Abstract
Adult mesenchymal stem cells, specifically adipose-derived stem cells have self-renewal and multiple differentiation potentials and have shown to be the ideal candidate for therapeutic applications in regenerative medicine, particularly in peripheral nerve regeneration. Adipose-derived stem cells are easily harvested, although they may show the effects of aging, hence their potential in nerve repair may be limited by cellular senescence or donor age. Cellular senescence is a complex process whereby stem cells grow old as consequence of intrinsic events (e.g., DNA damage) or environmental cues (e.g., stressful stimuli or diseases), which determine a permanent growth arrest. Several mechanisms are implicated in stem cell senescence, although no one is exclusive of the others. In this review we report some of the most important factors modulating the senescence process, which can influence adipose-derived stem cell morphology and function, and compromise their clinical application for peripheral nerve regenerative cell therapy.
Keywords: aging, adipose-derived stem cell, DNA damage, p38, p53, mitochondria, Sirtuins, peripheral nervous system
Introduction
During the past decade, the field of stem cell biology has developed considerably following reports demonstrating that adult stem cells possess great abilities in tissue regeneration and regenerative medicine. Many efforts have been focused on understanding the molecular mechanisms that regulate their plasticity in order to exploit this capacity for a therapeutic use. Stem cells are classified as embryonic stem cells (ES cells)[1], adult stem cells[2,3], and induced pluripotent stem cells (iPS cells)[4]. Stem cells can be defined as (i) undifferentiated (lacking a tissue-specific differentiation marker), (ii) capable of proliferation, (iii) self-renewable, (iv) able to produce a large number of differentiated functional progeny and (v) able to regenerate tissue after injury[5]. A large number of studies suggested that stem cells from one tissue can cross lineage and differentiate into cells of other lineages either in vitro or in vivo after transplantation. Recent studies support the hypothesis that stem cells in several tissues are largely retained in a quiescent state but can be induced to re-enter into the cell cycle in response to extracellular cues, injuries or diseases, even after a long period of dormancy. In order to promote regeneration or repair processes, the stem cells reactivate their proliferative cycle through signal stimulations, while the somatic cells remain inactive. This concept suggests that stem cells are capable of self-regulating the cells cycle[6]. However, it is still unclear whether the stem cells are influenced by extracellular signals from a “niche”, or their stimulation is dependent by the aging process. The plasticity, or ability of stem cells to trans-differentiate, has been a source of great interest for its therapeutic potential in tissue engineering, in particular in peripheral nerve regeneration[7,8]. Adipose tissue has been shown to contain adult mesenchymal stem cells (MSC) that have therapeutic applications in regenerative medicine[7,9]. In previous studies, the use of MSC isolated from bone marrow (BM-MSC), or adipose-derived stem cells (ASC) have demonstrated successful differentiation towards the Schwann cell-like (SC) lineage following the exposure to the mitogen glial growth factor (GGF), and incubation of the cultures with β-mercaptoethanol and all-trans-retinoic acid[7,10,11]. However, it is becoming increasingly clear that their therapeutic potential may be limited by senescence, or by the age of the donor.
Aging is a complex process in which gradual deterioration of physiological function involves virtually all cells and tissues in an organism. This functional decline is associated with a diminished capacity to maintain normal tissue homeostasis and by a reduced regenerative capacity of tissues in old organisms in response to injury. Currently, it is not completely clear how aging may influence nerve regeneration. For instance, Bharali and Lisney[12] (1990) showed that in rats of 4 to 40 weeks of age, the nerve regeneration potential was weakly affected by the age at which injury occurred, whereas the rats injured at 2 weeks of age showed significantly reduced peripheral nerve regeneration. Changes in the peripheral neural pathway are most likely caused by modifications in the extracellular matrix. Kovacic et al.[13] demonstrated that the amount of laminin present in intact nerves from aged rats was significantly less than that from young adults rats. It is also known that during aging, there is a reduction in the level of peripherin, which is involved in axonal growth[14]. It is clear that a comprehensive understanding of the importance of age-related differences in nerve regeneration capacity, as well as of the effects of modulatory factors, is essential for the development of tissue engineering solutions[12].
