Abstract
Objective
The aim of this study was to assess disease features in Sle1.Yaa mice with genetic deficiency of IL-6.
Methods
Sera and tissues were collected from B6, Sle1.Yaa and Sle1.Yaa.IL-6−/− mice and analyzed for various features of disease. Serum samples were used to determine autoantibody specificities by ELISA and indirect immunofluorescence, cytokine production by Luminex and ELISA and levels of blood urea nitrogen by ELISA. Renal, lung and salivary gland tissue sections were evaluated for pathologic changes. Lymphocyte phenotypes, including CD4+ T cell cytokine production, and those of follicular and extrafollicular T helper subsets, germinal center B cells and plasma cells, were determined using flow cytometry.
Results
IL-6 deficiency not only ameliorated autoantibody production and renal disease in this model, but also effectively reduced inflammation of lungs and salivary glands. Furthermore, IL-6 deficiency abrogated differentiation of Th1 and extrafollicular T helper cells, germinal center B cells and plasma cells in spleen and eliminated renal T cells with IL-17, IFN-γ and IL-21 production potential.
Conclusions
This study highlights IL-6-mediated T cell aberrations in Yaa-driven autoimmunity and supports the concept of therapeutic IL-6/IL-6R blockade in SLE and Sjogren’s Syndrome by impairing the production of autoantibodies and lymphocytic infiltration of kidney, lung and salivary gland.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that affects multiple tissues and exhibits extensive clinical heterogeneity. Some of the most prevalent manifestations of this disease include antibodies to dsDNA, nucleosomes and RNA-associated nuclear and cytoplasmic antigens, nephritis, cardiovascular and musculoskeletal manifestations, hematological abnormalities and pleuritis (1).
In response to injury or inflammation, interleukin (IL)-6 acts as an acute phase cytokine that can directly stimulate lymphocytes (2). Overexpression of IL-6 has been associated with chronic heart and coronary artery disease (3), proliferative mesangial cell glomerulonephritis (4), significantly increased numbers of plasma cells (5, 6), malignancy (7) and hematologic abnormalities (8, 9).
IL-6 is one of multiple inflammatory cytokines elevated in the serum of SLE patients and is associated with B cell hyperactivity and the mediation of organ damage (10, 11). This cytokine promotes the terminal differentiation of pre-activated B cells into IgG-secreting cells in SLE patients through upregulation of, and increased stimulation through, the IL-6 receptor α (10). Increased levels of IL-6 have been detected in renal glomeruli and tubules and excreted in the urine of lupus patients with renal disorders. The source of IL-6 is thought to be infiltrating inflammatory monocytes/macrophages and renal mesangial cells (4, 10, 12–14). Interestingly, antibodies to dsDNA can induce upregulation of inflammatory cytokine expression including IL-6 by endothelial and mononuclear cells, suggesting a positive pathogenic autoinflammatory feedback loop in the context of SLE (15).
We have shown previously that IL-6 promotes myeloid cell differentiation at the expense of B cell differentiation through IL-6Rα and STAT-3-mediated expression of Id1 in bone marrow lymphoid-myeloid progenitors in B6.Sle1.Yaa (hereafter referred to as SY) mice (9). In addition to effects on B cell differentiation and function and renal damage in SLE, IL-6 promotes Th17 and T follicular helper cell (Tfh) differentiation (16–18). Furthermore, Gong et al. recently showed that salivary gland epithelial cells can promote Tfh differentiation through the production of IL-6 (19). However, effects of IL-6 deficiency or inhibition on T cell phenotypes in the context of lupus or secondary Sjogren’s syndrome have not been well studied.
Morel et al. described a murine model of lupus in which B6 mice congenic for the SLAM/CD2-containing Sle1interval and the Y-linked autoimmune accelerator locus, Yaa (SY), demonstrate many of the pathologic features observed in human SLE (20). Males expressing Yaa, a duplication of several genes including a functional genetic duplication of the Tlr7 gene (21–23), spontaneously develop splenomegaly, increased T and B cell activation and produce autoantibodies and immune complex deposition, progressing to fatal lupus nephritis as early as 6 months of age. Both serum IL-6 and expression of IL-6Rα are significantly increased in SY mice compared to wild type B6 controls and single congenic B6.Sle1 and B6.Yaa mice (9).
Because IL-6 is a therapeutic target in SLE (24, 25) and is currently being investigated as a therapeutic target in Sjogren’s Syndrome (SS), a disorder of autoimmune exocrinopathy (26–28) in which IL-6 is elevated (29, 30), we used the SY model to investigate the effect of IL-6 deficiency on features of SLE and secondary SS, with increased emphasis on T cell phenotypes.
Materials and Methods
Mice
SY and IL-6-deficient SY (SY6KO) mice were obtained by intercrossing B6.Sle1 mice (generously provided by Edward Wakeland, University of Texas, Southwestern Medical Center, Dallas, TX, USA) with B6.Yaa and IL6−/− mice on the C57BL/6J background (The Jackson Laboratory) as described (9). Mice were housed under specific pathogen-free barrier conditions in the Laboratory Animal Resource Center of the Oklahoma Medical Research Foundation (OMRF) and given food and water ad libitum. All studies were performed between 20–32 weeks of age and were approved by the OMRF Institutional Animal Care and Use Committee.
