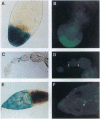
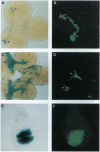
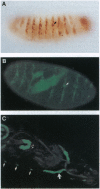
Abstract
We have used the green fluorescent protein (GFP) from the jellyfish Aequorea victoria as a vital marker/reporter in Drosophila melanogaster. Transgenic flies were generated in which GFP was expressed under the transcriptional control of the yeast upstream activating sequence that is recognized by GAL4. These flies were crossed to several GAL4 enhancer trap lines, and expression of GFP was monitored in a variety of tissues during development using confocal microscopy. Here, we show that GFP could be detected in freshly dissected ovaries, imaginal discs, and the larval nervous system without prior fixation or the addition of substrates or antibodies. We also show that expression of GFP could be monitored in intact living embryos and larvae and in cultured egg chambers, allowing us to visualize dynamic changes in gene expression during real time.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellen H. J., O'Kane C. J., Wilson C., Grossniklaus U., Pearson R. K., Gehring W. J. P-element-mediated enhancer detection: a versatile method to study development in Drosophila. Genes Dev. 1989 Sep;3(9):1288–1300. doi: 10.1101/gad.3.9.1288. [DOI] [PubMed] [Google Scholar]
- Bier E., Vaessin H., Shepherd S., Lee K., McCall K., Barbel S., Ackerman L., Carretto R., Uemura T., Grell E. Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 1989 Sep;3(9):1273–1287. doi: 10.1101/gad.3.9.1273. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993 Jun;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Budnik V., Zhong Y., Wu C. F. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990 Nov;10(11):3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan C. A., Thomas J. B. Tau-beta-galactosidase, an axon-targeted fusion protein. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5972–5976. doi: 10.1073/pnas.91.13.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Irvine K. D., Wieschaus E. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development. 1994 Apr;120(4):827–841. doi: 10.1242/dev.120.4.827. [DOI] [PubMed] [Google Scholar]
- Jaglarz M. K., Howard K. R. Primordial germ cell migration in Drosophila melanogaster is controlled by somatic tissue. Development. 1994 Jan;120(1):83–89. doi: 10.1242/dev.120.1.83. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Klämbt C., Glazer L., Shilo B. Z. breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 1992 Sep;6(9):1668–1678. doi: 10.1101/gad.6.9.1668. [DOI] [PubMed] [Google Scholar]
- Krasnow M. A., Cumberledge S., Manning G., Herzenberg L. A., Nolan G. P. Whole animal cell sorting of Drosophila embryos. Science. 1991 Jan 4;251(4989):81–85. doi: 10.1126/science.1898782. [DOI] [PubMed] [Google Scholar]
- Montell D. J., Rorth P., Spradling A. C. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell. 1992 Oct 2;71(1):51–62. doi: 10.1016/0092-8674(92)90265-e. [DOI] [PubMed] [Google Scholar]
- O'Kane C. J., Gehring W. J. Detection in situ of genomic regulatory elements in Drosophila. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman-Fried M., Dickson B., Hafen E., Shilo B. Z. Elucidation of the role of breathless, a Drosophila FGF receptor homolog, in tracheal cell migration. Genes Dev. 1994 Feb 15;8(4):428–439. doi: 10.1101/gad.8.4.428. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Beckwith J. R. Uses of lac fusions for the study of biological problems. Microbiol Rev. 1985 Dec;49(4):398–418. doi: 10.1128/mr.49.4.398-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Bernholz J. T., Wilson C., Gibson G., Schuh R., Gehring W. J. Identification of target genes of the homeotic gene Antennapedia by enhancer detection. Genes Dev. 1991 Dec;5(12B):2467–2480. doi: 10.1101/gad.5.12b.2467. [DOI] [PubMed] [Google Scholar]
- Wang S., Hazelrigg T. Implications for bcd mRNA localization from spatial distribution of exu protein in Drosophila oogenesis. Nature. 1994 Jun 2;369(6479):400–403. doi: 10.1038/369400a0. [DOI] [PubMed] [Google Scholar]