Summary
Dendritic cells (DCs) are professional antigen (Ag)-presenting cells capable of inducing immune responses to tumor Ags and, therefore, play a central role in the induction of antitumor immunity. There is a large amount of evidence, however, about paucity of tumor-associated DCs and that DCs’ immunogenic functions are suppressed in a tumor environment. Here we describe a potent in situ vaccine targeting tumoral DCs in vivo. This vaccine comprised of an oncolytic adenovirus expressing RANTES (regulated upon activation, normally T expressed, and presumably secreted) (Ad-RANTES-E1A), enhanced tumor infiltration, and maturation of Ag-presenting cells in vivo. In this study, we show that intratumoral vaccinations with Ad-RANTES-E1A induced significant primary tumor growth regression and blocked metastasis formation in JC and E.G-7 murine tumor models. This vaccine recruited DCs, macrophages, natural killer cells, and CD8+ T cells to the tumor site, and thus enhanced Ag-specific cytotoxic T lymphocyte responses and natural killer cell responses. DCs purified from the Ad-RANTES-E1A–treated E.G-7 tumors secreted significantly higher levels of interferon-γ and interleukin-12, as compared with control groups and more efficiently enhanced CD8+ T-cell response. This in situ immunization strategy could be a potent antitumor immunotherapy approach for aggressive established tumors.
Keywords: oncolytic adenoviral vaccine, RANTES, tumor immunotherapy
Dendritic cells (DCs) play a pivotal role in antitumor immunity.1,2 These cells are known as the most potent antigen-presenting cells (APCs), uniquely capable of inducing immunity to self and foreign antigens (Ags).3 DCs can break tolerance to tumor Ags and induce tumor regression.4–6 After capture of Ags in the periphery, DCs mature and modulate their chemokine receptor profile, down-regulating CCR1 and CCR5, which recognize RANTES (regulated upon activation, normally T expressed, and presumably secreted) and other proinflammatory chemokines, and up-regulating CCR7, which directs cells into secondary lymphoid tissues.1,7 After stimulation, DCs migrate to secondary lymphoid organs to initiate immune responses.7
As DCs play a central role in the initiation of immune responses, a variety of immunotherapeutic strategies are based on activation and maturation of DCs ex vivo and their adoptive transfer to tumor-bearing recipients after pulsing the DCs with tumor Ags.8,9 However, cultured DCs have demonstrated poor homing to the lymph nodes, inconsistent processing and presentation of Ags, and, depending on DCs’ maturation status, the ability to either enhance or inhibit the generation of tumor Ag-specific immunity.10,11 Therefore, new therapeutic approaches for manipulation of host immune responses against tumors, such as stimulating DCs in vivo, may allow natural maturation and retention of the most potent DC functions and may represent more rational methods of immunotherapy.11
Several animal models have already demonstrated that chemokine expression within tumors can induce the migration of T cells and APCs to tumor sites and can mediate modest antitumor responses.12,13 Thus, local expression of chemokines can alter the types of cell present within tumors and modulate the immune responses in vivo. Once appropriate cells are mobilized, they can be further activated or expanded by the use of other potent immune stimulatory molecules, such as cytokines and costimulatory molecules. In the case of DCs, this allows for uptake and presentation of tumor Ags directly from established tumors, avoiding the need for defined Ags. Furthermore, the DCs can be activated at the tumor site through the coexpression of local costimulatory molecules,14 cytokines,15 and Toll-like receptor agonists.16,17
RANTES, also termed CCL5, is a proinflammatory C-C chemokine that exerts chemotactic activities on DCs,18 macrophages, monocytes,19 natural killer (NK) cells,20,21 leukocytes,22 and T cells23 by binding CCR1, CCR3, and CCR5 on cell surfaces.24 It was reported recently that, after RANTES stimulation, DCs respond by inducing an amplification cascade that results in the synthesis of several proinflammatory cytokines and maturation.25 Moreover, several studies have demonstrated that intratumoral expression of RANTES delays or inhibits tumor growth and enhances lymphocyte infiltration of tumors.26–28 Here we present an in situ vaccine with the ability to elicit tumor-specific immunity by intratumoral expression of mouse RANTES to recruit and stimulate the maturation of host APCs. Specifically, a recombinant oncolytic adenovirus expressing RANTES would be produced for intratumoral immunization of tumor-bearing mice, which would have several effects: (1) oncolytic adenoviral activity against local tumor, releasing a broad spectrum of tumor Ags; (2) RANTES-mediated induction of systemic antitumor responses by attraction of endogenous DCs to the site of tumor; and (3) facilitation of DC maturation through amplified local expression of proinflammatory cytokines. Consequently, maturing DCs will uptake tumor Ags, migrate into lymph organs or tissues, and prime tumor-specific naive T cells, resulting in the activation of systemic tumor Ag-specific immune responses. Our data demonstrate that such a vaccine is effective in inducing tumor-specific cellular immunity by recruiting myeloid DCs and macrophages to the tumor site.
MATERIALS AND METHODS
Cell Lines and Animals
JC murine mammary adenocarcinoma, CT26 murine colon carcinoma, EL-4 lymphoma, E.G-7 lymphoma [ovalbumin (OVA)-transfected clone derived from murine EL-4 lymphoma], B16 melanoma, human embryonic kidney cells (HEK-293), and Yac-1 cells were purchased from American Type Culture Collection Inc (Manassas, VA).
Four to 6-week-old Balb/c and C57BL/6J female mice used in this study were purchased from Harlan Laboratories Inc (Indianapolis, IN). All mice were housed in a specific pathogen-free rodent facility at Baylor College of Medicine (Houston, TX).
Generation of the Recombinant Adenoviral Vectors
Generation and evaluation of the Ad-E1A pShuttle transfer vector (Fig. 1A) was previously described.29 To generate recombinant oncolytic adenovirus encoding mouse RANTES, that is Ad-RANTES-E1A, a pShuttle transfer vector carrying RANTES and E1A expression cassette under the cytomegalovirus promoter was constructed by transcriptionally linking the RANTES gene to the E1A gene via an encephalomyocarditis virus internal ribosome entry site (IRES) (Fig. 1A). Mouse RANTES cDNA was amplified by polymerase chain reaction (PCR) using the primer sets, 5′-AAT TAA AGA TCT ATG AAG GTC TCC GCG GCA-3′ and 5′-TAT ATT GTC GAC CTA GCT CAT CTC CAA AGA-3′, using mouse splenic cDNA pool as a template. The resulting cDNA was 276 bp and had sticky 5′ BglII/SalI ends. IRES of encephalomyocarditis virus was amplified with the primer set 5′-AAT TAA GTC GAC GCC CCT CTC CCT CCC CCC-3′ and 5′-TAT ATT GCG GCC GCT GTG GCC ATA TTA TCA TC-3′ on pIRES template (BD Biosciences, Palo Alto, CA). The resulting cDNA was 610 bp and had sticky 5′ SalI/NotI ends. RANTES and IRES PCR products were inserted into the Ad-E1A pShuttle vector by ligation of 3 fragments with cohesive ends. The sequences of the inserts in the plasmid were confirmed by sequencing.
