Abstract
Purpose
We investigated the outcome of suppression of renin angiotensin system (RAS) using Captopril combined with an antioxidant (EUKarion-207) for mitigation of radiation-induced lung damage in rats.
Materials and Methods
The thoracic cavity of female Sprague-Dawley (SD) rats was irradiated with single dose of 11 Gy. Treatment with Captopril at a dose of 40 mg/kg/day in drinking water and EUK-207 given by subcutaneous injection (8 mg/kg daily) was started 1 week (wk) post-irradiation (PI) and continuing until 14 wks PI. Breathing rate was monitored until the rats were sacrificed at 32 wks PI when lung fibrosis was assessed by lung hydroxyproline content. Lung levels of the cytokine, Transforming Growth Factor (TGF)-β1, and macrophage activation were analyzed by immunohistochemistry. Oxidative DNA damage was assessed by 8-hydroxy-2-deoxyguanosine (8-OHdG) levels and lipid peroxidation was measured by a T-BARS assay.
Results
The increase in breathing rate in the irradiated rats was significantly reduced by the drug treatments. The drug treatment also significantly decreased the hydroxyproline content, 8-OHdG and malondialdehyde levels, and levels of activated macrophages and the cytokine TGF-β1 at 32 wks. Almost complete mitigation of these radiation effects was observed by combining Captopril and EUK-207.
Conclusion
Captopril and EUK-207 can provide mitigation of radiation-induced lung damage out to at least 32 wks PI following treatment given 1–14 wks PI. Overall the combination of Captopril and EUK-207 was more effective than the individual drugs used alone.
Keywords: Radiation, Lung, Mitigation, Captopril, EUK-207
Introduction
There is significant concern regarding accidental overexposure to radiation but our knowledge of the best clinical management of such exposures remains uncertain1. Radiation injury to the lung is one of the major concerns due to its sensitivity. Mechanisms are still poorly understood, although several studies have implicated a prolonged inflammatory response, the upregulation of pro-inflammatory cytokines and reactive oxygen species (ROS), hypoxia and loss of alveolar epithelial cells as well as arterioles and influx of fibrocytes as major factors in causing pneumonitis and fibrosis in irradiated lung2–4. Effective measures to mitigate or treat such exposures are currently very limited and no agent has yet been approved by the US Federal Drug Agency (FDA) for "radiation mitigation" to lung when administered after radiation5–7.
Classes of drugs studied for protection/mitigation of radiation-induced late injuries include suppressors of the renin-angiotensin system (RAS) and anti-oxidants. Initial studies reported promise with RAS inhibitors as protectors8 and more recent work has reported that various angiotensin converting enzyme (ACE) inhibitors can also act as mitigators of radiation damage in lung4,9–11. Anti-oxidants have also shown promise as potential mitigators of radiation damage and acute pneumonitis can be partially mitigated with salen-Mn complexes such as the compound, EUK-207 with administration initiated at 1–2 weeks (wks) after radiation exposure12–14. Salen-Mn complexes are a class of synthetic low molecular weight agents that mimic the antioxidant enzymes superoxide dismutase (SOD) and catalase, scavenging superoxide and hydrogen peroxide, respectively15,16. EUK-207 is well-tolerated, suggesting low toxicity17. In this study, we examined the combination of the ACE inhibitor Captopril and EUK-207 in SD rats, a well-established model for radiation-induced lung damage. Captopril has widespread clinical use, is orally available and is well tolerated.
Materials and Methods
Animal treatments
Female SD rats (Charles River Laboratories International, Inc., Wilmington, MA, USA) aged 7–8 wks and weighing 150–160g were used in all experiments. They were housed in animal facilities accredited by the Canadian Council on Animal Care and treated in accordance with approved protocols. This strain was chosen for the study because they tend to show early increases in breathing rate associated with acute pneumonitis and robust radiation-induced fibrosis at late time points following irradiation13. The experimental groups of rats (8 rats per group) included an untreated control group maintained throughout the study, whole thoracic radiation alone (WTR), radiation with EUK-207 (WTR+EUK), radiation with Captopril (WTR+CAPT), radiation with Captopril+EUK-207 (WTR+Capt+EUK) and Captopril and EUK-207 without radiation (Capt+EUK). All surviving rats were euthanized at 32 weeks post irradiation (PI).
Drug treatments
Drug treatments were initiated 1 week PI and terminated 14 weeks PI. EUK-207 was custom-synthesized and characterized as described previously18. The animals received a dose of 8 mg/kg/day in saline by daily subcutaneous (sbc) injection as reported previously13. EUK-207 is water-soluble and given by sbc injection results in readily detectable plasma levels that persist for several hours19. Pharmaceutical-grade captopril (Sigma, USA) was dissolved in the drinking water at 300 mg/L, which is expected to deliver ~40 mg/kg/day or 236 mg/m2/day to a rat, a dose in the approved range for use of captopril in humans9.