Studies on stem cell senescence started almost half a century ago, when the first report describing the limited replicative potential of primary cells in culture has been published[15]. Mammalian aging has been defined as a reduction in the capacity to maintain adequate tissue homeostasis or to repair tissue after an injury[16]. Tissue homeostasis and regenerative capacity are nowadays considered to be related to the stem cell pool present in every tissue. For this reason, when an organism undergoes unfavourable physiopathological conditions the reduction in stem cells number and/or function may develop[17,18]. However, the correlation between organism aging and cell senescence remains controversial despite decades of studies[16,19,20].
It is to be considered that the cell type, the nature and the magnitude of the damage or stress might be responsible for the switch between the biological processes of senescence and apoptosis[21]. In order to evaluate the relationship between physiological process of aging and the biological mechanisms regulating senescent cells, different intracellular factors have been proposed to influence the cell cycle in tissue from organism of different ages. It should be highlighted that any of these factors may be exclusive of the senescent state, whereas all of them require an adequate time to exert their action. During the last few years researchers have investigated the pathways involved in telomeric shortening, as well as the regulation of some important intracellular proteins like p38 mitogen activated protein kinase (p38 MAPK), p53 MAPK (also known as protein 53 or tumor protein 53) and other senescence factors[22]. Also, DNA damage and oxidative stress may account for an age-related decline in cellular proliferation. It has also to be considered that ASC produce various growth factors supporting peripheral nerve regeneration [e.g., GGF, brain derived neurotrophic factor (BDNF), nerve growth factor (NGF)], whose secretion seems to be maintained regardless of donor age[23].
DNA damage
Cellular senescence can be classified as replicative senescence and stress induced premature senescence. The first process is strictly related to telomere dysfunction, the second is the result of different mechanisms like mitochondrial damage, activated oncogenes and epigenetic changes. Telomeres are defined as the end part of eukaryotic chromosomes, and consist of nucleotide repeats that are shortened after each replication cycle. Telomerase is a nuclear enzyme deputed to elongate and protect telomeres, and the telomerase that no longer accomplish an end-protective function is defined as dysfunctional. Therefore, the reduced length of telomeres is indicative of the proliferative stage of the cells, hence the measure of telomere length and telomerase activity provides an early marker for cellular senescence[24].
However, no studies have been reported yet on the possible relationship between telomerase activity and ASC damaged by stress or isolated from elderly donor. Therefore, it would be interesting to investigate whether this type of DNA damage influences the biological features of ASC isolated from individual of different ages in relation to their possible therapeutical application.
p38 MAPK
The protein p38 MAPK mediates important intracellular mechanisms. It is strongly activated by stress, playing an important role also in immune response, and in the regulation of cell survival, differentiation and apoptosis induction[25]. p38 MAPK, also known as RK, CSBP and SAPK2a, was initially described as a 38-kDa protein mediating the inflammatory response of several cytokines. Overall, the role of p38 MAPK signalling in cellular responses is complex, depending upon stimulus and cell types.
The stress activated p38 MAPK may contribute to cellular senescence by up-regulating p16INK4A, possibly through an indirect mechanism[26]. Additionally, a role for p38 MAPK in aging process has been implied in several cell types, including haematopoietic stem cells[27], MSC as well as epithelial stem/progenitor cells[28]. Indeed, the regulation of proliferation, survival and differentiation of normal haematopoietic stem cells by different cytokines and growth factors occurs via p38 MAPK signalling[28].
It should be highlighted that among stem cell regulators, Wnt and Notch refer to intracellular signalling which are not completely elucidated. Conversely, p38 MAPK, which lie downstream to those regulators, is able to promote adult neural differentiation by activating transcription factors such as transforming growth factor-β (TGF-β). Through this pathway p38 MAPK is also involved in retinal ganglion cell differentiation and neuronal development[29].