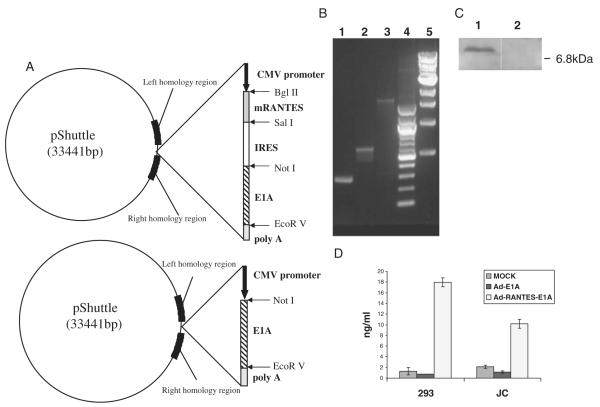
FIGURE 1.
Construction and characterization of Ad-RANTES-E1A and Ad-E1A vectors. A, Schematic representation of pShuttle vectors is shown. IRES indicates internal ribosome entry site; mRANTES, mouse regulated upon activation, normally T expressed, and presumably secreted. B, The integrity of the RANTES, IRES, and E1A in the produced adenovirus was verified by PCR on viral DNA template. Lane 1, PCR product of mRANTES (276 bp); lane 2, PCR product for IRES (610 bp); lane 3, PCR product for E1A (2100 bp); lanes 4 and 5, 100 bp ladder and 1 kb ladder (New England Biolabs), respectively. C, In vitro expression of RANTES. Western blot analysis of cell extracts from JC cells infected with Ad-RANTES-E1A (lane 1) and Ad-E1A (lane 2). Western blots were performed as described in Materials and Methods. Molecular weight in kDa is indicated on the right and the RANTES band (~7.9 kDa) is indicated by the arrow on the left. D, Secretion of RANTES from the transduced cells by the recombinant adenoviruses was quantitated by ELISA in the supernatants of 293 HEK and JC tumor cells. ELISA indicates enzyme-linked immunosorbent assay; HEK, human embryonic kidney cell; PCR, polymerase chain reaction.
Homologous recombination and subsequent amplification of the recombinant viruses were carried out in HEK-293 cells according to the manufacturer’s instructions (Qbiogene Inc, Carlsbad, CA). Final stocks of the amplified recombinant virus Ad-RANTES-E1A and Ad-E1A were titrated by using the Adeno-X Rapid Titer Kit (BD Biosciences, Palo Alto, CA), and stored in −80°C.
Western Blot
Western blot was used to test RANTES expression in mammary carcinoma JC cells that support human adenovirus replication.30 Briefly, JC cells were infected with Ad-RANTES-E1A or Ad-E1A (negative control) for 48 hours. Cells were lysed in a buffer containing 1% Triton X-100, 150 mM NaCl, 50 mM tris-HCl (pH 7.4), and 1 × mammalian protease inhibitor cocktail (Sigma-Aldrich Inc, St. Louis, MO). Samples were electrophoresed in a 4% to 20% gradient sodium dodecyl sulfate polyacryl-amide gel electrophoresis gel and electrotransferred onto 0.22-μm nitrocellulose membrane (Hybond, Amersham Biosciences Inc, Sunnyvale, CA). Immunodetection was performed with rabbit anti-RANTES antibodies (Abs) (R&D Systems Inc, Minneapolis, MN) at 1 μg/ml (1:500) in phosphate buffered saline (PBS) with 0.1% Tween 20 (PBS-T) and 2.5% nonfat dried milk overnight at 4°C. The membrane was then incubated with antigoat IgG-horse-radish peroxidase (HRP)–conjugated Ab (Amersham Biosciences Inc, Sunnyvale, CA) diluted 1:5000 in PBS-T for 1 hour at room temperature. Chemiluminescent signal was detected with ECL Plus Western blotting detection kit (Amersham Biosciences Inc, Sunnyvale, CA).
Tumor Challenge
About 5 × 105 JC and E.G-7 tumor cells were inoculated subcutaneously in Balb/c or C57BL/6 mice, respectively. When tumors reached approximately 7 mm in diameter (7 d after tumor inoculation), mice were intratumorally injected with 1010 i.f.u. of Ad-RANTES-E1A, Ad-E1A, or PBS (mock control). The injections were repeated on days 8 and 14 after tumor inoculation. Tumor volumes were measured every 2 days with a caliper. Tumors were surgically resected when they grew larger than 10 mm in diameter to avoid unnecessary animal suffering (day 21 to 25 postinoculation). In rechallenge experiments for JC model, 105 JC cells were inoculated 2 days after the tumor resections.
DC Isolation
DCs were purified from the spleens and tumors following the protocol for mouse CD11c micromagnetic beads (Miltenyi Biotec Inc, Auburn, CA). Cells were >90% pure and cultured for 1 day before the assays in Roswell Park Memorial Institute (RPMI)-1640 with 10% fetal bovine serum, 20 ng/ml murine granulocyte macrophage-colony stimulating factor (Biosource International Inc, Camarillo, CA), and 20 ng/ml murine interleukin-4 (R&D Systems Inc, Minneapolis, MN). Purified DCs were CD11c+MHCII+ cells, as confirmed by flow cytometry.
Enzyme-linked Immunosorbent Assay
Production of RANTES by the recombinant adenovirus-transduced cells was quantified by enzyme-linked immunosorbent assay (ELISA) following the manufacturers’ instructions (R&D Systems Inc, Minneapolis, MN). Levels of mouse interleukin (IL)-2, interferon (IFN)-γ, and IL-12 in the supernatants of cultured cells and blood serum were measured by ELISA following the manufacturers’ instructions (BD Biosciences, Palo Alto, CA).
Immunohistochemistry
Freshly isolated organ or tumor tissue was embedded in OCT (Sakura Finetek USA Inc, Torrance, CA), snap frozen on dry ice, and stored at −20°C. Cryostat sections (5-μm thick) were air dried at room temperature, fixed in cold acetone (10 min), rinsed in PBS (pH 7.4), and the nonspecific binding was blocked by incubation with 1% goat serum (Vector Laboratories Ltd, Burlingame, CA). Sections were then stained for 1 hour in the presence of antimouse monoclonal Abs conjugated with the fluorochromes: CD11c-phycoerythrin (PE), CD86-fluorescein isothiocyanate (FITC), CD80-FITC, and CD40-FITC (BD Biosciences, Palo Alto, CA) diluted 1:50 in PBS.
Isolation of Peripheral Blood Lymphocytes and Tetramer Staining
Peripheral blood was collected by retroorbital bleeding of mice 10 days after the last adenoviral immunization. Lymphocytes were isolated using Histopaque-1083 gradient following the manufacturer’s instructions (Sigma-Aldrich, Steinheim, Germany). OVA-specific CD8+ T cells were detected by staining with SIINFEKL-H2-Kb murine tetramer-PE (Beckman Coulter Inc, Fullerton, CA) and anti–CD8-FITC (BD Biosciences, Palo Alto, CA) and flow cytometric analysis.
