Abstract
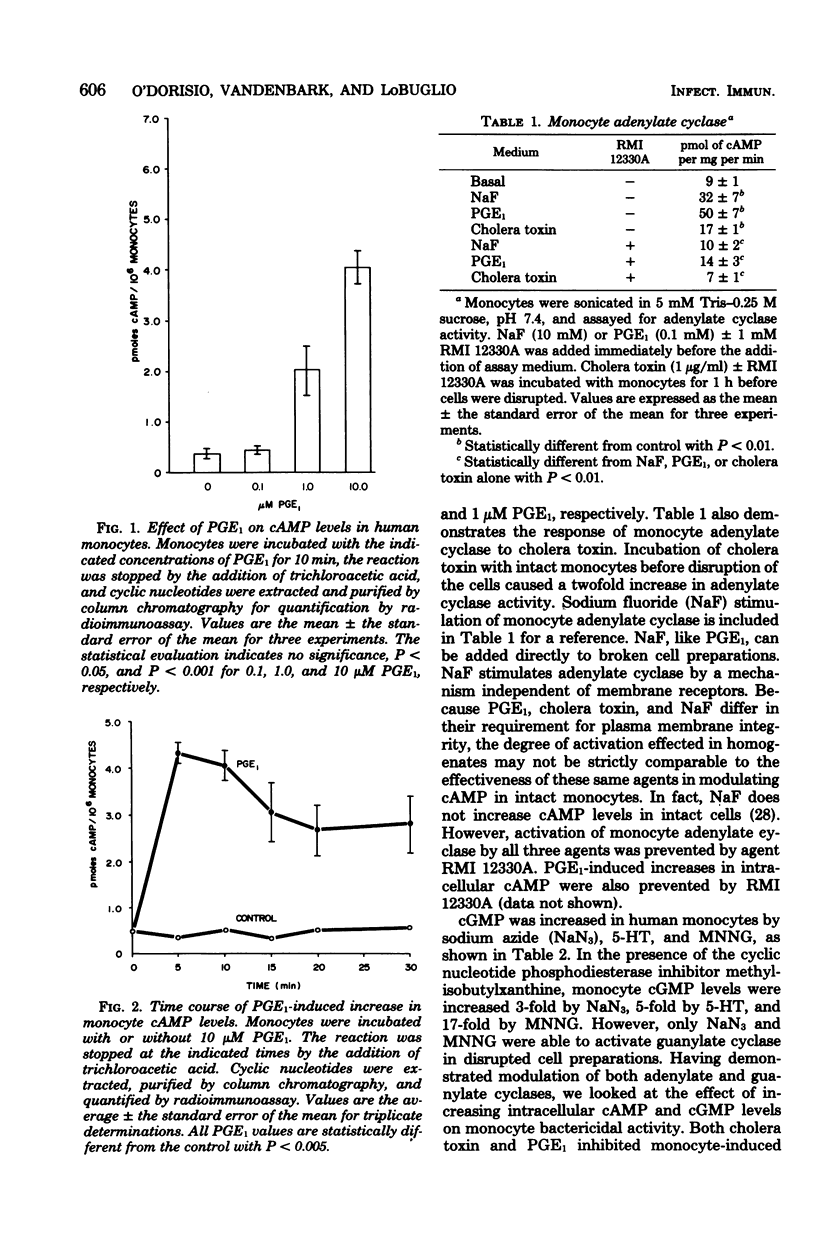
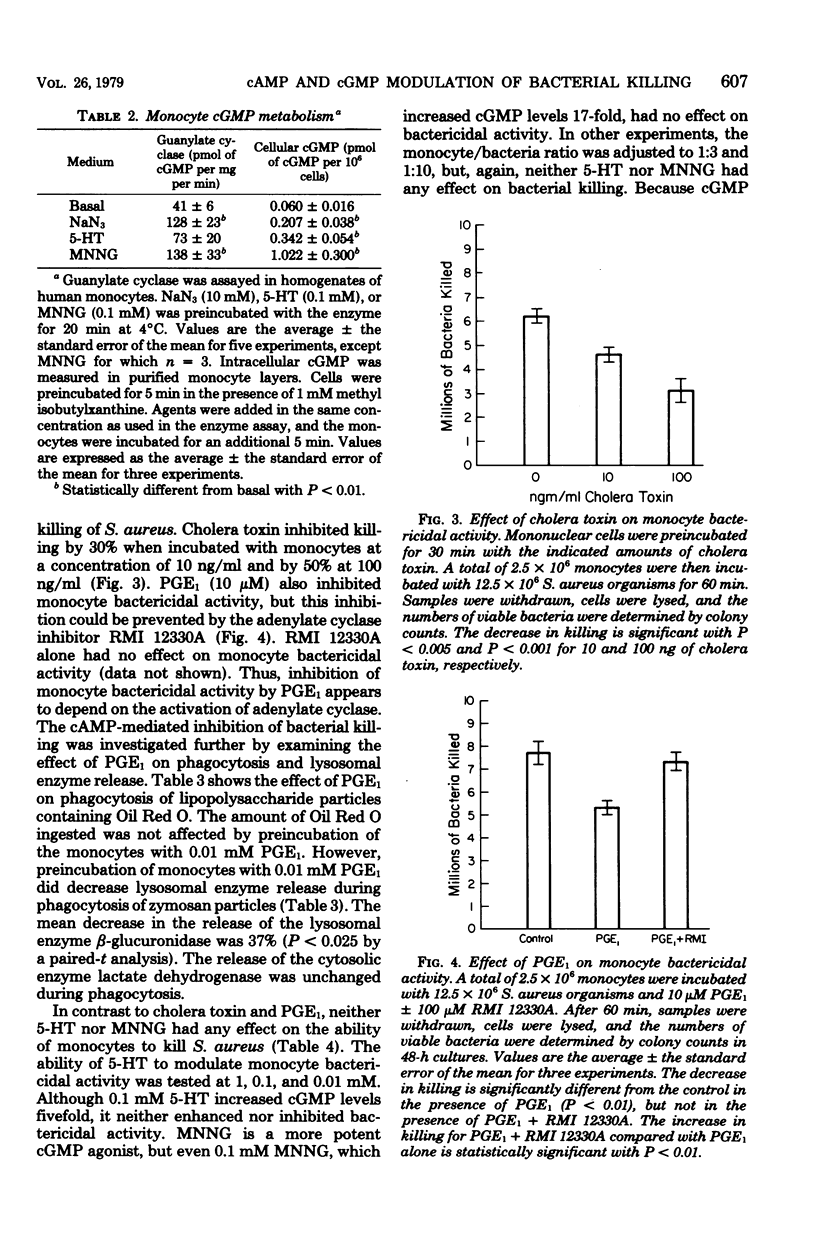
This study was designed to test whether cyclic nucleotides play a role in the regulation of bacterial killing by human monocytes. Agents were tested for their ability to activate monocyte adenylate or guanylate cyclase in cell-free preparations, to increase cyclic adenosine 3',5'-monophosphate (cAMP) or cyclic guanosine 3',5'-monophosphate (cGMP) in intact human monocytes, and to modulate monocyte-induced killing of Staphylococcus aureus in vitro. Prostaglandin E1 and cholera toxin activated monocyte adenylate cyclase and inhibited monocyte killing of S. aureus. An adenylate cyclase inhibitor, RMI 12330A, reversed the prostaglandin E1-mediated inhibition of bacterial killing, thus implicating cAMP as the intracellular mediator of this inhibition. In contrast, monocyte cGMP levels were increased 5- and 17-fold by 5-hydroxytryptamine and N-methyl-N' -nitro-N-nitrosoguanidine, respectively, but neither agent was effective in modulating monocyte bactericidal activity. Thus, modulation of bactericidal activity in human monocytes did not conform to the yin/yang theory of opposing actions by cAMP and cGMP, for although monocyte-mediated killing of S. aureus was inhibited by cAMP agonists, it was not enhanced by cGMP agonists.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block L. H., Aloni B., Biemesderfer D., Kashgarian M., Bitensky M. W. Macrophage migration inhibition factor: interactions with calcium, magnesium, and cyclic AMP. J Immunol. 1978 Oct;121(4):1416–1421. [PubMed] [Google Scholar]
- Chervenick P. A., LoBuglio A. F. Human blood monocytes: stimulators of granulocyte and mononuclear colony formation in vitro. Science. 1972 Oct 13;178(4057):164–166. doi: 10.1126/science.178.4057.164. [DOI] [PubMed] [Google Scholar]
- Clyman R. I., Sandler J. A., Manganiello V. C., Vaughan M. Guanosine 3',5'-monophosphate and adenosine 3',5'-monophosphate content of human umbilical artery. J Clin Invest. 1975 May;55(5):1020–1025. doi: 10.1172/JCI108002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droller M. J., Schneider M. U., Perlmann P. A possible role of prostaglandins in the inhibition of natural and antibody-dependent cell-mediated cytotoxicity against tumor cells. Cell Immunol. 1978 Aug;39(1):165–177. doi: 10.1016/0008-8749(78)90091-6. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Jenkin C. R. The role of the macrophage in immunity to Trypanosoma lewisi infections in the rat. Cell Immunol. 1979 Feb;42(2):327–335. doi: 10.1016/0008-8749(79)90198-9. [DOI] [PubMed] [Google Scholar]