Serologic analyses
Cytokine and chemokine levels (IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40/p70, IL-13, IL-17, IFN-γ, TNF-α, GM-CSF, bFGF, IP-10, KC, MCP-1, MIG, MIP-1α and VEGF) were quantified in sera from 9–10 animals per group (diluted 1:2) using a Mouse Cytokine 20-plex Panel according to the manufacturer’s protocols and analyzed using the Luminex detection platform (Invitrogen, Grand Island, NY). BAFF levels were measured from serum samples (diluted 1:10) of 10 mice per genotype by ELISA (R&D Systems) according to the manufacturer’s instructions, with the exception of generating a more concentrated standard curve.
Serum IgG autoantibodies to dsDNA and extractable nuclear antigens (9–10 mice per group) were determined using commercially available kits and were performed according to the manufacturer’s suggested protocols (Alpha Diagnostic International, San Antonio, TX). Sera were screened in duplicate for the presence of antibodies to purified bovine Ro/SS-A and La/SS-B antigens (9–10 mice per group) (Immunovision, Springdale, AR) and individual histones (6 mice per group) (H1, H2a, H2b, H3 and H4; Roche Diagnostics, Indianapolis, IN) using traditional methods. Briefly, polystyrene plates were coated with 5 Qg/ml of Ro or La, or 10 Qg/ml of individual histone antigens in carbonate coating buffer (pH 9) overnight at 4°C. Following coating, plates were blocked in PBS containing 0.1% BSA for 2 hours at RT, incubated with sera for 2 hours at RT, washed and incubated for 45 minutes with an alkaline phosphatase secondary antibody. After washing, PNPP substrate was added to each well and the plates read following 1 hour of incubation for Ro and La and following 2 hours for histones. The absorbance at 405nm was averaged between duplicate samples.
Salivary flow
Saliva from 15–18 individual mice per group was collected as previously described with modifications (31). Briefly, animals were fasted overnight prior to saliva collection. Each animal was anesthetized with an i.p. injection of 2.5% 2,2,2-tribromoethanol (Avertin, Sigma Chemical Co) at 0.10 ml/g body weight followed by an i.p. injection of 50 µg of pilocarpine/100 g of body weight to stimulate saliva flow. Saliva was obtained from the oral cavity over a 10 minute period using a pipettor and the quantity of the sample determined based on weight in mg.
Flow cytometry
Spleens were removed from 10 mice per group and single cell suspensions were made using a 40 Qm nylon filter. Red cells were lysed using tris ammonium chloride (0.14M NH4Cl in 17mM Tris, pH 7.2). Mononuclear cells were washed twice with Dulbecco’s Modified Eagle Medium (Sigma-Aldrich, Inc, St. Louis, MO) supplemented with 10% fetal calf serum (Atlanta Biologicals, Flowery Branch, GA), 1X non-essential amino acids (Gibco, Grand Island, NY), 2mM L-glutamine (Corning/Cellgro, Manassas, VA), 10µg/ml penicillin-streptomycin (Corning/Cellgro), 50µM β-mercaptoethanol (Sigma-Aldrich, Inc.) and 2mM sodium pyruvate (Gibco), then quantified using trypan blue exclusion.
Mononuclear cells were purified from 10 kidneys from each group using a procedure adapted from Sekine et al. (32). Briefly, kidneys were minced and digested in RPMI 1640 containing 10mM HEPES and 1.0 mg/mL collagenase for 15 minutes at 37°C. Tissue fragments were homogenized between the frosted section of two sterile glass slides, filtered through a 40 Qm cell strainer and the mononuclear cells isolated using Lympholyte M (Cedarlane Laboratories). The intermediate layer was removed, washed twice with RPMI 1640/HEPES and the cells quantified as described above.
Prior to intracellular cytokine staining, spleen and kidney mononuclear cells were re-stimulated for 5 hours in culture with 50 ng/ml PMA (Sigma-Aldrich, Inc.), 1 Qg/ml ionomycin (Sigma-Aldrich, Inc.) and 3 Qg/ml Brefeldin A (eBioscience, Inc., San Diego, CA). After stimulation, Fc receptors were blocked with anti-CD16/32 monoclonal antibody (mAb) for 5 minutes and cells were surface stained for 30 minutes on ice, permeabilized with fixation/permeabilization buffer (eBioscience, Inc.) and stained with antigen-specific or isotype control antibodies for 30 minutes at room temperature. Cells were washed in permeabilization buffer then resuspended in FACS-Fix (1% paraformaldehyde in 0.15M NaCl) for analysis.
Spleen cells were stained on ice for extrafollicular Th (Texfh) and Tfh cell subsets as described (33, 34) by anti-CD16/32 blocking for 5 minutes, incubating with primary antibodies for 1 hour at 37°C followed by washing and incubation with a streptavidin secondary antibody for 15 minutes. After a final wash, cells were resuspended in wash buffer without fixation and immediately evaluated by flow cytometry. All samples were collected on a LSRII cytometer (BD Biosciences, San Jose, CA) and analyzed with FACS Diva software (BD Biosciences).