Irradiation
The thoracic cavity in each animal was irradiated using an image-guided small animal-irradiator (CX-Rad-225, Precision X-ray Inc. USA). The description and calibration of this unit has been described previously13, 20. The dose rate at 225kVp, 13mA (HVL: 0.93mm Cu, added filtration: 0.3mm Cu) was estimated as 3.51 Gy/min at the depth of 1.5cm in solid water for the whole thorax irradiation. Each rat was set up individually for irradiation, using the imaging capability of the irradiator, and given a dose of 11Gy to the whole thorax (WTR) using an anterior and posterior beam.
Breathing Rate
We measured the breathing frequency of rats using a respiration rate monitor (Columbus Instruments, Columbus, OH, USA) as described previously13,21.
Tissue Analysis
After euthanasia of the animals at 32 weeks the lobes of the right lung were flash frozen and kept for biochemical analyses and the left lobe of the lung was used for histopathology. A volume (0.5–1.0 ml) of 10% formalin was injected into the left lobe to expand the alveoli and the lobes were placed in 10% formalin for fixation. The antibodies used for the immunohistochemical staining and Masson’s Trichrome staining have been described previously13,21,22. Sections were cut from the left lobe of the lung in the coronal plane. We analyzed five sections from the lungs of different surviving rats. Following staining, the slides were scanned using the ScanScope XT (Aperio Technologies, USA). The images were then viewed using ImageScope (Aperio Technologies) for quantitative analysis of the positive staining (the ratio of positive pixels/total number of positive and negative pixels, with air spaces excluded, as described previously13, 21). Since the staining in these sections is primarily cellular the quantitative analysis gives an estimate of the density of stained cells over the whole section. Tissue Studio (Definiens AG, Munich, Germany) was also used to measure percent of cells stained with TGF-β1 (at different levels) in the whole tissue section. Nuclei were identified (hematoxylin staining) and a circle with a radius of 3 µm greater than the nucleus was drawn to define the cell area. Four levels of cell staining were defined (high, medium, low, none).
Lipid peroxidation
Lipid peroxidation was assessed by measuring Malondialdehyde (MDA) levels in part of the right lung (25 mg wet wt.) of each rat using a T-BARS assay kit (Cayman Chemical Company, Ann Arbor, MI, USA). The flash-frozen sample was thawed, sonicated for 15s in RIPA buffer with protease inhibitors (Roche Applied Science, Laval, Quebec), and then centrifuged (1600g). The supernatant containing the lipid fraction was used for the assay as described previously13,21.
Hydroxyproline estimation
Fibrosis was assessed by analyzing part (100 mg wet wt.) of the upper right lung for hydroxyproline content using a colorimetric assay as described previously13,21.
Statistical analysis