- Frandsen E. K., Krishna G. A simple ultrasensitive method for the assay of cyclic AMP and cyclic GMP in tissues. Life Sci. 1976 Mar 1;18(5):529–541. doi: 10.1016/0024-3205(76)90331-3. [DOI] [PubMed] [Google Scholar]
- Gemsa D., Woo C. H., Webb D., Fudenberg H. H., Schmid R. Erythrophagocytosis by macrophages: suppression of heme oxygenase by cyclic AMP. Cell Immunol. 1975 Jan;15(1):21–36. doi: 10.1016/0008-8749(75)90161-6. [DOI] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Guellaen G., Mahu J. L., Mavier P., Berthelot P., Hanoune J. RMI 12330 A, an inhibitor of adenylate cyclase in rat liver. Biochim Biophys Acta. 1977 Oct 13;484(2):465–475. doi: 10.1016/0005-2744(77)90102-4. [DOI] [PubMed] [Google Scholar]
- Guellaen G., Mahu J. L., Mavier P., Hanoune J., Berthelot P. Non-specific inhibition of some rat liver membrane-bound enzymes by an adenylate cyclase inhibitor RMI 12330 A. Biochem Pharmacol. 1978 Mar 1;27(5):641–645. doi: 10.1016/0006-2952(78)90498-7. [DOI] [PubMed] [Google Scholar]
- Hanley M. R., Iversen L. L. Muscarinic cholinergic receptors in rat corpus striatum and regulation of guanosine cyclic 3',5'-monophosphate. Mol Pharmacol. 1978 Mar;14(2):246–255. [PubMed] [Google Scholar]
- Hern E. P., Schultz J., O'Dorisio M. S., Moore R. O., Serif G. S. The effects of fasting, diabetes, and hypophysectomy on adipose lactic dehydrogenase isozymes. Arch Biochem Biophys. 1975 Jul;169(1):331–338. doi: 10.1016/0003-9861(75)90348-3. [DOI] [PubMed] [Google Scholar]
- Kimura H., Murad F. Evidence for two different forms of guanylate cyclase in rat heart. J Biol Chem. 1974 Nov 10;249(21):6910–6916. [PubMed] [Google Scholar]
- King G. W., Bain G., LoBuglio A. F. The effect of tuberculosis and neoplasia on human monocyte staphylocidal activity. Cell Immunol. 1975 Apr;16(2):389–395. doi: 10.1016/0008-8749(75)90127-6. [DOI] [PubMed] [Google Scholar]
- Kohl S., Starr S. E., oleske J. M., Shore S. L., Ashman R. B., Nahmias A. J. Human monocyte-macrophage-mediated antibody-dependent cytotoxicity to herpes simplex virus-infected cells. J Immunol. 1977 Mar;118(3):729–735. [PubMed] [Google Scholar]