For detection of plasma cells (PC) and germinal center B cells (GCB), splenocytes were blocked with anti-CD16/32 for 5 minutes followed by the addition of cell surface staining antibodies. After washing, cells stained with the GCB antibody panel were resuspended in FACS-Fix until analyzed on an LSRII cytometer. Samples stained with the PC antibody panel were washed and permeabilized with Cytofix/Cytoperm buffer (BD Biosciences) for 20 minutes. Following permeabilization, anti-Ig antibodies were added to the samples and incubated overnight at 4°C. Cells were resuspended in FACS-Fix and collected on an LSRII cytometer.
Antibodies used were: PSGL1/CD162-PE (2PH1), CXCR5-Biotin (2G8), CXCR4-V450 (2B11/CXCR4), B220-PETxRed (RA3-6B2), CD138-PE (281-2), IgD-V450 (11-26c.2a), CD19-PECy7 (ID3), GL-7-PE (GL7), TCRβ-PECy7 (H57-597), CD4-APC-eFluor 780 (RM4-5), CD44-PECy7 (IM7), CD62L-APC (MEL-14), CD19-FITC (MB19-1), CD11c-APC-eFluor 780 (N418), CD4-eFluor 450 (RM4-5), CD44-APC-eFluor 780 (IM7), IL-21-APC (FFA21), IL-17A-AlexaFluor 488 (eBio17B7) and IFN-γ-PerCPCy5.5 (XMG1.2) purchased from BD Biosciences, eBiosciences or BioLegend. Anti-IgM F(ab’)2-Cy5 and anti-IgG F(ab’)2-FITC were purchased from Jackson ImmunoResearch (West Grove, PA), and PNA-FITC was purchased from Vector Laboratories (Burlingame, CA). Isotype control mAbs were purchased from BD Biosciences or eBiosciences.
Histology (kidney, lung, salivary gland)
Lung, salivary gland and kidney tissues were collected from individual animals, fixed in 10% neutral-buffered formalin then paraffin-embedded for histological analysis. Paraffin-embedded samples were sectioned at 5 µm, collected on positively-charged glass slides (4 to 8 sections per slide, with each section separated by 50 Qm), and stained with hematoxylin and eosin. Two slides from each animal were evaluated by a professional veterinary pathologist (AWC) blinded to the groups using coded slides. Renal sections from 10–15 mice per group were graded for glomerular involvement using a scale from 0–4: 0=0% affected; 1=1–10% affected; 2=11–25% affected; 3=26–50% affected; 4=>50% affected. Global, segmental, mesangial, proliferative or hyaline changes and the location and severity of infiltrating foci were noted if observed for scores of 2–4. Lung sections from 18–20 SY and SY6KO mice were compared using a 12-point system based on 4 criteria. The total percentage of lung affected was scored using the following scale: 0=0% affected; 1=1–33% affected; 2=34–66% affected; 3=67–100% affected. The severity of inflammatory changes, vasculitis and fibrosis were scored from 0–3 (no, mild, moderate or severe lesions). The sum of scores for each criterion was combined to obtain a total severity score. Other abnormal observations were noted as observed.
To quantify salivary gland infiltrates, glands were collected from 9–10 SY and SY6KO mice and the same section number on the same slide for every sample was selected for measurement. Color images were captured with the Zeiss Axioplan 2i microscope equipped with an MR camera (Zeiss, Inc., Thornwood, NY) using light microscopy at low (20x) and high (40x) power. The total area of the evaluated sections was similar between mice and amongst groups. The number of focal infiltrates (defined as clusters of ≥ 50 lymphocytes) per section is reported.
Blood urea nitrogen and urine albumin
Blood urea nitrogen levels were assessed in mouse sera from 12–17 animals of each genotype using the QuantiChrom Urea Assay Kit (BioAssay Systems, Hayward, CA). Spot urine samples were collected from 18–20 B6, SY and SY6KO mice to determine urine albumin levels by sandwich ELISA using the Mouse Albumin ELISA Quantitation Kit (Bethyl Laboratories, Inc., Montgomery, TX). Serum and urine samples were sufficiently diluted to fall within the linear range of the curve for both assays.