Flow Cytometric Analysis of Tumor Suspension Cells
Single-cell tumor suspensions were prepared by crushing tumors through a 70-μm pore nylon cell strainer (Falcon; BD Biosciences, Franklin Lakes, NJ) using a 3-mL syringe plunger and rinsing with cold PBS/5 mM ethylene diamine tetraacetic acid. Cells were pelleted by centrifugation at 4°C at 400 × g for 5 minutes, and then red blood cells were lysed in ammonium chloride cell lysis buffer for 5 minutes at room temperature. Cells were incubated with 0.5 μg of Fc block/106 cells (BD Biosciences, San Diego, CA). Antimouse CD11c-PE, CD11b-APC, and CD8-FITC (all from BD Biosciences, San Diego, CA) were used for staining the tumor suspension cells. NK cells and CD8+ T cells were detected using antimouse pan-NK cell Ab (clone DX5 from eBioscience, San Diego, CA) and antimouse CD8 Ab (BD Biosciences, San Diego, CA), respectively.
IFN-γ Enzyme-linked Immunosorbent Spot Assay
The enzyme-linked immunosorbent spot (EliSpot) assay was used to determine the frequency of T cells producing IFN-γ in response to tumor-specific stimulus. Briefly, EliSpot plates (Millipore, Bedford, MA) were coated with monoclonal anti–IFN-γ AN18 (Mabtech AB, Sweden) (75 ng/well) for overnight and then blocked with RPMI supplemented with 10% fetal bovine serum. Splenocytes (1 × 105) were cultured in the presence of JC or CT26 cell lysates (irrelevant control) for JC model or 10 μg/mL of OVA peptide (SIINFEKL) or TRP-2 peptide (irrelevant control) for E.G-7 model in the coated plate for 20 hours at 37°C. After wash, biotinylated antimouse IFN-γ was added into wells and then incubated for 2 hours. After another wash, HRP-conjugated avidin was added into the wells and incubated for 1 hour. Finally, spots were developed by the addition of HRP substrate (Vectastain ABC Kit from Vector Laboratories). After spot development, the plate was rinsed thoroughly with ddH2O and allowed to dry. Spots were analyzed by Zellnet Consulting Inc (Fort Lee, NJ).
Cytotoxic T Lymphocyte Cytotoxicity Assay
Cytotoxic T lymphocyte (CTL) assay was used to detect cytolytic activity of CD8+ T cells from differently treated mice. Briefly, splenocytes from differently treated mice were restimulated in vitro with JC tumor lysates at a splenocyte/tumor cell ratio of 2:1 (for JC tumor model) or 10 μg/mL OT-I peptide (for E.G-7 tumor model) in RPMI-1640 culture medium. JC and CT26 (for JC tumor model) or E.G-7 and EL-4 (for E.G-7 tumor model) were labeled with 150 μCi of sodium 51Cr chromate solution (Amersham International, Arlington Heights, IL) for 90 minutes. Different numbers of effector cells were incubated with a constant number of target cells (1 × 104/well) in 96-well U-bottom plates (200 μL/well) for 6 hours at 37°C. About 2 μg of monoclonal antimouse CD8α (clone 53-6.7 BD Biosciences, San Diego, CA) were added for blocking assays. An excess of Yac-1 cells was used for blocking the NK activity at a Yac-1:JC cell ratio of 50:1. The supernatants from triplicate cultures were collected, and the amount of 51Cr radioactivity released was measured by LS 6500 Multi-Purpose Scintillation Counter (Beckman Coulter Inc, Hialeah, FL). The percentage lysis was defined as (experimental release–spontaneous release)/(maximum release–spontaneous release) × 100. Maximum release was determined by cell lysis by 1% Triton X-100.
NK Assay
The splenocytes were cultured for 24 hours in RPMI-1640 culture medium with 20 U/mL of IL-2. Target cells Yac-1 and CT26 cells (irrelevant control) were labeled for 90 minutes at 37°C with 150 μCi of 51Cr. The splenocytes were cocultured with the labeled Yac-1 (104) or CT26 (104) cells in 96-well U-bottom plates at an effector-target ratio of 100:1, 50:1, 25:1, and 12:1. Plates were incubated for 6 hours at 37°C under 5% CO2. The supernatant from each well was harvested, the amount of released 51Cr radio-activity was measured, and percentage of specific lysis was calculated as described for the CTL assay. About 1.5 μg of monoclonal antimouse NK Ab (PK136) was added in each well for the NK blocking assay.
T-cell Proliferation Assay
DCs were purified by using mouse CD11c micro-magnetic beads (Miltenyi Biotec Inc, Auburn, CA) from E.G-7 tumors that were resected 48 hours after the intratumoral injections of adenoviruses. Graded numbers of CD11c+-enriched tumoral DCs were cocultured with 3 × 105 of purified naive CD8+ T cells in 96-well flat-bottom plates coated with antimouse CD3 (clone 17A2 BD Biosciences, San Diego, CA) for 3 days. In the last 16 hours, 3H-thymidine (1 μCi/well) was added into the cell culture to monitor the 3H-thymidine incorporation.
Statistical Analysis
All data are reported as mean ± SE. Statistical comparison was made using the 2-tailed Student t test, and a value of P<0.05 was accepted as significant.
RESULTS
Generation and Characterization of the Recombinant Oncolytic Adenovirus Expressing RANTES (Ad-RANTES-E1A)
The replication-competent E1B-deleted adenoviruses were found to selectively destroy tumor cells with minimal toxicity to normal cells.31,32 In our study, we generated the recombinant replication-competent E1B-deleted adenovirus Ad-RANTES-E1A by inserting an expression cassette consisting of murine RANTES cDNA transcriptionally linked to E1A via encephalomyocarditis virus IRES into the E1 and E3-deleted adenovirus (Fig. 1A). As a control for Ad-RANTES-E1A, we used the previously generated recombinant replication-competent adenovirus encoding E1A (Ad-E1A),29 which is E1B-deleted and resembles the E1B-deleted ONYX-015.33 The recombinant adenoviruses were produced29,34 and analyzed by PCR (Fig. 1B). The expression of RANTES in JC mammary carcinoma cells was verified by Western blot (Fig. 1C), and the secretion of RANTES from the Ad-RANTES-E1A–transduced cells was quantified by ELISA (Fig. 1D).