Given that ASC/BM-MSC are important for nerve regeneration, the attention has been focused on the role of p38 MAPK in undifferentiated (uASC and uBM-MSC) and differentiated types (dASC and dBM-MSC) of mesenchymal stem cells. In uASC isolated from animals of different ages (neonatal, young and old), p38 MAPK was present in all the groups of cells analyzed and its expression was increased with the aging of rats[9]. This result strengthens the hypothesis that the cellular senescence may be linked to the age of the donor. Interestingly, in the neonatal dASC there are significant differences in p38 expression levels when compared to cells from young/old rats, but the difference is not significant when compared cells from the young to the old rat groups. It was suggested that the last absence of effect may result from the compensatory action of fibroblast growth factor type 2 (FGF2) and platelet-derived growth factor (PDGF), which were used for feeding and stem cells differentiation in culture[30].
In conclusion, the mechanisms at the base of p38 MAPK regulation of cellular senescence is likely to be complex and it is still an open to debate. For example, it has been recently shown that p38 MAPK inhibits Sirtuin-1[31], whose involvement in aging process is becoming increasingly important.
p53 MAPK
Many studies have demonstrated that senescence and apoptosis share the signalling pathway p53 MAPK as a common regulator. It plays an important role in activating genes that arrest cell proliferation, or it facilitates those genes that are involved in apoptosis[32]. As with p38 MAPK, p53 MAPK is also activated by various stimuli, such as stress, DNA damage or inappropriate expression of oncogenes, which can lead to tumorigenesis and cell apoptosis[33]. p53 MAPK activation may also depend on other factors, such as the cell type, the different p53 MAPK post-translational modifications or the recruitment of different co-factors. There are emerging data indicating that p53 MAPK and NF-ĸB signalling are linked to each other, and this relationship is important in the regulation of cellular senescence and aging process[34].
p53 MAPK is normally expressed at low levels in the cells due to the action of an ubiquitin ligase called MDM2, which promotes p53 MAPK degradation. When the binding between p53 MAPK and MDM2 is disrupted by adverse stimuli, the accumulation of p53 MAPK in the nucleus modifies the expression of genes inducing either apoptosis or cell cycle arrest. This process can be transient (quiescent) or permanent (senescence). For these reasons, p53 MAPK is so-called “guardian of the genome” or “a key regulator of cell death”[35]. In line with this correlation, the deactivation of the mammalian target of rapamycin (mTOR) prevented senescence, causing cellular quiescence[36]. Different studies have shown that there is a cross-talk between p53 MAPK and mTOR signalling pathways. Nevertheless, Blagosklonny and colleagues[37] noted that p53 MAPK induction does not always lead to cellular senescence, thus questioning the role of p53 MAPK in the senescence. Indeed, this study indicates that p53 MAPK suppresses senescence and promotes cellular quiescence, in a way that the cells remain in a state of transient cell arrest induction.
In vivo experiments have shown that p53 MAPK is involved in cell differentiation as well as in cell-fate decisions that occur during development of the nervous system. A few studies have shown that p53 MAPK reaches a maximum level of mRNA expression during embryonic development of several tissues, including early neuronal precursor cells in the mouse brain[38].
Since the efficiency of p53 MAPK-mediated responses to cellular stresses was shown to be decreased during aging[39], it has been hypothesized that p53 MAPK signalling lessenes the aging process of an organism. In line with this hypothesis, and considering the involvement of p53 MAPK in MSC, we expected that in neonatal, young and old uMSC, the expression of p53 MAPK changes with aging. Despite a slight increase of p53 MAPK expression was observed in dASC and dMSC, its expression in the undifferentiated cells does not change. Based on this concept, we suggested that possible mutations of p53 MAPK are implicated in age-related MSC transformation, either in the self-renewal and differentiation of MSC[9]. Hence, the pharmacological modulation of p53 MAPK signalling may represent a target and a challenge for the application of ASC and MSC in nerve regenerative medicine, although this would require further investigations.