ANOVA and Tukey’s method for the adjustment of least square means in multiple comparisons were used for analysis of the data sets. The WTR-only group was set as the primary comparison group for these analyses since the purpose of the study was to determine the efficacy of treatments relative to this group. Linear mixed effects modeling was used to examine trends in the breathing rate data over fixed time windows of 0–6, 6–10, 10–14 and 14–32 weeks. We also did a spot time point comparisons at 32 weeks.
Results
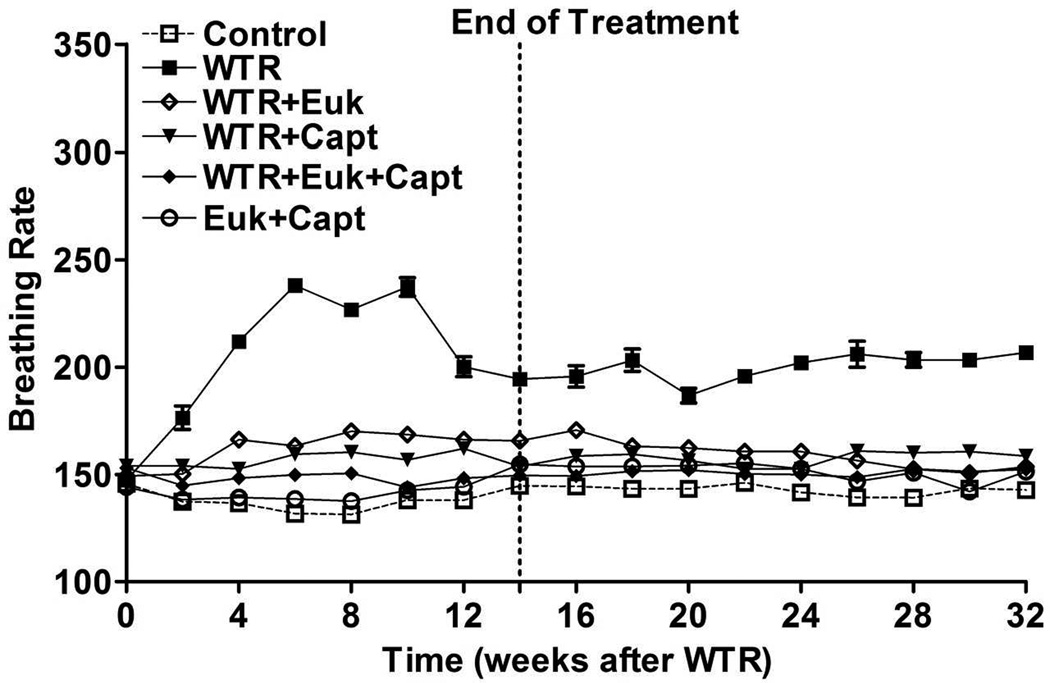
Figure 1 shows the average breathing rate for the rats following irradiation. For the control rats and those given the combined drug treatment alone, the breathing rate stayed at approximately 130–150 breaths per minutes (bpm), over the whole 32 wks. The rats given WTR alone demonstrated an initial increase in bpm over 2–14 wks which peaked at approximately 240 bpm at 6–10 wks PI. The bpm of this group of rats declined at later times but remained significantly (p < 0.01) above the control level out to 32 weeks. The rats given WTR plus captopril, or EUK-207 or their combination all showed a significant reduction (p<0.01) in bpm relative to the WTR group in all time windows. The combination drug treatment showed levels significantly lower than EUK treatment alone in the 6–10 and 14–32 wk time windows (p<0.05 and p<0.01) but was significantly different from Captopril treatment alone only during the 6–10 wk time window (p<0.05). At 32 weeks only the WTR group was significantly different from the other groups.
Figure 1.
Breathing rate (mean breaths per minute) as a function of time after irradiation. Control = No irradiation; WTR = whole thorax irradiation (11Gy); WTR+Euk = whole thorax irradiation + Euk-207; WTR+Capt = whole thorax irradiation + Captopril; WTR+Euk+Capt = whole thorax irradiation + Euk-207 + Captopril; Euk+Capt = Euk-207 + Captopril. The drug treatments were started 1 week post-irradiation (PI) and terminated at 14 weeks PI. The rats were euthanized at 32 weeks PI. Each point represents the mean (±SEM) for all rats available for analysis at the different times. All groups contained 8 rats at the start of the experiment. At 8 weeks this was reduced to 6 rats for the WTR group and to 7 rats for the WTR + Euk-207 group and the WTR + Capt group due to morbidity requiring euthanasia. These numbers remained constant out to sacrifice at 32 weeks.
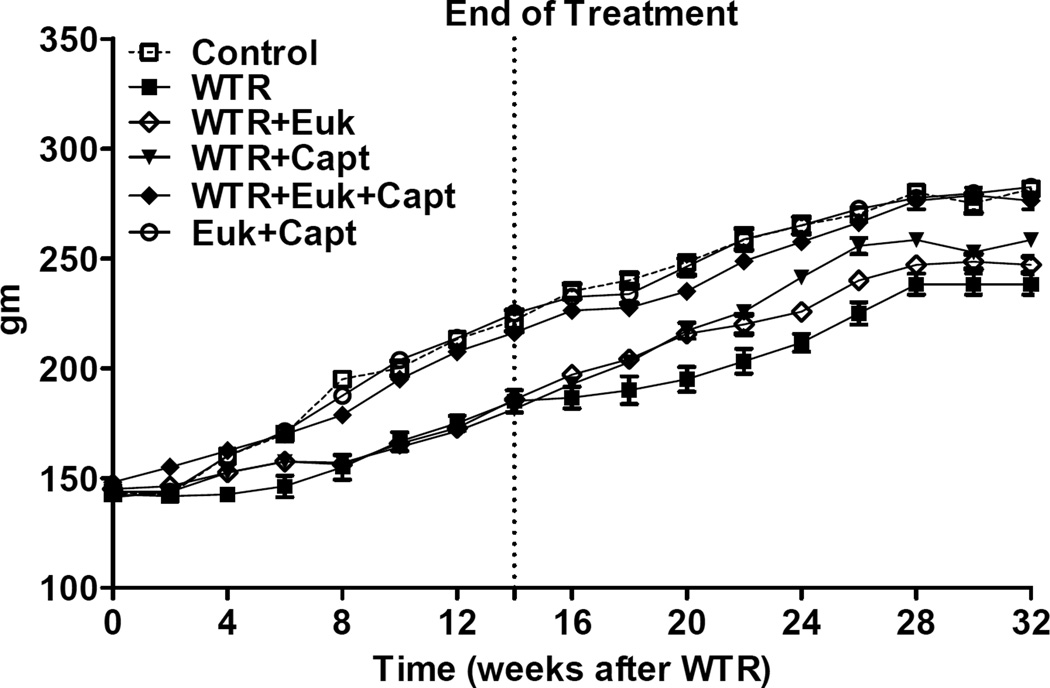
Irradiation affected the increase in body weight of the rats (Figure 2). The two non-irradiated groups showed a steady increase until about 28 weeks. The WTR group showed a 3–4 wk delay and this was similar for the WTR + EUK and WTR+ captopril groups. However, the rats given WTR + captopril + EUK-207 were similar to the unirradiated groups and significantly better than WTR plus either drug treatment alone (p<0.01). That the dose of 11Gy (225kVp X-rays) is close to the lung tolerance limit for the SD strain of rats is illustrated by the fact that at the peak of the breathing rate increase (6–10 wks PI) we observed some morbidity in the WTR group (2 rats), the WTR + Captopril group (1 rat) and the WTR + EUK-207 group (1 rat) which required euthanasia. No other rats died during the course of the experiment.