RANTES Expression at the Tumor Site Led to the Recruitment of APCs to the Tumor
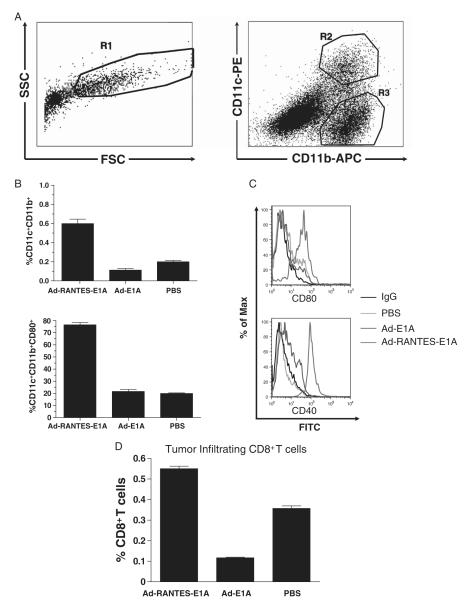
To determine whether RANTES expression in tumors enhances tumor infiltration of APCs, we injected JC tumor-bearing mice intratumorally with 1010 i.f.u. of Ad-RANTES-E1A, Ad-E1A, or PBS. Tumor-associated APCs (DCs and macrophages) were analyzed by flow cytometry 48 hours after the injections (Fig. 2A). As shown in Figure 2B and Supplementary Figure 1, intratumoral injection of Ad-RANTES-E1A resulted in a 3-fold increase in the percentage of tumoral CD11c+CD11b+DCs (cell count data are summarized in Supplementary Table 1) and CD11c− CD11b+ macrophages compared with the control groups. Interestingly, we found that the tumor-mobilized DCs were of myeloid origin, as they expressed both CD11c and CD11b (Fig. 2A). Moreover, the tumor-infiltrating DCs were mature, compared with the control groups, as they expressed significantly higher levels of surface costimulatory molecule CD80 and CD40 (Fig. 2B and C). The expression of CD80 was up-regulated on intratumoral CD11c− CD11b+ macrophages (Supplementary Fig. 1); however, the levels of its expression were significantly lower than on DCs, suggesting that DCs somehow are more responsive than macrophages to RANTES-induced in situ maturation. Thus, APCs’ recruitment to the tumor site was dependent on expression of RANTES by Ad-RANTES-E1A, but not owing to nonspecific adenovirus replication, as there was no significant difference in APC infiltration between Ad-E1A and PBS control group. In addition, significantly higher CD8+ T-cell infiltration was observed in the tumors of Ad-RANTES-E1A–treated mice (Fig. 2D).
FIGURE 2.
RANTES expression in the tumor site attracts the antigen-presenting cells into the tumor mass. A, 5 × 105 JC tumor cells were subcutaneously inoculated and the established tumors were injected with 1010 i.f.u. of Ad-RANTES-E1A, Ad-E1A, or PBS when they reached 5 to 7 mm in diameter. Tumors were established in 3 mice for each treatment group and processed separately. Forty-eight hours later, tumors were resected and total tumor single-cell suspensions (side and forward) scatter analyzed by flow cytometry depicted on left panel, APCs (gated as R1) were stained with antimouse CD11c, CD11b, and CD80 mAbs (right panel, flow cytometry results for CD11c and CD11b expression). DCs were gated as CD11c+ CD11b+ cells (gate R2); macrophages were gated as CD11c− CD11b+ (gate R3). Mean percentage of DCs (B, upper panel). Maturation status of DCs was determined by staining with monoclonal antibodies to mouse CD80 and CD40 [B (lower panel) and C]. D, Percentage of tumor infiltrating CD8+ T cells. Data presented for 3 mice. DC indicates dendritic cell; mAbs, monoclonal antibodies; PBS, phosphate buffered saline; RANTES, regulated upon activation, normally T expressed, and presumably secreted.
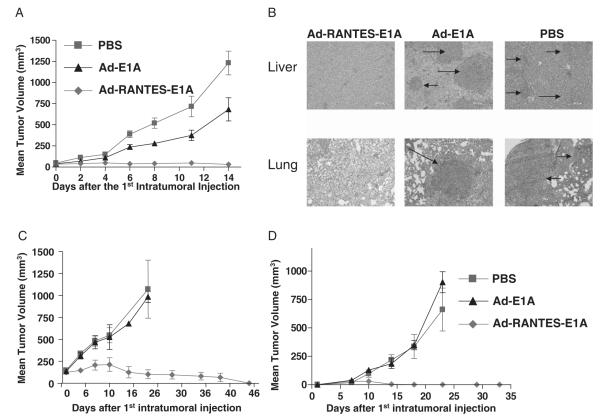
Intratumoral Administration of Ad-RANTES-E1A Induced Significant Tumor Regression and Suppression of Metastasis of JC Mammary Tumors
JC mammary carcinoma is a weakly immunogenic and highly metastatic mammary adenocarcinoma model.35 Treatment with adenovirus started when subcutaneously established tumors reached approximately 5 to 7 mm in diameter. Six mice of each group were intratumorally vaccinated 3 times (days 7, 8, and 14 after tumor inoculation) with 1010 i.f.u. of Ad-RANTES-E1A, Ad-E1A, or 50 μL of PBS (mock). Vaccination with Ad-RANTES-E1A significantly inhibited JC tumor growth, compared with vaccination with Ad-E1A or PBS (Fig. 3A). Mice were observed for 80 days after the final treatment. Rechallenge with JC cells 15 days after the treatment revealed the protective immunity in approximately 75% of Ad-RANTES-E1A–treated mice (data not shown). Tumor challenge and rechallenge experiments with the Ad-RANTES-E1A vaccine were repeated in 3 independent experiments.
FIGURE 3.
Treatment with Ad-RANTES-E1A eradicates established tumors and protects from metastasis. A, 5 × 105 JC cells were injected subcutaneously into the right flank of Balb/c mice. Groups of mice (N = 6) were injected intratumorally with 1 × 1010 i.f.u. of Ad-RANTES-E1A, Ad-E1A, or PBS on days 7, 8, and 14 after JC cell inoculation. Tumors were resected when they reached about 10 mm in diameter in control group (day 15 to 16 after the first adenoviral treatment). Error bars represent SEM. B, Mice were euthanized on day 21 after the first adenoviral injection. Organs from 3 mice of each group were collected and fixed in Bouin’s solution. Hematoxylin-eosin staining was performed on formalin-fixed paraffin-embedded lung and liver tissues from mice bearing JC breast tumors. Representative pictures of 9 sections (3 sections, 5-μm thick from 3 different mice of each group) at 20 × magnification. Upper panel, Liver sections; lower panel, lung sections. Arrows indicate JC metastatic tumor nodules. C, Parental E.G7 tumors were established SC into the right flank. Graph shows tumor growth in mice intratumorally injected with Ad-RANTES-E1A, Ad-E1A, and PBS as described above. D, Mice were inoculated with E.G-7 tumor cells (distant tumors) subcutaneously into the left flank before first treatment with adenovirus. Tumors were resected when they reached about 10 mm in diameter in PBS group (day 15 to 16 after the first adenoviral treatment). Error bars represent SE. PBS indicates phosphate buffered saline; RANTES, regulated upon activation, normally T expressed, and presumably secreted.
We then tested whether intratumoral treatment with Ad-RANTES-E1A could block metastasis formation. After tumor resections, mice were killed and their lungs and livers were collected for hematoxylin and eosin staining (Fig. 3B) and tumor nodule enumeration (Supplementary Fig. 2). qWe found that metastasis formation was suppressed in Ad-RANTES-E1A–treated mice, unlike Ad-E1A and mock-treated mice.