Results
Normalization of serum cytokines and autoantibodies by IL-6 deficiency in SY mice
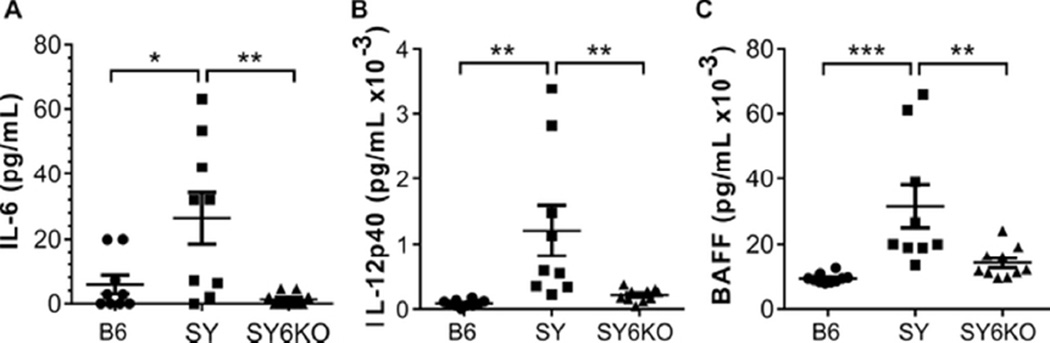
Serum IL-6 and IL-12 were previously reported to be elevated in SY mice compared to B6 controls (9, 23). After generation of IL-6-deficient SY mice, we first evaluated a panel of inflammatory cytokines and chemokines to verify the IL-6-deficient phenotype and to determine the effect of IL-6 gene deletion. We confirmed elevated levels of the inflammatory cytokines IL-6 and IL-12 in SY mice compared to B6 controls (Fig 1). Not only were IL-6 levels in SY6KO mice reduced below those observed in B6 controls, serum IL-12 levels were normalized by IL-6 deficiency, as well (Fig 1). Additionally, due to the reported interplay between IL-6 and B cell activating factor/B lymphocyte stimulator (BAFF/BLyS) particularly in the context of SS (30), as well as the importance of this cytokine in B cell differentiation and survival (35, 36), we measured BAFF levels from sera of 10 B6, SY and SY6KO mice. We observed elevated serum BAFF concentration in SY mice, which was significantly decreased in SY6KO animals, further supporting a feedback system between IL-6 and BAFF (Fig 1). No other tested cytokines or chemokines were significantly elevated in SY mice (not shown).
Fig. 1.
IL-6 deficiency normalizes serum IL-12 and BAFF in B6.Sle1.Yaa (SY) mice. Serum levels of IL-6 (A) and IL-12 (B) as determined by Luminex assay and BAFF (C) as determined by ELISA in C57BL/6 (B6), B6.Sle1.Yaa (SY) and B6.Sle1.Yaa.Il6−/− (SY6KO) mice are shown. Each symbol represents results of individual mice of the indicated genotype (n=9–10 mice per group). * p<0.05; **p<0.01 by one-way ANOVA with Bonferroni’s test for multiple comparisons. Mean±SEM are shown.
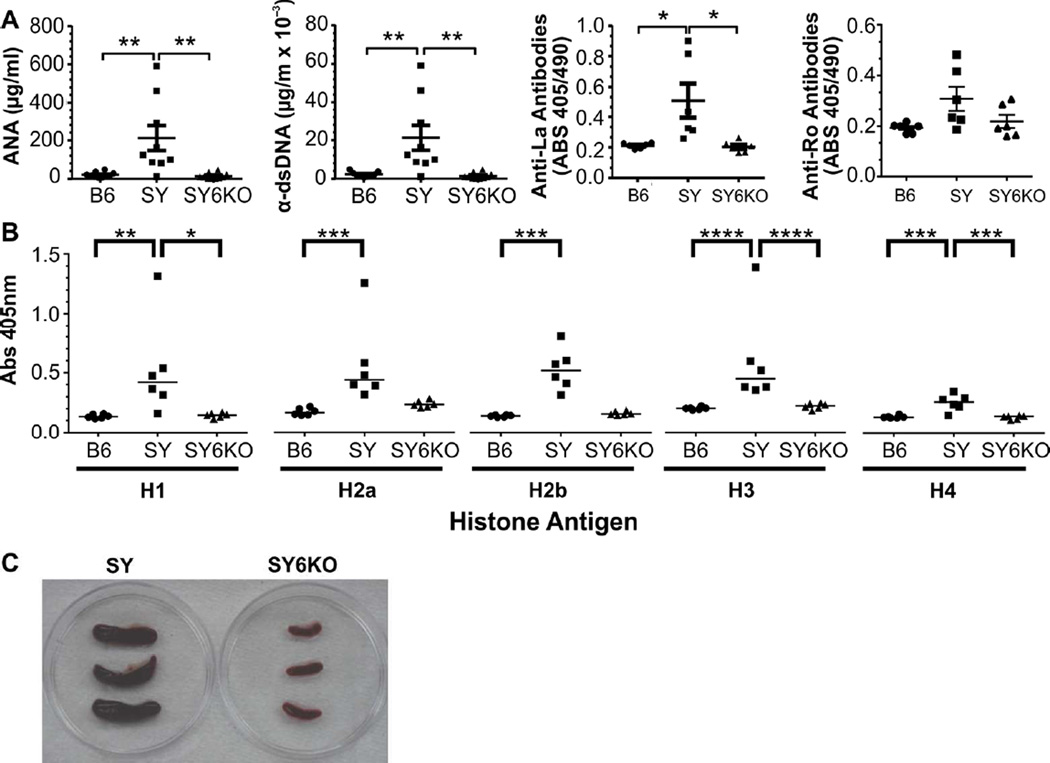
Autoantibodies are a defining feature of lupus-like autoimmunity, and increased IL-6 can drive terminal differentiation of activated IgG-producing B cells leading to autoantibody production. To determine whether genetic deficiency of IL-6 would ameliorate autoantibody production and reduce splenomegaly in SY mice, we analyzed serum samples from B6, SY and SY6KO mice for anti-nuclear, -dsDNA, -histone, -Ro and -La antibodies by ELISA, and qualitatively evaluated spleen size in SY and SY6KO mice. Deficiency of IL-6 in SY mice significantly reduced anti-nuclear, anti-dsDNA and anti-La antibody levels to that of control B6 mice as measured by ELISA (Fig 2A). Anti-Ro antibodies were elevated at low penetrance in SY mice and were not significantly different from that of B6 or SY6KO mice (Fig 2A, right panel). Antibodies against all histone subunits (H1, H2a, H2b, H3 and H4) were significantly increased in SY mice, and antibodies to H1, H3 and H4 were significantly reduced to B6 levels in SY6KO mice, although H2a and H2b antibodies showed non-significant trends towards a reduction, as well (Fig 2C). The spleens removed from SY6KO mice were definitively smaller than those of SY mice, indicating that IL-6 deficiency does abrogate splenomegaly (Fig 2C).