- Koller C. A., King G. W., Hurtubise P. E., Sagone A. L., LoBuglio A. F. Characterization of glass adherent human mononuclear cells. J Immunol. 1973 Nov;111(5):1610–1612. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawley P. D., Thatcher C. J. Methylation of deoxyribonucleic acid in cultured mammalian cells by N-methyl-N'-nitro-N-nitrosoguanidine. The influence of cellular thiol concentrations on the extent of methylation and the 6-oxygen atom of guanine as a site of methylation. Biochem J. 1970 Feb;116(4):693–707. doi: 10.1042/bj1160693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E., Lefkowitz R. J. Myocardial guanylate cyclase: properties of the enzyme and effects of cholinergic agonists in vitro. Biochim Biophys Acta. 1975 Jan 23;377(1):186–196. doi: 10.1016/0005-2744(75)90299-5. [DOI] [PubMed] [Google Scholar]
- Muschel R. J., Rosen N., Rosen O. M., Bloom B. R. Modulation of Fc-mediated phagocytosis by cyclic AMP and insulin in a macrophage-like cell line. J Immunol. 1977 Nov;119(5):1813–1820. [PubMed] [Google Scholar]
- Nesbitt J. A., 3rd, Anderson W. B., Miller Z., Pastan I., Russell T. R., Gospodarowicz D. Guanylate cyclase and cyclic guanosine 3':5'-monophosphate phosphodiesterase activities and cyclic guanosine 3':5'-monophosphate levels in normal and transformed fibroblasts in culture. J Biol Chem. 1976 Apr 25;251(8):2344–2352. [PubMed] [Google Scholar]
- Perkins J. P. Adenyl cyclase. Adv Cyclic Nucleotide Res. 1973;3:1–64. [PubMed] [Google Scholar]
- Quayle E. S., Pagel J., Monti J. A., Christian S. T. A serotonin sensitive guanylate cyclase associated with specific neurotransmitter binding sites on isolated synaptic membranes from mature rat brain. Life Sci. 1978 Jul 10;23(2):159–165. doi: 10.1016/0024-3205(78)90265-5. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Remold H. G. The enhancement of macrophage adenylate cyclase by products of activated lymphocytes. J Biol Chem. 1974 Jun 10;249(11):3622–3627. [PubMed] [Google Scholar]
- Rinehart J. J., Vessella R., Lange P., Kaplan M. E., Gormus B. J. Characterization and comparison of human monocyte- and macrophage-induced tumor cell cytotoxicity. J Lab Clin Med. 1979 Mar;93(3):361–369. [PubMed] [Google Scholar]
- Rosenthal A. S., Lipsky P. E., Shevach E. M. Macrophage-lymphocyte interaction and antigen recognition. Fed Proc. 1975 Jul;34(8):1743–1748. [PubMed] [Google Scholar]