Intratumoral Administration of Ad-RANTES-E1A Induced Therapeutic Antitumor Immunity to E.G-7 Lymphoma
To determine whether the antitumor immunity induced by Ad-RANTES-E1A is not specific for JC tumors, we inoculated mice (6 mice in each treatment group) subcutaneously with E.G-7 tumors (5 × 105 cells). When tumors reached 5 to 7 mm (day 6 after first inoculation), we inoculated 5 × 105 E.G-7 cells into the contralateral flank (distant tumors), followed by 3 treatments of primary E.G-7 tumors with Ad-RANTES-E1A, Ad-E1A, or PBS (as described for JC model), and then monitored primary (Fig. 3C) and distant tumor growth (Fig. 3D). We found that intratumoral Ad-E1A and PBS injections did not inhibit primary and distant tumors’ growth. By contrast, injections with Ad-RANTES-E1A led to a significant regression of primary tumors and abrogation of distant tumor growth in 100% of experimental mice. Thus, Ad-RANTES-E1A induced systemic immune response against previously established, nontreated tumors.
CTLs and NK Cells Mediated the Antitumor Immunity Response Induced by Ad-RANTES-E1A Vaccination
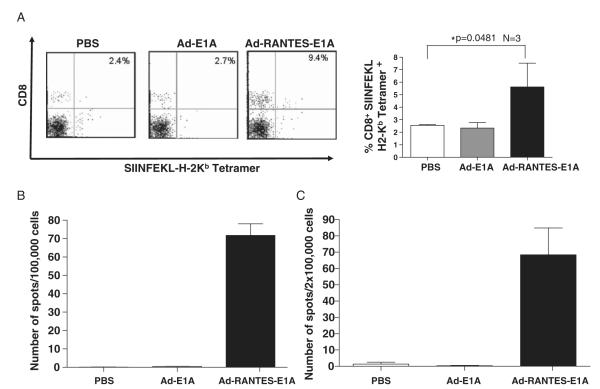
In mice treated with Ad-RANTES-E1A, expanded tumor-specific CTL populations were easily detected in the peripheral blood, as SIINFEKL-tetramer staining revealed a higher frequency of Ag-specific CTLs in the peripheral blood of Ad-RANTES-E1A–treated mice compared with Ad-E1A and PBS groups (Fig. 4A and Supplementary Fig. 3). The observation is consistent with tumor growth inhibition found in the E.G-7 model. Moreover, total percentages of CD8+ T cells were higher in both spleens and tumors of Ad-RANTES-E1A–treated mice compared with those from the Ad-E1A and PBS groups (Fig. 2D and data not shown). IFN-γ EliSpot assay further confirmed significantly higher frequency of tumor Ag-specific T cells in Ad-RANTES E1A–treated mice (compared with the control Ad-E1A and PBS groups) for both JC (Fig. 4B) and E.G-7 models (Fig. 4C). IFN-γ secretion was JC specific, as the splenocytes derived from JC-bearing mice treated with Ad-RANTES-E1A did not respond to CT26 stimulation (data not shown). In addition, splenocytes derived from E.G-7–bearing mice treated with Ad-RANTES-E1A did not secrete IFN-γ in response to TRP-2 peptide (negative control) stimulation.
FIGURE 4.
Expansion of antigen-specific CTLs in Ad-RANTES-E1A–treated mice. A, Peripheral blood was collected from the E.G-7 mice 10 days after the final adenoviral immunization. Left panel, Peripheral blood lymphocytes were stained with CD8-FITC and SIINFEKL-H2-Kb tetramer. The percentage of double-positive cells is indicated in the quadrants. Right panel, Percentage of tetramer-positive CD8+ T cells in the peripheral blood of 3 mice in each group. *P<0.05 between tetramer-positive CD8 T cells in the blood of Ad-RANTES-E1A–treated group compared with Ad-E1A and PBS groups. B, Spleens were harvested from the JC-bearing mice 10 days after final immunization and frequency of IFN-γ secreting T cells in response to JC lysates was measured by EliSpot assay. Millipore MultiScreen-HA plates were coated with antimouse IFN-γ Ab. Splenocytes (105 cells/well) were cultured for 20 hours at 37°C in a 5% CO2 incubator in the complete medium with tumor lysates of JC. C, Total splenocytes from E.G-7–bearing mice were prepared 10 days after the last immunization and cocultured with 10 μg/ml of OT-I peptide for 20 hours. The frequency of SIINFEKL-specific T cells was determined by EliSpot assay. The results were evaluated with an automated EliSpot reader system. The number of IFN-γ–producing lymphocytes was evaluated in triplicate wells. Average ± SD for each mouse was calculated and the assays were repeated twice. Ab indicates antibody; CTL, cytotoxic T lymphocyte; EliSpot, enzyme-linked immunosorbent spot; FITC, fluorescein isothiocyanate; IFN, interferon; PBS, phosphate buffered saline; RANTES, regulated upon activation, normally T expressed, and presumably secreted.
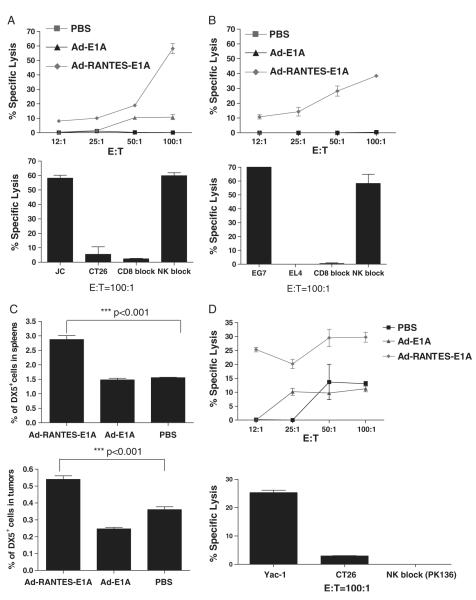
The expansion of tumor-specific CTLs was accompanied by enhanced antitumor cytotoxicity, as measured by the in vitro chromium release assay for both JC-bearing (Fig. 5A) and E.G-7–bearing mice (Fig. 5B). CTL activity was tumor Ag specific, as it was abrogated in response to irrelative control cell line CT26 for the JC model and EL-4 for the E.G-7 model (Fig. 5A, B, lower panel). These CTL responses were CD8-dependent and NK and CD4 cell-independent, as they were abolished in presence of CD8 neutralizing Ab (Fig. 5A, B, lower panels) and not changed in the presence of excess of Yac-1 cells and CD4 Ab (data not shown). Interestingly, the results of the IFN-γ EliSpot assay were less sensitive for JC-bearing mice, probably owing to soluble immunosuppressive factors present in the tumor lysates. Additional studies are needed to elucidate the underlying mechanism.
FIGURE 5.