Fig. 2.
IL-6 deficiency eliminates serum autoantibodies and splenomegaly in B6.Sle1.Yaa (SY) mice. A, Serum IgG levels of antibodies to nuclear antigens (n=9–10) and dsDNA (n=9–10) and serum IgG reactivity to La/SSB (n=6) and Ro/SSA (n=6) as determined by ELISA. B, Serum IgG reactivity to individual histones (n=6) as determined by ELISA. C, Representative images of SY and SY6KO spleens. *p<0.05; **p<0.01; ***p<0.001 by one-way ANOVA with Bonferroni’s test for multiple comparisons for ANA and dsDNA and by Kruskall-Wallis test with Dunn’s multiple comparisons post-test for Ro, La and histones. Mean±SEM are shown for ANA and dsDNA, and horizontal lines depict median values in for Ro, La and histones.
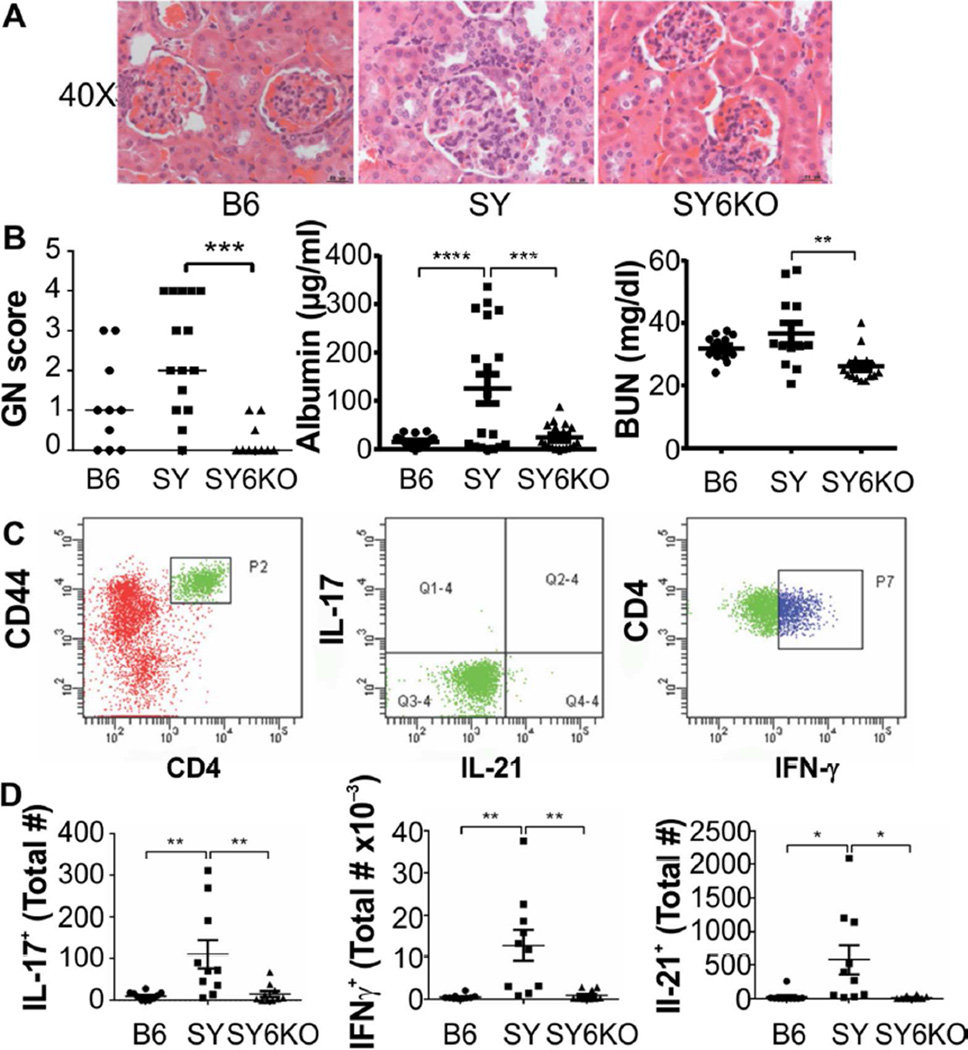
SY-associated tissue pathology is dependent upon IL-6
IL-6 deficiency profoundly improved glomerulonephritis scores in the SY model (Fig 3A and B). Where noted, patterns of SY-specific glomerulonephritis were of the global proliferative and global hyaline phenotype. These patterns were completely absent in SY6KO mice (not shown). Lymphocytic foci in the renal peripelvis were noted in SY but not B6 or SY6KO kidney sections (not shown). Furthermore, serum albumin and blood urea nitrogen (BUN) levels were reduced in SY6KO mice compared to SY mice (Fig 3B, center and right panels), indicating significant improvement in renal function in the absence of IL-6.