Figure 2.
Body weight (mean in grams) as a function of time after irradiation. Labeling of the treatment groups (and number of rats per group) as indicated in the legend to Figure 1. Each point represents the mean (±SEM) for all rats available for analysis at the different times.
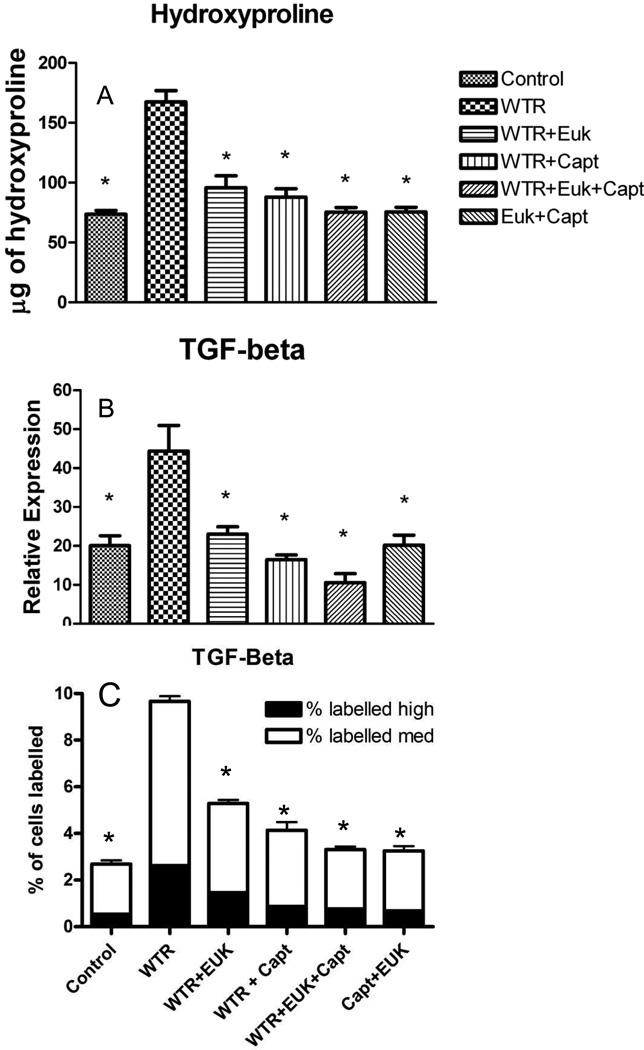
We assessed fibrosis in the lungs of the rats using both Masson-Trichrome (M-T) staining and hydroxyproline levels. Images of M-T staining in the lungs (see Supplementary Figure S1) showed large increases in staining for rats given WTR alone. These staining levels were substantially reduced in the rats given the drug treatments. Consistent with the M-T levels there was a large increase in hydroxyproline levels in the WTR only rats (Figure 3A) relative to both unirradiated groups (p<0.01). All the drug treatment groups had significantly (p<0.01) lower hydroxyproline content than the WTR group but there was no significant differences among the groups. The drug combination showed a trend as the most effective treatment.
Figure 3.
A) Hydroxyproline (µg of hydroxyproline/100mg of wet lung tissue) content of the lung tissue at 32 weeks after irradiation; B) Aperio analysis of TGF-beta staining (percent positivity) at 32 weeks after irradiation. C) Definiens analysis of TGF-beta staining (percent of cells stained). Each bar represents the mean (±SEM) for all rats available for analysis. Labeling of the treatment groups (and number of rats per group) as indicated in the legend to Figure 1. The asterisks indicate groups that are significantly different from the WTR group in each panel.
TGF-β1 is believed to play an important role in stimulating collagen deposition. Images of TGF-β1 staining are shown in Figures S2 and S3. There were increased levels of both localized and regional staining in the irradiated animals. We analysed the staining levels for this cytokine using both Image Scope (Figure 3B and S2) and Tissue Studio (Figures 3C and S3) to assess both regional and local cellular staining levels. Both analyses give very similar results. In the WTR group there was a significant (p<0.01) increase in levels of TGF-β1 expression at 32 weeks post-irradiation. All the drug treatment groups showed a significant (p<0.01) reduction in TGF-β1 staining to levels similar to those observed in the two unirradiated groups but differences between the drug treatment groups were not significant.