CTL and NK cell responses were induced by Ad-RANTES-E1A intratumoral vaccination in JC and E.G-7 tumor models. Spleens were harvested from tumor-bearing mice. Splenocytes were tested for cytolytic activity in a standard 6-hour 51Cr-release assay. A, Upper panel, CTLs (1.5 × 106 cells) were stimulated with JC tumor lysates at a T cell:JC tumor cell ratio of 2:1 for 5 days. Target cells labeled with 51Cr were placed in each well of 96-well plates, and 50 μL of effector T cells for each dilution was added. The supernatant from each well was harvested, and the amount of 51Cr radioactivity released was measured in a γ counter. Lower panel, JC-specific CTL response in Ad-RANTES-E1A–treated mice. 51Cr-labeled CT26 colon carcinoma cells were used as syngeneic negative control. About 2 μg of monoclonal antimouse CD8 mAbs were added for the blocking assay; excess of Yac-1 cells was added to block NK cell activity (negative control). E:T ratio is 100:1. B, Spleens were harvested from E.G-7–bearing mice. Upper panel, CTLs were stimulated with 10 μg/mL of OT-I peptide for 5 days. E.G-7 target cells labeled with 51Cr were placed in each well of 96-well plates, and 50 μl of effector T cells for each dilution was added. The supernatant from each well was harvested, and the amount of 51Cr radioactivity released was measured in a γ counter. Lower panel, E.G-7–specific CTL response in Ad-RANTES-E1A–treated mice. 51Cr-labeled EL-4 cells were used as negative control. About 2 μg of monoclonal antimouse CD8 mAbs were added for the blocking assay; excess of Yac-1 cells was added to block NK cell activity; E:T ratio is 100:1. The assays were performed 3 times or more. C. Total spleen (upper panel) and tumor (lower panel) cell suspensions were stained with antimouse DX5+ Ab and percentage of DX5+ NK cells was evaluated by flow cytometry in 3 mice of each group. D, NK functional response was induced by Ad-RANTES-E1A intratumoral vaccination. Upper panel, splenocytes from Ad-RANTES-E1A, Ad-E1A, or PBS-treated mice were purified and cultured overnight in complete medium with 20 U/mL of IL-2. About 2 × 105 splenocytes were plated in 96-well plates and incubated with 51Cr labeled Yac-1 cells. Lower panel, NK cells from Ad-RANTES E1A mice were cocultured with 51Cr-labeled CT26 (negative control) and with antimouse NK blocking Ab (PK 136) for 6 hours at 37°C and 5% CO2. The assays were performed 3 times in triplicates. CTL indicates cytotoxic T lymphocyte; E:T, effector:target; IL, interleukin; mAbs, monoclonal antibodies; NK, natural killer; PBS, phosphate buffered saline; RANTES, regulated upon activation, normally T expressed, and presumably secreted.
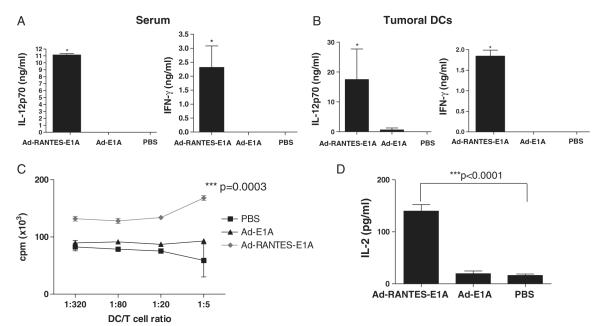
In addition, significantly higher (P<0.001) percentages of NK cells were detected in the spleens and tumors of Ad-RANTES-E1A–treated, EG-7–bearing mice when the tissues were analyzed 48 hours after intratumoral injections (Fig. 5C). This high percentage of NK cells correlated with enhanced NK lytic activity to Yac-1 cells, which was blocked by antimouse NK-neutralizing Ab (Fig. 5D). We also tested whether the enhanced activities of CD8+ T and NK cells in Ad-RANTES-E1A–treated mice were accompanied by higher levels of cytokines in the blood serum. Significant elevations of serum IFN-γ and IL-12 were detected for Ad-RANTES-E1A (Fig. 6A) mice compared with control groups.
FIGURE 6.
Elevated levels of IL-12 and IFN-γ in tumor infiltrating DCs and blood serum of Ad-RANTES-E1A–treated mice and high immunostimulatory function of tumor-infiltrating DCs. A, Blood serum was collected from 3 mice of each group (Ad-RANTES-E1A, Ad-E1A, and PBS) 5 days after the last adenoviral treatment. Levels of IL-12 and IFN-γ were measured by ELISA. B, Tumors were established subcutaneously into the right and left flanks of 6 mice of each treatment group. Forty-eight hours after the intratumoral adenoviral injections, tumors were resected and tumoral DCs were purified as described in Materials and Methods. Purified DCs were cultured overnight in a 96-well plate in triplicates and level of the secreted cytokines in DCs-conditioned medium was measured by ELISA. *P<0.05 between levels in Ad-RANTES-E1A group compared with Ad-E1A and PBS groups. C and D, E.G-7 tumors were established subcutaneously into the right and left flanks of 6 mice of each treatment group. Forty-eight hours after the intratumoral adenoviral injections, tumors were resected and tumoral DCs were purified as described in Materials and Methods. Graded numbers of CD11c+-enriched tumoral DCs cultured for 3 days in different culture were cocultured with 3 × 105 purified naive CD8+ T cells in the presence of anti-CD3 for 3 days. In the last 16 hours, 3H-thymidine (1 μCi/well) was added into the cell culture to monitor the T-cell proliferation (C) and IL-2 secretion (D) was measured 16 hours later. Results shown are representative of 3 different experiments. ***P<0.0001 and *P<0.05 for cytokine secretion and proliferation primed by tumoral DCs of Ad-RANTES-E1A–treated group compared with Ad-E1A and PBS groups. DC indicates dendritic cell; ELISA, enzyme-linked immunosorbent assay; IFN, interferon; IL, interleukin; PBS, phosphate buffered saline; RANTES, regulated upon activation, normally T expressed, and presumably secreted.
Thus, Ad-RANTES-E1A intratumoral injection induced both innate and adaptive immune responses, specifically NK and CD8+ T-cell immune responses, for its antitumor immunity.
Ad-RANTES-E1A Treatment Endowed Tumor-mobilized DCs With Superior Ability to Enhance CD8+ T-cell Responses
To explore whether the Ad-RANTES-E1A enhances function of tumor-infiltrating DCs, 48 hours after the intratumoral injections, tumor-infiltrating DCs were purified. Interestingly, Ad-RANTES-E1A not only significantly increased the levels of CD80, CD86, and CD40 costimulatory molecule on tumoral DCs (Fig. 2), but also enhanced production of IL-12 and IFN-γ by tumoral DCs (Fig. 6B). As a result, Ad-RANTES-E1A treatment boosted the capacity of tumor-mobilized DCs in promotion of CD8+ T-cell response, as CD8+ T cells exhibited higher proliferation (Fig. 6C) and more robust IL-2 secretion (Fig. 6D) when cocultured with tumor-infiltrating DCs from Ad-RANTES-E1A group compared with from Ad-E1A and PBS groups in the presence of anti-CD3. Tumoral DCs of all groups did not produce IL-2, as measured by ELISA (data not shown). These results suggest that Ad-RANTES-E1A enables tumor-infiltrating DCs to efficiently enhance CD8+ T-cell proliferation and cytokine production.