The mitochondria
Mitochondria are central organelles of the cells that during normal activity supply energy in the form of ATP. They also synthesize lipids and produce unstable reactive oxygen species (ROS)[40]. Therefore, the number of mitochondria in a cell is determined by the specific cell function and by its energy needs. Cells such as highly metabolic heart muscle cells have numerous mitochondria, whereas red blood cells have none[41]. Mitochondria are also involved in the process of cell death and apoptosis. In a model of mitochondrial participation to apoptosis, Bcl-2 family members (Bax and Bak) have been shown to control this process by regulating mitochondrial morphogenetic pathways[42]. Based on the correlation between aging and apoptosis, mitochondria may assume an important role in development of aging.
In addition to the their traditionally described anabolic/catabolic roles, mitochondria appear to regulate a variety of cellular processes, such as cell proliferation and aging in many cell types[43]. Generally, aging is characterized both by an impairment of the mitochondrial respiratory function and by an increased production of ROS, leading to a cumulative oxidative damage which may affect either the mitochondrion or other intracellular components[44,45].
According to the literature, many researchers are supporting the concept that intact mitochondrial function is crucial for the maintenance of stem cell pluripotency and renewal[46]. In particular, it has been suggested a strict correlation between DNA damage, p53 MAPK activation and mitochondrial dysfunction. Above all, p53 MAPK activation would impair mitochondrial function either directly and indirectly, increasing ROS levels and leading to DNA damage (Figure 1).
Figure 1.
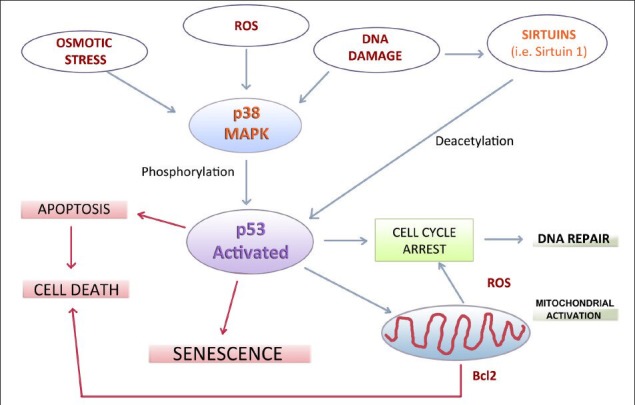
Schematic representation of the most important intracellular signalling pathways which correlate cellular senescence, cell death and apoptosis to environmental stimuli.
These mechanisms may be potentially relevant for the biology of adipose-derived stem cells and for their application in regenerative medicine. p38 MAPK: Protein 38 mitogen activated protein kinase; ROS: reactive oxygen species; Bcl2: B-cell lymphoma 2.
In ASC from old animals, most mitochondria were clustered around the nucleus, while in the neonatal animals mitochondria are homogenously distributed along the cells[9]. The aggregation of mitochondria in the perinuclear region of post-mitotic cells, observed in apoptosis and in necrosis[47] may be due to a variety of reasons. As a consequence of these structural changes, the diminished expression of the respiratory chain complex results in a reduction of membrane potential[48]. Moreover, the reduced mitochondrial fusion and their perinuclear clustering, observed in old animals, might indicate a decreased energy production in aging cells. Interestingly, a study performed in primate adult ASC reported that low-passage cell cultures, containing a high proportion of undifferentiated stem cells, show significant perinuclear clustering of mitochondria when compared to late-passage cells[49]. Additionally, a study conducted on embryonic stem cell undergoing differentiation has shown high level of mitochondrial activity, which may be due to mitochondrial DNA replication[50]. We can speculate that the degree of mitochondrial activity in adult stem cells may also be strongly dependent upon the target lineages into which these cells differentiate.
Altogether, the differences observed among these studies may be ascribed to the age of the donor tissues from which the stem cells were derived. In agreement with this hypothesis, our preliminary data[9] suggested a correlation between mitochondrial functions and age-related origin of ASC/MSC.