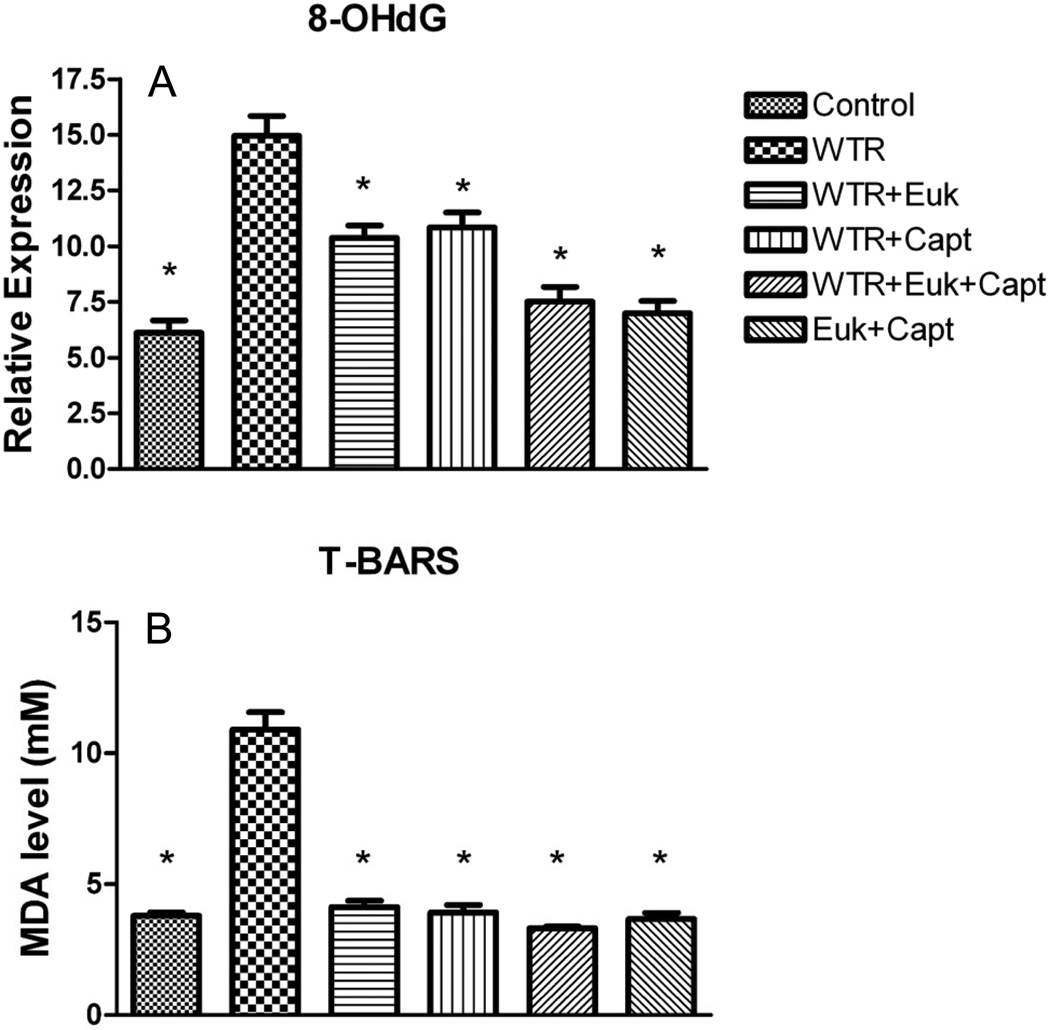
The presence of oxidative damage was measured using two markers of oxidative lesions. In the DNA we assessed 8-hydroxy-2-deoxyguanosine (8-OHdG) staining (using Image Scope), and in the tissue we measured levels of malondialdehyde (MDA) to determine lipid peroxidation. Quantitative analysis of the lung sections stained for 8-OHdG (see Figure S4) showed that the WTR group demonstrated a significant (p < 0.01) increase relative to the control (Figure 4A). All the drug treatment groups showed significant reduction in 8-OHdG levels (p<0.01), and the WTR+Capt+EUK group showed a significantly greater decrease (p<0.05) than the WTR+Capt group. Relative to the WTR+EUK group the greater decrease with the combination treatment was on the borderline of significance (p = 0.066).
Figure 4.
A) Analysis of 8-OHdG staining (percent positivity assessed using Tissue Scope) at 32 weeks after irradiation; B) Analysis of MDA levels (mM) in the lung tissue at 32 weeks after irradiation. Each bar represents the mean (±SEM) for all rats available for analysis. Labeling of the treatment groups (and number of rats per group) as indicated in the legend to Figure 1. The asterisks indicate groups that are significantly different from the WTR group in each panel.