DISCUSSION
Until recently, tumors were considered as a nonrewarding target for immune therapy because of tumor immunosuppressive environments.11 It has now become clear that tumor cells do express tumor Ags and should be targeted for immunotherapeutic purposes. Some of these Ags are unique to the tumor, such as mutated36 or aberrantly processed (generated by peptide splicing) proteins,37 whereas other tumor Ags can be normally differentiated proteins that are overexpressed in tumor cells.38 Despite their potential antigenicity, most tumors grow, apparently without eliciting a strong immune response.
One primary reason for the failure of the immune system is suppression of tumor infiltrating APCs by the tumor environment.11 The ability to manipulate host DCs’ maturation status and functional activity at tumor sites is a new therapeutic strategy for enhancement of host immune responses against tumors. The migration of DCs and other immune cells is mediated by chemokines through a concentration-dependent process attracting cells to sites of chemokine secretion.39 Recent evidence suggests that chemokines induce motility, and possibly other proinflammatory and activating processes, through the selected expression of specific membrane receptors on immune cells.25 Several studies have shown that antitumor immune responses can be primed by attracting host DCs to the tumor site.12,13,16 However, access of DCs to tumor Ags is not sufficient to elicit potent antitumor immunity in all tumor models, especially in those that are weakly immunogenic and secrete immunosuppressive factors. It has been shown that the ability of the APCs to mature is critical for the induction of efficient antitumor immune responses. For example, in the study of Furumoto et al,16 CT26 colon carcinoma was easily eradicated by CCL20 intratumoral injections; however, B16 tumors, which produce high levels of transformation growth factor-β, were resistant to CCL20 treatment. Therefore, additional injection of CpG oligos was critical for stimulating DC maturation in the tumor milieu to prime a strong antitumor immunity.16
As described above, RANTES is a member of the intercrine or chemokine family of proinflammatory basic polypeptides.40 RANTES is a prototype of the C-C chemokine subfamily that acts as a selective chemoattractant of human monocytes, DCs, macrophages, and T lymphocytes.41,42 Recent studies reported that DCs selectively respond to RANTES by induction of chemokine and proinflammatory cytokine synthesis, and that RANTES is involved in the amplification cascade of proinflammatory cytokines in DCs, thus contributing to their maturation.25 Some studies, however, consider potential involvement of RANTES in cancer progression.43 On the basis of the promiscuous functions of RANTES in tumor immunology, we developed an oncolytic adenoviral vaccine expressing mouse RANTES. We tested whether intratumoral injection of the viral vaccine could elicit potent systemic antitumor immunity for control of tumor growth via recruitment of DCs and macrophages to the tumor site and promotion of these APC functions. We have found that the number and maturation status of tumoral APCs can be enhanced, and proinflammatory cytokine production by tumoral DCs can be potentiated by intratumoral RANTES expression. We further found that intratumoral injection of the viral vaccine elicits potent systemic CTL and NK immune responses and prevents tumor growth and metastasis in different tumor mouse models. Importantly, we show that intratumoral treatment with Ad-RANTES-E1A is protecting mice from growing distant tumors, implying this approach might be applicable to treatment of patients with metastatic disease. Our in vivo data is in line with previously reported in vitro studies, in which RANTES stimulated a cascade of proinflammatory cytokine production in DCs and contributed to their maturation status.25 Also, our data suggest that tumor-infiltrating APCs might play an important role in triggering antitumor immunity in Ad-RANTES-E1A–treated mice.
Tumor-infiltrating DCs from Ad-RANTES-E1A–treated mice not only expressed high levels of costimulatory molecules, but also secreted high level of IL-12 and IFN-γ. Moreover, elevated systemic levels of IL-12 and IFN-γ were detected in the serum of Ad-RANTES-E1A–treated mice compared with controls. As IL-12 and IFN-γ are 2 key factors in the induction of innate and adaptive immunity cells, such as T-helper 1 cells, CTLs, and NK cells,44,45 the unique features associated with the RANTES-mobilized DCs hint their role in facilitating expansion and activation of CTLs and NKs observed in Ad-RANTES-E1A–treated mice. To confirm this, we found that the tumoral DCs isolated from Ad-RANTES-E1A–treated mice were more potent in enhancing T-cell proliferation and boosting IL-2 secretion ex vivo triggered by anti-CD3. Furthermore, we also demonstrated that expression of RANTES attracts NK cells to the tumor site in vivo. As NK cells participate in adaptive immune responses, mainly by interacting with DCs,46 it is very likely that RANTES-mobilized APCs play a role in the attraction and activation of NK to tumor site. However, additional studies are necessary to find out whether the NK activation was facilitated via the Ad-RANTES-E1A directly or mediated by tumoral DCs.
The expression of RANTES at the tumor site preferentially mobilized the CD11c+CD11b+ myeloid subset of DCs (Fig. 2), and RANTES-mobilized DCs expressed high level of IL-12. It was recently reported that myeloid DCs specifically inhibit tumor neovascularization by secretion of IL-12.47 Thus, the mobilization of myeloid DCs secreting IL-12 at the tumor site by intratumoral injection of Ad-RANTES-E1A may contribute to tumor regression through the inhibition of tumor vasculogenesis. Additional studies are underway to understand the detailed molecular mechanism of tumor regression with intratumoral expression of RANTES.
Currently, oncolytic adenoviral vaccines show encouraging therapeutic results in clinical trials. Although promising, the limitations of in situ adenoviral treatment include preexisting immunity to adenovirus in human, restriction to patients with solid tumors, and lack of target cell specificity. In the present study, we reported development of a novel oncolytic adenoviral vaccine that specifically targets and activates tumor-infiltrating APCs without the need for ex vivo manipulation of APCs. The in situ, adenovirus-based vaccine combines the oncolytic activity of the adenovirus to release unique tumor Ags and the RANTES-chemokine–mediated attraction and activation of APCs. This vaccine, capable of inducing potent tumor-specific immunity mediated by expansion and activation of both innate and adaptive immune cells, represents an attractive strategy for tumor immunotherapy.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Wenhong Ren in the laboratory for technical assistance.
Supported by grants from the US National Institutes of Health (R01CA90427, R01CA116677, and R01AI68472 to S.-Y. C. and R01 CA100841 to X. F. H.) and US Army Prostate Cancer program (DOD W81XWH-04-1-0194 to X.F.H.).
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
Supplementary data are available online at: http://www.immunotherapy-journal.com.