Fig. 3.
IL-6 deficiency normalizes renal disease in B6.Sle1.Yaa (SY) mice. A, Representative glomerular images and B, glomerulonephritis (GN) scores (n=10–15) (left), serum albumin levels (n=18–20) (center) and serum BUN levels (n=12–17) (right) from mice of the indicated genotype are shown. C, Representative flow cytometry gating scheme. D, Absolute numbers of T cells secreting IL-17 (left), IFN-γ (center) or IL-21 (right), per kidney in response to in vitro PMA/ionomycin stimulation are shown (n=10). Each symbol represents results of individual mice of the indicated genotype. * p<0.05; **p<0.01; ***p<0.001; ****p<0.0001 by Kruskall-Wallis test with Dunn’s multiple comparison post-test for GN score and by one-way ANOVA with Bonferroni’s test for multiple comparisons for Albumin and BUN. Horizontal lines indicate median values for GN score, while mean±SEM are shown for Albumin, BUN and panel C. Bars in A. are 20 Qm.
Activated, kidney-infiltrating CD4+ T cells have been demonstrated in SY mice (23). Here, we evaluated the cytokine secretion potential of CD4+ T cells infiltrating the kidneys in B6, SY and SY6KO mice. Significantly increased numbers of CD4+ T cells capable of secreting IL-17, IFN-γ or IL-21, were isolated from kidneys of SY mice but not from B6 or SY6KO mice (Fig 3C and D). Together with observations of significantly reduced lymphocytic infiltration in SY6KO mice, these data indicate that IL-6 can promote the accumulation of CD4+ T cells with effector cytokine production potential in the kidneys of SY mice.
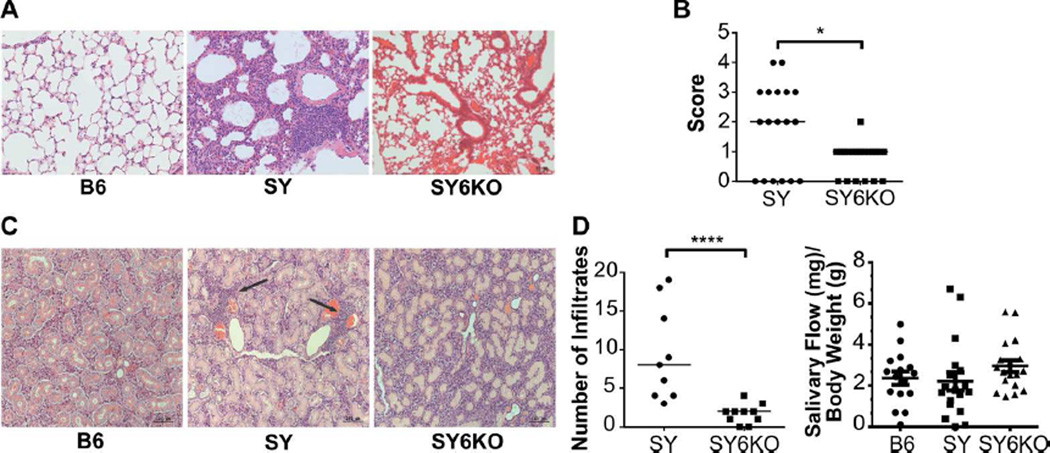
Lung pathology was evident in SY mice compared to B6 controls and was characterized by consistent, multifocal lesions of mild to moderate peribronchial lymphoplasmacytic accumulations with no evidence of etiologic agents (Fig 4A). Lungs of IL-6-deficient SY mice exhibited minimal pathology, appearing similar to B6 control animals. Although SY6KO mice had evidence of focal to multifocal peribronchial lymphoplasmacytic accumulations, they were significantly reduced compared to those of the SY mice and were considered to be within normal limits (Fig 4A, right panel). Lung sections from B6 mice were essentially normal with the exception of one mouse demonstrating a mild neutrophilic vasculitis of undetermined cause.
Fig. 4.
IL-6 deficiency ameliorates inflammation of lung and salivary gland tissue in B6.Sle1.Yaa (SY) mice. A, Representative sections of hematoxylin and eosin-stained lung tissue from mice of the indicated genotypes showing peribronchial lymphocytic infiltrates. B, Quantitation of lung inflammation in individual mice of the indicated genotype (n=18–20). C, Representative sections of hematoxylin and eosin-stained salivary gland tissue from mice of the indicated genotype. D, Number of salivary gland focal lymphocytic infiltrates (≥ 50 lymphocytes/focus) per section in mice of the indicated genotype (n=9–10, left panel) and saliva flow measurement from mice of the given genotype (n=15–18, right panel). Lymphocytic infiltrates in salivary glands of B6 mice were absent. *p<0.05; **p<0.01; ***p<0.00 by Mann-Whitney U-test. Horizontal lines depict median values for Score in B and Number of Infiltrates in D. Horizontal lines depict mean ± SEM for Salivary Flow in D. Bar in A is 50 Qm and Bars in C are 100 Qm.