The T-BARS assay used to quantify the concentrations of MDA in the lung (Figure 4B) showed a significant (p<0.01) increase in the WTR group relative to the unirradiated groups and all the drug treatment groups showed significant (p<0.01) reduction of MDA concentration to levels similar to those in the control. There were no significant differences between the three treatment groups but again the combination drug treatment group showed the lowest value.
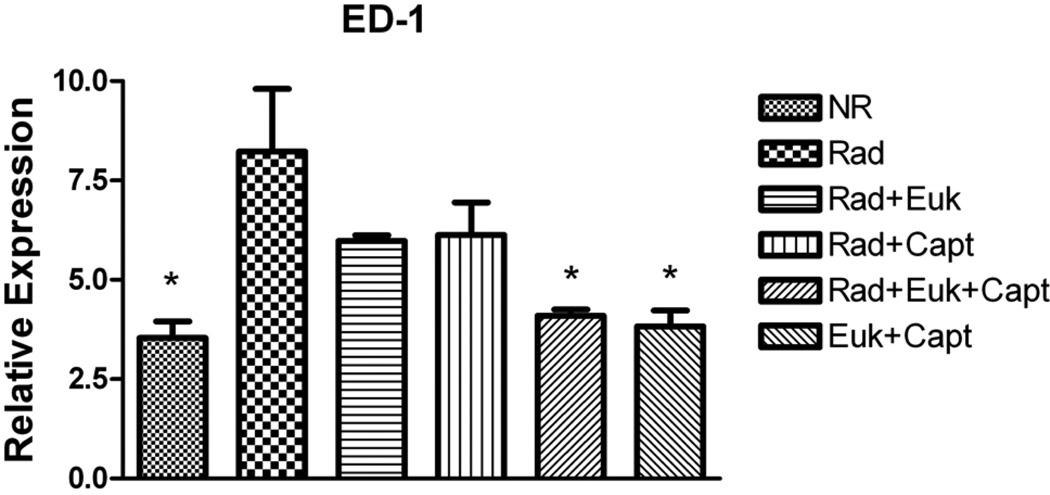
We measured overall levels of the inflammatory cytokines IL-1α, IL-1β, IL-6 and TNF-α in irradiated lung using Image Scope (see Figure S5). IL-1β, IL-6 and TNF-α levels were significantly (p<0.01) increased at 32 weeks post-irradiation relative to the unirradiated controls but only IL-1β was significantly (p<0.01) affected by drug treatment and only in the groups treated with Captopril. Levels of activated macrophages (ED-1 staining assessed using Image Scope) are shown in Figure 5 (and Figure S6). In the WTR group ED-1 staining was significantly (p<0.05) elevated relative to the non-irradiated groups. The WTR + drug treatment groups showed reduced levels but only that for the combined drug treatment group was significant (p<0.05). The difference between the combination and the individual drug treatment groups was not significant.
Figure 5.
Analysis of macrophage activation (ED-1 staining) at 32 weeks after irradiation as percent positivity (assessed using Tissue Scope). Each bar represents the mean (±SEM). Labeling of the treatment groups (and number of rats per group) as indicated in the legend to Figure 1. The asterisks indicate groups that are significantly different from the WTR group.
Discussion
The lung is one of the most susceptible organs to radiation toxicity and after radiation accidents lung injury may play an important role in multi-organ failure7. There remains a need to develop effective mitigators against radiation-induced lung damage. The ideal such agents must be easy to deliver, inexpensive and relatively non-toxic. Our current study suggests a significant step in this direction. The combination of EUK-207 and Captopril gave almost complete mitigation of acute pneumonitis and fibrosis using a treatment schedule that started 1 week after radiation exposure (PI) and was continued through the pneumonitis phase but stopped at 14 weeks PI, well before the measurements (at 32 wks) of fibrosis.
Our current experiments were based on recent work which demonstrated that different agents with anti-oxidant properties, such as EUK-207 and genistein, are potential mitigators of radiation-induced lung damage in animal models12,13,22. Similar results have been reported using chronic administration of the novel catalytic antioxidant, AEOL 1015023. Various renin angiotensin converting enzyme inhibitors have also been found to mitigate various aspects of radiation-induced lung damage including improvement of pulmonary vascular hemodynamics altered by radiation injury, even when treatment was started 2 weeks after radiation exposure4, 9–11. Furthermore the increase in lung collagen at 30 weeks after a single dose of 13 Gy X-rays to the whole thorax of WAG/RijCmcr rats was mitigated by ACE inhibitors, enalapril, captopril and fosinopril using a different strain of rats (Wistar) than in the current study9. Both captopril and enalapril improved survival during pneumonitis between 6–10 weeks when drug treatment was started 1 week after irradiation.