The Sirtuins
Sirtuins are important molecules correlated to the aging process, as well as to metabolism, stress tolerance, apoptosis and inflammation in several organisms[51,52]. In mammalian cells, Sirtuins are a group of NAD-deacetylases-dependent proteins that have been defined as Sirtuin 1, 2, 3, 6, and possibly 5 and 7. Sirtuins regulate the circadian clocks and the mitochondrial biogenesis, as well as controlling the cell energy efficiency and alertness during low-calorie situations[53,54]. Moreover, it has been demonstrated that Sirtuins can influence adipocyte, myocyte and neuronal differentiation through the activation of different signalling pathways such as p53 MAPK, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and NF-κB,[55]. Notably, Sirtuin1 can decrease p53 MAPK activity, or stimulate mitochondrial biogenesis through PGC-1α activation[56]. Although this molecular mechanism correlating p53 and mitochondrial dysfunction is supported by in vitro and in vivo studies, some aspects remain to be elucidated. Also, the inhibitory effect of p38 MAPK on Sirtuin 1[31] in regulating cellular senescence needs further investigation. Recently, it has been reported that Sirtuins induce a neuroprotective effect in acute and chronic neurological diseases[57]. Unfortunately, there are no recent studies reporting the role of Sirtuins in peripheral nerve regeneration.
Conclusions
The changes in stem cell functions, related to aging can be attributed to a decline in the effectiveness of regenerative capability and to environmental cues. This determines a lack of response of stem cells to extrinsic signals, so that they are unable to participate in the tissue repair processes. In this review, we presented some of the most important intracellular signalling pathways that challenge and regulate cellular senescence.
Many studies have addressed questions relating to stem cell rejuvenation and their protection from insults, although other studies showed that age does not affect the ability of ASC to support regeneration following an injury[58,59]. Altogether these data emphasize the complexity and controversy of the mechanisms regulating stem cell senescence.
Given that ASC are important for peripheral nervous system regeneration, the study of all the factors described in this reviews is of particular relevance and fundamental for setting the protocols to apply for stem cells therapy.
Acknowledgments:
We are grateful to Willan A for paper revision. We also acknowledge Fondazione Cariplo grant no. 2010-0501 for financial support.
Footnotes
Conflicts of interest: None declared.
Copyedited by Li CH, Song LP, Liu WJ, Zhao M
References
- [1].Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- [2].Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- [3].Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- [4].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- [5].Loeffler M, Bratke T, Paulus U, et al. Clonality and life cycles of intestinal crypts explained by a state dependent stochastic model of epithelial stem cell organization. J Theor Biol. 1997;186(1):41–54. doi: 10.1006/jtbi.1996.0340. [DOI] [PubMed] [Google Scholar]
- [6].Rubin GM, Yandell MD, Wortman JR, et al. Comparative genomics of the eukaryotes. Science. 2000;287(5461):2204–2215. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].di Summa PG, Kingham PJ, Raffoul W, et al. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010;63(9):1544–1552. doi: 10.1016/j.bjps.2009.09.012. [DOI] [PubMed] [Google Scholar]
- [8].Tohill M, Terenghi G. Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol Appl Biochem. 2004;40(Pt 1):17–24. doi: 10.1042/BA20030173. [DOI] [PubMed] [Google Scholar]
- [9].Mantovani C, Raimondo S, Haneef MS, et al. Morphological, molecular and functional differences of adult bone marrow- and adipose-derived stem cells isolated from rats of different ages. Exp Cell Res. 2012;318(16):2034–2048. doi: 10.1016/j.yexcr.2012.05.008. [DOI] [PubMed] [Google Scholar]
- [10].Kingham PJ, Kalbermatten DF, Mahay D, et al. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207(2):267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- [11].Brohlin M, Mahay D, Novikov LN, et al. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci Res. 2009;64(1):41–49. doi: 10.1016/j.neures.2009.01.010. [DOI] [PubMed] [Google Scholar]