Due to the association of anti-Ro/SS-A and La/SS-B antibodies with Sjogren’s syndrome, an autoimmune disease affecting exocrine gland tissue resulting in dryness, the effects of IL-6 on salivary gland infiltration and function were assessed. Focal lymphocytic infiltrates were observed in the salivary glands of SY mice that were significantly reduced in SY6KO and absent in B6 mice (Fig 4 D, left panel). Unexpectedly, no significant defects were detected in saliva production in SY mice compared to B6 controls (Fig 4D, right panel). Thus, IL-6 promotes glandular inflammation in the SY model.
Correction of lymphocytic alterations in spleens of IL-6 deficient SY mice
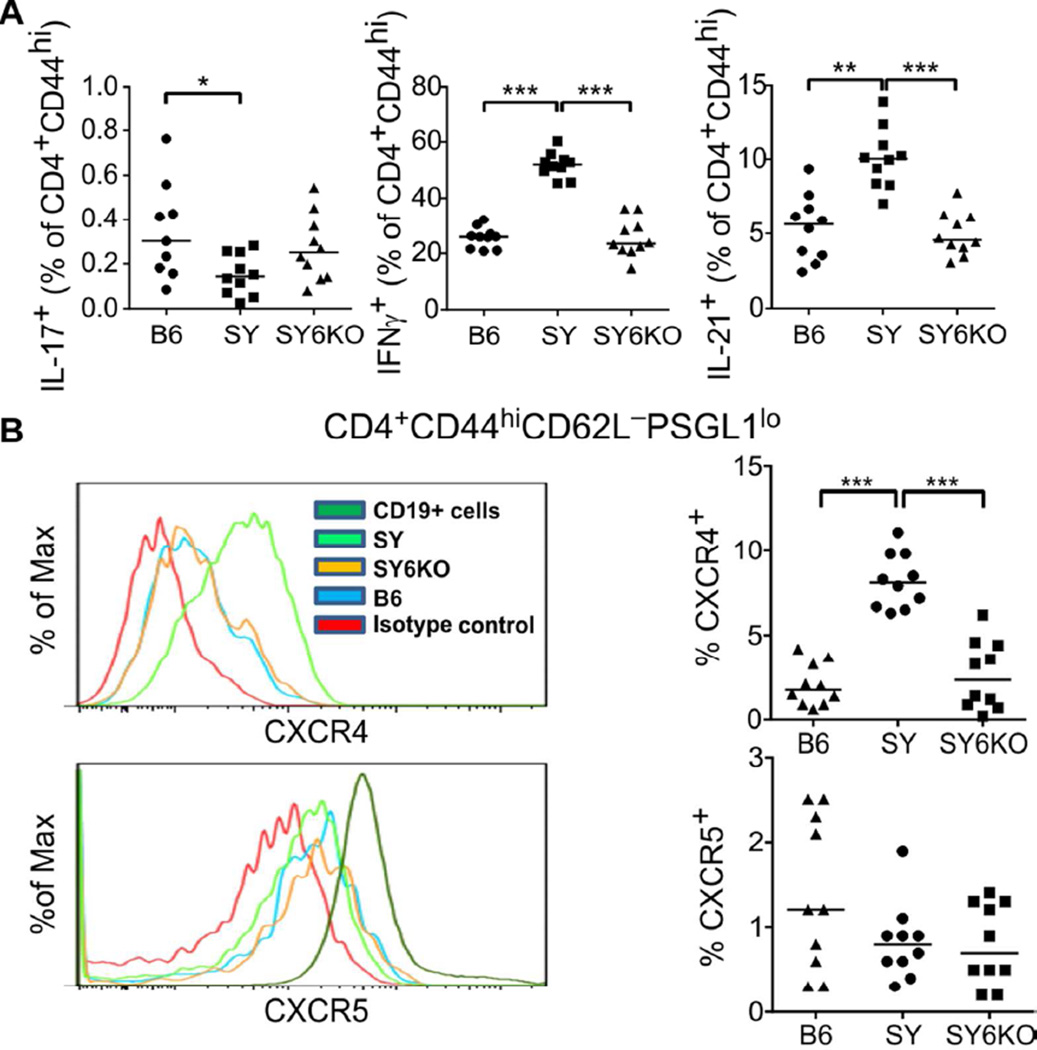
Lymphocytes play an important role in lupus-like pathogenesis of the SY mouse model (21, 23), as well as its predecessor, BXSB (37). The splenic CD4+ T cell compartment of SY mice exhibited alterations in T helper cell differentiation patterns as compared to B6 controls. SY animals showed elevated frequencies of IFN-γ-secreting CD4+ T cells, CXCR4+PSGL-1lo extrafollicular T helper cells (Texfh) and IL-21-producing CD4+ T cells, a reduction in IL-17-secreting CD4+ T cells and no change in CXCR5+PSGL-1lo T follicular helper (Tfh) cells (Fig 5A and B). Genetic deficiency of IL-6 restored the frequencies of T helper cell populations to those observed in control B6 mice (Fig 5A and B). IL-6 deficiency did not significantly impact the frequency of IL-17-producing T cells in SY6KO spleens, which were already significantly lower in SY than B6 mice (Fig 5A, left panel).
