-dependent synaptic plasticity and spatial memory decay. Neurobiol Aging. 2011;32(11):2061–2074. doi: 10.1016/j.neurobiolaging.2009.12.007. [DOI] [PubMed] [Google Scholar]
- [62].Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1(5):2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- [63].Edwards D, Das M, Molnar P, et al. Addition of glutamate to serum-free culture promotes recovery of electrical activity in adult hippocampal neurons in vitro. J Neurosci Methods. 2010;190(2):155–163. doi: 10.1016/j.jneumeth.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