- [12].Bharali LA, Lisney SJ. Success of regeneration of peripheral nerve axons in rats after injury at different postnatal ages. J Neurol Sci. 1990;100(1-2):203–210. doi: 10.1016/0022-510x(90)90034-k. [DOI] [PubMed] [Google Scholar]
- [13].Kovacic U, Zele T, Mars T, et al. Aging impairs collateral sprouting of nociceptive axons in the rat. Neurobiol Aging. 2010;31(2):339–350. doi: 10.1016/j.neurobiolaging.2008.03.020. [DOI] [PubMed] [Google Scholar]
- [14].Troy CM, Brown K, Greene LA, et al. Ontogeny of the neuronal intermediate filament protein, peripherin, in the mouse embryo. Neuroscience. 1990;36(1):217–237. doi: 10.1016/0306-4522(90)90364-a. [DOI] [PubMed] [Google Scholar]
- [15].Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- [16].Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech Ageing Dev. 2008;129(7-8):467–474. doi: 10.1016/j.mad.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8(9):703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- [18].Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132(4):681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- [19].Hayflick L. New approaches to old age. Nature. 2000;403(6768):365. doi: 10.1038/35000303. [DOI] [PubMed] [Google Scholar]
- [20].Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [21].Rebbaa A, Zheng X, Chou PM, et al. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene. 2003;22(18):2805–2811. doi: 10.1038/sj.onc.1206366. [DOI] [PubMed] [Google Scholar]
- [22].Kiyono T, Foster SA, Koop JI, et al. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396(6706):84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- [23].Sowa Y, Imura T, Numajiri T, et al. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev. 2012;21(11):1852–1862. doi: 10.1089/scd.2011.0403. [DOI] [PubMed] [Google Scholar]
- [24].Belair CD, Yeager TR, Lopez PM, et al. Telomerase activity: a biomarker of cell proliferation, not malignant transformation. Proc Natl Acad Sci U S A. 1997;94(25):13677–13682. doi: 10.1073/pnas.94.25.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].De Paula D, Bentley MV, Mahato RI. Effect of iNOS and NF-kappaB gene silencing on beta-cell survival and function. J Drug Target. 2007;15(5):358–369. doi: 10.1080/10611860701349695. [DOI] [PubMed] [Google Scholar]
- [26].Bulavin DV, Fornace AJ., Jr p38 MAP kinase's emerging role as a tumor suppressor. Adv Cancer Res. 2004;92:95–118. doi: 10.1016/S0065-230X(04)92005-2. [DOI] [PubMed] [Google Scholar]
- [27].Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12(4):446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- [28].Oeztuerk-Winder F, Ventura JJ. The many faces of p38 mitogen-activated protein kinase in progenitor/stem cell differentiation. Biochem J. 2012;445(1):1–10. doi: 10.1042/BJ20120401. [DOI] [PubMed] [Google Scholar]
- [29].Walshe TE, Connell P, Cryan L, et al. Microvascular retinal endothelial and pericyte cell apoptosis in vitro: role of hedgehog and Notch signaling. Invest Ophthalmol Vis Sci. 2011;52(7):4472–4483. doi: 10.1167/iovs.10-7061. [DOI] [PubMed] [Google Scholar]
- [30].Baron W, Metz B, Bansal R, et al. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci. 2000;15(3):314–329. doi: 10.1006/mcne.1999.0827. [DOI] [PubMed] [Google Scholar]
- [31].Hong EH, Lee SJ, Kim JS, et al. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2010;285(2):1283–1295. doi: 10.1074/jbc.M109.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jackson JG, Pereira-Smith OM. p53 is preferentially recruited to the promoters of growth arrest genes p21 and GADD45 during replicative senescence of normal human fibroblasts. Cancer Res. 2006;66(17):8356–8360. doi: 10.1158/0008-5472.CAN-06-1752. [DOI] [PubMed] [Google Scholar]
- [33].Batchelor E, Loewer A, Mock C, et al. Stimulus-dependent dynamics of p53 in single cells. Mol Syst Biol. 2011;7:488. doi: 10.1038/msb.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Le Guezennec X, Bulavin DV. WIP1 phosphatase at the crossroads of cancer and aging. Trends Biochem Sci. 2010;35(2):109–114. doi: 10.1016/j.tibs.2009.09.005. [DOI] [PubMed] [Google Scholar]