In radiation-induced lung damage, prolonged expression of pro- and anti-inflammatory cytokines and ROS production together with epithelial cell death and potential influx of fibrocytes from the bone marrow are believed to form the basis for the inflammatory and fibrogenic processes. Recent studies investigating the effect of lipopolysaccharide (LPS) treatment on radiation-induced lung response in mice knocked-out for various components of the Tumour Necrosis Factor (TNF) alpha pathway have suggested a role for this cytokine pathway24. It is thus of interest that in the current study we observed increased levels of TNF-alpha and IL-6 expression at 32 weeks post irradiation despite observing almost complete mitigation of fibrosis. Levels of activated macrophages were reduced, indicating that other cells within the lung may be maintaining the high levels of these cytokines, as has been suggested by others2,25. While it is well recognized that expression of inflammatory cytokines is temporally variant after irradiation, our findings suggest the possibility that prolonged expression of certain inflammatory cytokines may not be directly associated with the development of radiation-induced fibrosis in all animal models, consistent with suggestions from a recent review of other syndromes resulting in lung fibrosis26. Notably, the cytokine most closely associated with radiation-induced fibrosis TGF-β127–29 was significantly increased in WTR group but was clearly reduced in the drug treated groups in parallel with reduced fibrosis.
It is possible that the two mitigating agents are acting directly on the oxidative stress associated with the inflammatory response. In this context both EUK-207 and captopril alone suppressed oxidative stress, based on staining of 8-OHdG and on MDA levels, in the irradiated lung. This suggests that the mitigating effects of captopril may involve some antioxidant mechanism(s). Angiotensin II is known to increase activation of NADPH oxidases30 so this is one possible mechanism by which an ACE inhibitor might have an antioxidant effect during an injury scenario. A direct antioxidant effect, while possible and not without precedent, seems less likely31. Captopril has a free SH group and, therefore, potential redox modulating properties, but enalapril does not, and, as discussed above, it is also a potent mitigator of radiation lung injury. As noted by Kma et al.9, collagen deposition is reported to be mediated by the AT1 and AT2 receptors present on fibroblasts. ACE inhibitors block synthesis of angiotensin II, the peptide ligand for these receptors, potentially attenuating fibrosis. These agents may thus work effectively together because EUK-207 (with help from Captopril) attenuates the early inflammation, while Captopril can also help to block collagen production.
Conclusion
In this study, we found significant mitigation of radiation-induced lung damage using a short schedule of Captopril and EUK-207 individually or their combinations in S-D rats. The combination of the two drugs, at the doses used, demonstrated effects that were generally greater than either drug alone suggesting strong potential for mitigating of radiation-induced lung damage following exposure in an event like terrorist attack or nuclear accident or for future clinical trial of these drugs among patients. The window of one week before starting of drug treatment should allow biodosimetry and other necessary evaluation in a mass casualty event. Further studies relating to the exact mechanism of action of these drugs in mitigating radiation-induced lung damage, including detailed cytokine analysis, remain necessary, as do studies of whether such treatment would increase the tolerated dose. Similarly, studies with fractionated treatments will be required to assess the therapeutic potential of this approach for reducing lung injury in cancer patients receiving thoracic irradiation, although reports on radiation therapy patients taking ACE inhibitors suggest they may reduce incidence of lung complications32–34.
Supplementary Material
Acknowledgements
We thank Melania Pintilie for help analyzing the breathing rate data. This work was supported by funds from an NIAID/NIH U19 program (U19 AI-067734) and by funds from the Canadian Institutes of Health Research. Partial support was also provided by the Princess Margaret Foundation and the Ontario Ministry of Health and Long Term Care. The views expressed do not necessarily reflect those of OMHLTC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drouet M, Herodin F. Radiation victim management and the haematologist in the future: time to revisit therapeutic guidelines? Int J Radiat Biol. 2010;86(8):636–648. doi: 10.3109/09553001003789604. [DOI] [PubMed] [Google Scholar]
- 2.Fleckenstein K, et al. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol. 2007;17(2):89–98. doi: 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Ghafoori P, et al. Radiation-induced lung injury. Assessment, management, and prevention. Oncology (Williston Park) 2008;22(1):37–47. discussion 52-3. [PubMed] [Google Scholar]
- 4.Molthen RC, et al. Mitigation of radiation induced pulmonary vascular injury by delayed treatment with captopril. Respirology. 2012;17(8):1261–1268. doi: 10.1111/j.1440-1843.2012.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hensley ML, et al. American Society of Clinical Oncology clinical practice guidelines for the use of chemotherapy and radiotherapy protectants. J Clin Oncol. 1999;17(10):3333–3355. doi: 10.1200/JCO.1999.17.10.3333. [DOI] [PubMed] [Google Scholar]