- Sandler J. A., Clyman R. I., Manganiello V. C., Vaughan M. The effect of serotonin (5-hydroxytryptamine) and derivatives on guanosine 3',5'-monophosphate in human monocytes. J Clin Invest. 1975 Feb;55(2):431–435. doi: 10.1172/JCI107948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Gayk H. E., Jakobs K. H., Hackenthal E. Cyclic AMP and phagocytosis in alveolar macrophages: influence of hormones and dibutyryl cyclic AMP. J Reticuloendothel Soc. 1975 May;17(5):251–261. [PubMed] [Google Scholar]
- Schultz R. M., Pavlidis N. A., Stoychkov J. N., Chirigos M. A. Prevention of macrophage tumoricidal activity by agents known to increase cellular cyclic AMP. Cell Immunol. 1979 Jan;42(1):71–78. doi: 10.1016/0008-8749(79)90222-3. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Levy P. C., LoBuglio A. F. Human lymphocyte, monocyte, and neutrophil antibody-dependent cell-mediated cytotoxicity toward human erythrocytes. Cell Immunol. 1978 Nov;41(1):122–133. doi: 10.1016/s0008-8749(78)80032-x. [DOI] [PubMed] [Google Scholar]
- Simon B., Dittrich J., Kather H., Encke A., Kommerell B. Inhibition of human colonic adenylate cyclase by RMI 12330 A. Digestion. 1978;18(3-4):213–219. doi: 10.1159/000198204. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Williams R. H., Little S. A. Studies on the assay and activities of guanyl and adenyl cyclase of rat liver. Arch Biochem Biophys. 1973 Nov;159(1):206–213. doi: 10.1016/0003-9861(73)90446-3. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Hamilton J., Reich E. Macrophage plasminogen activator: modulation of enzyme production by anti-inflammatory steroids, mitotic inhibitors, and cyclic nucleotides. Cell. 1976 Jun;8(2):271–281. doi: 10.1016/0092-8674(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Vesely D. L., Graves W. R., Lo T. M., Fletcher M. A., Levey G. S. Isolation of a guanylate cyclase inhibitor from the balsam pear (Momordica charantia abreviata). Biochem Biophys Res Commun. 1977 Aug 22;77(4):1294–1299. doi: 10.1016/s0006-291x(77)80120-4. [DOI] [PubMed] [Google Scholar]
- Vesely D. L., Levey G. S. Saccharin inhibits guanylate cyclase activity: possible relationship to carcinogenesis. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1384–1389. doi: 10.1016/0006-291x(78)91289-5. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Goldstein I., Hoffstein S., Chauvet G., Robineaux R. Yin/Yang modulation of lysosomal enzyme release from polymorphonuclear leukocytes by cyclic nucleotides. Ann N Y Acad Sci. 1975 Jun 13;256:222–232. doi: 10.1111/j.1749-6632.1975.tb36049.x. [DOI] [PubMed] [Google Scholar]