REFERENCES
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003;51:59. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 4.Godelaine D, Carrasco J, Lucas S, et al. Polyclonal CTL responses observed in melanoma patients vaccinated with dendritic cells pulsed with a MAGE-3A1 peptide. J Immunol. 2003;171:4893. doi: 10.4049/jimmunol.171.9.4893. [DOI] [PubMed] [Google Scholar]
- 5.Kugler A, Stuhler G, Walden P, et al. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 6.Thurner B, Haendle I, Roder C, et al. Vaccination with MAGE-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caux C, Ait-Yahia S, Chemin K, et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- 8.Figdor CG, de Vries IJ, Lesterhuis WJ, et al. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 9.Reichardt VL, Brossart P, Kanz L. Dendritic cells in vaccination therapies of human malignant disease. Blood Rev. 2004;18:235. doi: 10.1016/j.blre.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Soruri A, Zwirner J. Dendritic cells: limited potential in immunotherapy. Int J Biochem Cell Biol. 2005;37:241. doi: 10.1016/j.biocel.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman HL, Disis ML. Immune system versus tumor: shifting the balance in favor of DCs and effective immunity. J Clin Invest. 2004;113:664. doi: 10.1172/JCI21148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crittenden M, Gough M, Harrington K, et al. Expression of inflammatory chemokines combined with local tumor destruction enhances tumor regression and long-term immunity. Cancer Res. 2003;63:5505. [PubMed] [Google Scholar]
- 13.Giovarelli M, Cappello P, Forni G, et al. Tumor rejection and immune memory elicited by locally released LEC chemokine are associated with an impressive recruitment of APCs, lymphocytes, and granulocytes. J Immunol. 2000;164:3200. doi: 10.4049/jimmunol.164.6.3200. [DOI] [PubMed] [Google Scholar]
- 14.Chiodoni C, Paglia P, Stoppacciaro A, et al. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med. 1999;190:125. doi: 10.1084/jem.190.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driessens G, Hamdane M, Cool V, et al. Highly successful therapeutic vaccinations combining dendritic cells and tumor cells secreting granulocyte macrophage colony-stimulating factor. Cancer Res. 2004;64:8435. doi: 10.1158/0008-5472.CAN-04-0774. [DOI] [PubMed] [Google Scholar]
- 16.Furumoto K, Soares L, Engleman EG, et al. Induction of potent antitumor immunity by in situ targeting of intratumoral DCs. J Clin Invest. 2004;113:774. doi: 10.1172/JCI19762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chicoine MR, Won EK, Zahner MC. Intratumoral injection of lipopolysaccharide causes regression of subcutaneously implanted mouse glioblastoma multiforme. Neurosurgery. 2001;48:607. doi: 10.1097/00006123-200103000-00032. [DOI] [PubMed] [Google Scholar]
- 18.Dieu-Nosjean MC, Vicari A, Lebecque S, et al. Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines. J Leukoc Biol. 1999;66:252. doi: 10.1002/jlb.66.2.252. [DOI] [PubMed] [Google Scholar]
- 19.Wang JM, McVicar DW, Oppenheim JJ, et al. Identification of RANTES receptors on human monocytic cells: competition for binding and desensitization by homologous chemotactic cytokines. J Exp Med. 1993;177:699. doi: 10.1084/jem.177.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taub DD, Sayers TJ, Carter CR, et al. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877. [PubMed] [Google Scholar]
- 21.Maghazachi AA, al-Aoukaty A, Schall TJ. C-C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. Role for G proteins. J Immunol. 1994;153:4969. [PubMed] [Google Scholar]
- 22.Kameyoshi Y, Dorschner A, Mallet AI, et al. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taub DD, Lloyd AR, Wang JM, et al. The effects of human recombinant MIP-1 alpha, MIP-1 beta, and RANTES on the chemotaxis and adhesion of T cell subsets. Adv Exp Med Biol. 1993;351:139. doi: 10.1007/978-1-4615-2952-1_15. [DOI] [PubMed] [Google Scholar]
- 24.Raport CJ, Gosling J, Schweickart VL, et al. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1 beta, and MIP-1 alpha. J Biol Chem. 1996;271:17161. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 25.Fischer FR, Luo Y, Luo M, et al. RANTES-induced chemokine cascade in dendritic cells. J Immunol. 2001;167:1637. doi: 10.4049/jimmunol.167.3.1637. [DOI] [PubMed] [Google Scholar]
- 26.Mulé JJ, Custer M, Averbook B, et al. RANTES secretion by gene-modified tumor cells results in loss of tumorigenicity in vivo: role of immune cell subpopulations. Hum Gene Ther. 1996;7:1545–1553. doi: 10.1089/hum.1996.7.13-1545. [DOI] [PubMed] [Google Scholar]
- 27.Lavergne E, Combadière C, Iga M, et al. Intratumoral CC chemokine ligand 5 overexpression delays tumor growth and increases tumor cell infiltration. J Immunol. 2004;173:3755–3762. doi: 10.4049/jimmunol.173.6.3755. [DOI] [PubMed] [Google Scholar]
- 28.Inoue H, Iga M, Xin M, et al. TARC and RANTES enhance antitumor immunity induced by the GM-CSF-transduced tumor vaccine in a mouse tumor model. Cancer Immunol Immunother. 2008;57:1399–1411. doi: 10.1007/s00262-008-0476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang XF, Ren W, Rollins L, et al. A broadly applicable, personalized heat shock protein-mediated oncolytic tumor vaccine. Cancer Res. 2003;63:7321. [PubMed] [Google Scholar]
- 30.Wang Y, Hallden G, Hill R, et al. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol. 2003;21:1328. doi: 10.1038/nbt887. [DOI] [PubMed] [Google Scholar]
- 31.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 32.Heise C, Sampson-Johannes A, Williams A, et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 33.Freytag SO, Rogulski KR, Paielli DL, et al. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9:1323. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- 34.Ren W, Strube R, Zhang X, et al. Potent tumor-specific immunity induced by an in vivo heat shock protein-suicide gene-based tumor vaccine. Cancer Res. 2004;64:6645. doi: 10.1158/0008-5472.CAN-04-1084. [DOI] [PubMed] [Google Scholar]
- 35.Chao TY, Chu TM. Characterization of a new spontaneously developed murine mammary adenocarcinoma in syngeneic BALB/c hosts. In Vitro Cell Dev Biol. 1989;25:621. doi: 10.1007/BF02623632. [DOI] [PubMed] [Google Scholar]
- 36.Lurquin C, Van Pel A, Mariame B, et al. Structure of the gene of tum-transplantation antigen P91A: the mutated exon encodes a peptide recognized with Ld by cytolytic T cells. Cell. 1989;58:293. doi: 10.1016/0092-8674(89)90844-1. [DOI] [PubMed] [Google Scholar]
- 37.Vigneron N, Stroobant V, Chapiro J, et al. An antigenic peptide produced by peptide splicing in the proteasome. Science. 2004;304:587. doi: 10.1126/science.1095522. [DOI] [PubMed] [Google Scholar]
- 38.Houghton AN, Guevara-Patino JA. Immune recognition of self in immunity against cancer. J Clin Invest. 2004;114:468. doi: 10.1172/JCI22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 40.Oppenheim JJ, Zachariae CO, Mukaida N, et al. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 41.Schall TJ, Bacon K, Toy KJ, et al. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 42.Schall TJ, Jongstra J, Dyer BJ, et al. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141:1018. [PubMed] [Google Scholar]
- 43.Azenshtein E, Luboshits G, Shina S, et al. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62:1093. [PubMed] [Google Scholar]
- 44.Verweij CL, Geerts M, Aarden LA. Activation of interleukin-2 gene transcription via the T-cell surface molecule CD28 is mediated through an NF-kB-like response element. J Biol Chem. 1991;266:14179. [PubMed] [Google Scholar]
- 45.Langrish CL, McKenzie BS, Wilson NJ, et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 46.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 47.Curiel TJ, Cheng P, Mottram P, et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.