- 6.Stone HB, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat Res. 2004;162(6):711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 7.Williams JP, McBride WH. After the bomb drops: a new look at radiation-induced multiple organ dysfunction syndrome (MODS) Int J Radiat Biol. 2011;87(8):851–868. doi: 10.3109/09553002.2011.560996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moulder JE, Fish BL, Cohen EP. ACE inhibitors and AII receptor antagonists in the treatment and prevention of bone marrow transplant nephropathy. Curr Pharm Des. 2003;9(9):737–749. doi: 10.2174/1381612033455422. [DOI] [PubMed] [Google Scholar]
- 9.Kma L, et al. Angiotensin converting enzyme inhibitors mitigate collagen synthesis induced by a single dose of radiation to the whole thorax. J Radiat Res. 2012;53(1):10–17. doi: 10.1269/jrr.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh SN, et al. Renin-Angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2009;75(5):1528–1536. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medhora M, et al. Radiation damage to the lung: mitigation by angiotensin-converting enzyme (ACE) inhibitors. Respirology. 2012;17(1):66–71. doi: 10.1111/j.1440-1843.2011.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langan AR, et al. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol. 2006;79(2):231–238. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Mahmood J, et al. Mitigation of radiation-induced lung injury by genistein and EUK-207. Int J Radiat Biol. 2011;87(8):889–901. doi: 10.3109/09553002.2011.583315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmood J, et al. Mitigation of radiation-induced lung injury with EUK-207 and genistein: effects in adolescent rats. Radiat Res. 2013;179(2):125–134. doi: 10.1667/RR2954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doctrow SR, et al. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activity relationship studies. J Med Chem. 2002;45(20):4549–4558. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- 16.Zhang HJ, et al. Redox modulation of the liver with chronic antioxidant enzyme mimetic treatment prevents age-related oxidative damage associated with environmental stress. Faseb J. 2004;18(13):1547–1549. doi: 10.1096/fj.04-1629fje. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal RA, et al. Orally available Mn porphyrins with superoxide dismutase and catalase activities. J Biol Inorg Chem. 2009;14(6):979–991. doi: 10.1007/s00775-009-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malfroy-Camine BDS. US Patent Number 7, 527., Editor. USA: 2006. Cyclic salen-Mn compounds as scavengers for oxygen radicals and useful as antioxidants in the treatment and prevention of disease. [Google Scholar]
- 19.Rosenthal RA, et al. Salen Mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med Chem. 2011;11(4):359–372. doi: 10.2174/187152011795677490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarkson R, et al. Characterization of image quality and image-guidance performance of a preclinical microirradiator. Med Phys. 2011;38(2):845–856. doi: 10.1118/1.3533947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmood J, et al. Mitigation of lung injury after accidental exposure to radiation. Radiat Res. 2011;176(6):770–780. doi: 10.1667/rr2562.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calveley VL, et al. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat Res. 2010;173(5):602–611. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabbani ZN, et al. Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radic Res. 2007;41(11):1273–1282. doi: 10.1080/10715760701689550. [DOI] [PubMed] [Google Scholar]
- 24.Zaidi A, et al. Effects of lipopolysaccharide on the response of C57BL/6J mice to whole thorax irradiation. Radiother Oncol. 2012;105(3):341–349. doi: 10.1016/j.radonc.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rube CE, et al. The bronchiolar epithelium as a prominent source of pro-inflammatory cytokines after lung irradiation. Int J Radiat Oncol Biol Phys. 2005;61(5):1482–1492. doi: 10.1016/j.ijrobp.2004.12.072. [DOI] [PubMed] [Google Scholar]
- 26.Todd NW, Luzina IG, Atamas SP. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair. 2012;5(1):11. doi: 10.1186/1755-1536-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anscher MS, et al. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2008;71(3):829–837. doi: 10.1016/j.ijrobp.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 28.Finkelstein JN, et al. Early alterations in extracellular matrix and transforming growth factor beta gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys. 1994;28(3):621–631. doi: 10.1016/0360-3016(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 29.Rube CE, et al. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47(4):1033–1042. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- 30.Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63(1):218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 31.Bartosz M, Kedziora J, Bartosz G. Antioxidant and prooxidant properties of captopril and enalapril. Free Radic Biol Med. 1997;23(5):729–735. doi: 10.1016/s0891-5849(97)00014-2. [DOI] [PubMed] [Google Scholar]
- 32.Cohen EP, et al. Mitigation of late renal and pulmonary injury after hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 2012;83(1):292–296. doi: 10.1016/j.ijrobp.2011.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kharofa J, et al. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84(1):238–243. doi: 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, et al. Do Angiotensin-Converting Enzyme Inhibitors Reduce the Risk of Symptomatic Radiation Pneumonitis in Patients With Non-Small Cell Lung Cancer After Definitive Radiation Therapy? Analysis of a Single-Institution Database. Int J Radiat Oncol Biol Phys. 2013 doi: 10.1016/j.ijrobp.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.