- [35].Efeyan A, Serrano M. p53: guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007;6(9):1006–1010. doi: 10.4161/cc.6.9.4211. [DOI] [PubMed] [Google Scholar]
- [36].Demidenko ZN, Zubova SG, Bukreeva EI, et al. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8(12):1888–1895. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- [37].Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012;4(3):159–165. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schmid P, Lorenz A, Hameister H, et al. Expression of p53 during mouse embryogenesis. Development. 1991;113(3):857–865. doi: 10.1242/dev.113.3.857. [DOI] [PubMed] [Google Scholar]
- [39].Feng Z, Hu W, Teresky AK, et al. Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci U S A. 2007;104(42):16633–16638. doi: 10.1073/pnas.0708043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52(3-5):159–164. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- [41].Zhang ZW, Cheng J, Xu F, et al. Red blood cell extrudes nucleus and mitochondria against oxidative stress. IUBMB Life. 2011;63(7):560–565. doi: 10.1002/iub.490. [DOI] [PubMed] [Google Scholar]
- [42].Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6(8):657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- [43].McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16(14):R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- [44].Navarro A, Torrejon R. Role of nitric oxide on mitochondrial biogenesis during the ovarian cycle. Front Biosci. 2007;12:1164–1173. doi: 10.2741/2134. [DOI] [PubMed] [Google Scholar]
- [45].Barja G. Free radicals and aging. Trends Neurosci. 2004;27(10):595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [46].Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464(7288):520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bereiter-Hahn J, Vöth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994;27(3):198–219. doi: 10.1002/jemt.1070270303. [DOI] [PubMed] [Google Scholar]
- [48].Jendrach M, Pohl S, Vöth M, et al. Morpho-dynamic changes of mitochondria during ageing of human endothelial cells. Mech Ageing Dev. 2005;126(6-7):813–821. doi: 10.1016/j.mad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- [49].Lonergan T, Brenner C, Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol. 2006;208(1):149–153. doi: 10.1002/jcp.20641. [DOI] [PubMed] [Google Scholar]
- [50].St John JC, Facucho-Oliveira J, Jiang Y, et al. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update. 2010;16(5):488–509. doi: 10.1093/humupd/dmq002. [DOI] [PubMed] [Google Scholar]
- [51].Calvanese V, Lara E, Suárez-Alvarez B, et al. Sirtuin 1 regulation of developmental genes during differentiation of stem cells. Proc Natl Acad Sci U S A. 2010;107(31):13736–13741. doi: 10.1073/pnas.1001399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rodriguez RM, Fraga MF. Aging and cancer: are sirtuins the link? Future Oncol. 2010;6(6):905–915. doi: 10.2217/fon.10.57. [DOI] [PubMed] [Google Scholar]
- [53].Satoh A, Brace CS, Ben-Josef G, et al. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010;30(30):10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Preyat N, Leo O. Sirtuin deacylases: a molecular link between metabolism and immunity. J Leukoc Biol. 2013;93(5):669–680. doi: 10.1189/jlb.1112557. [DOI] [PubMed] [Google Scholar]
- [55].Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008;20(3):303–309. doi: 10.1016/j.ceb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Guarente L. Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132(2):171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang F, Wang S, Gan L, et al. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011;95(3):373–395. doi: 10.1016/j.pneurobio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Morrison EE, Costanzo RM. Regeneration of olfactory sensory neurons and reconnection in the aging hamster central nervous system. Neurosci Lett. 1995;198(3):213–217. doi: 10.1016/0304-3940(95)11943-q. [DOI] [PubMed] [Google Scholar]
- [59].Luo G, Long J, Zhang B, et al. Developmental plasticity of stem cells and diseases. Med Hypotheses. 2010;75(6):507–510. doi: 10.1016/j.mehy.2010.07.007. [DOI] [PubMed] [Google Scholar]