Fig. 5.
IL-6 deficiency corrects T lymphocytic alterations in spleens of B6.Sle1.Yaa (SY) mice. A, Frequencies of splenic CD4+CD44hi T cells secreting IL-17 (left), IFN-γ (center) or IL-21 (right), following in vitro restimulation with PMA/ionomycin. B, Representative histograms showing expression levels of CXCR4 and CXCR5 on PSGL-1lo memory CD4+ T cells from spleen (left) and percentages of splenic PSGL-1lo memory CD4+ T cells expressing surface CXCR4 (upper right) or CXCR5 (lower right) in individual mice of the indicated genotype. * p<0.05; **p<0.01; ***p<0.001 by Kruskall-Wallis test with Dunn’s multiple comparison post-test (n=10 mice/group). Horizontal lines depict median values.
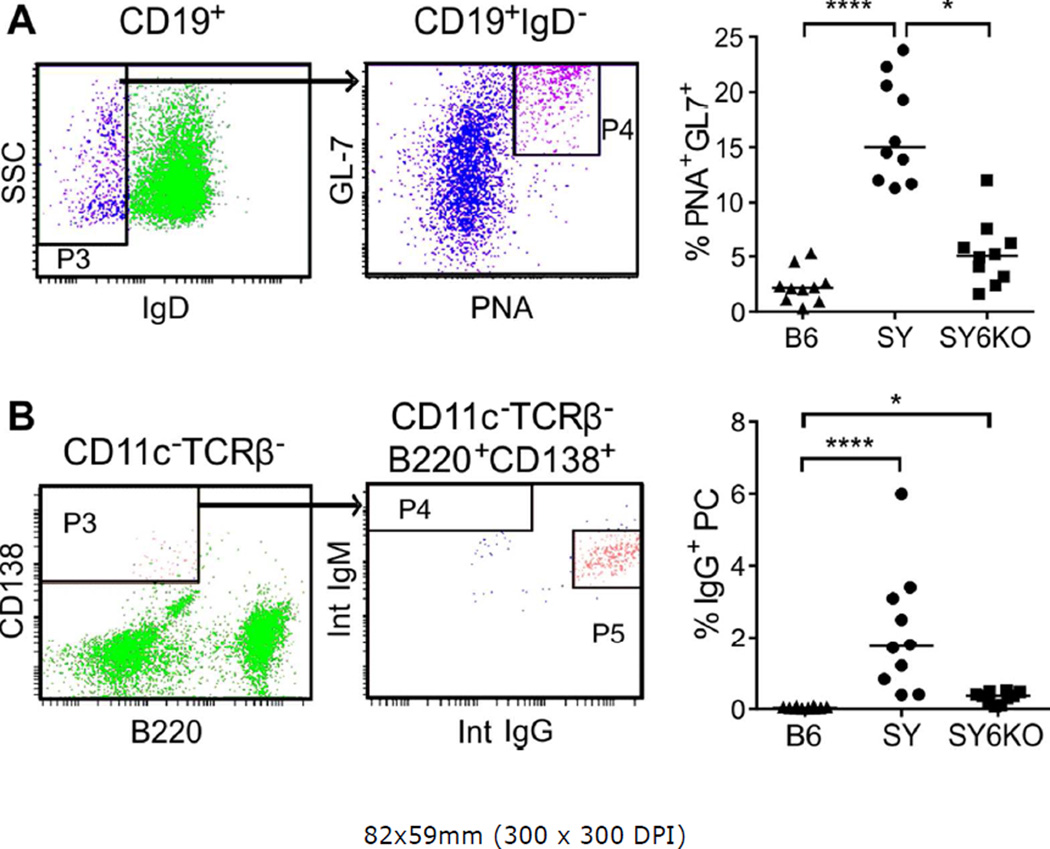
Despite the absence of increased frequencies of CXCR5+PSGL1lo Tfh cells in SY mice, we observed elevated frequencies of germinal center B cells (CD19+IgD−PNA+GL-7hi; Fig 6A) and IgG-producing plasma cells (CD11c−TCRβ−B220+CD138+; Fig 6B) in spleens of SY mice. IL-6 deficiency reduced the frequency of GCB and IgG+ plasma cells by 3-fold and 2-fold, respectively (Fig 6, right panels). The significant reductions in GCB and antibody-secreting cells are consistent with the reduction of autoantibody production observed in SY6KO mice.
Fig. 6.
IL-6 deficiency corrects B lymphocytic alterations in spleens of B6.Sle1.Yaa (SY) mice. A, Representative flow cytometry gating scheme for identification of B cells with a germinal center phenotype (left) and percentages of class-switched B cells expressing germinal center markers in individual mice of the indicated genotype (right) are shown. B, Representative flow cytometry gating scheme for the identification of plasma cells (left) and percentages of CD11c− TCRβ−B220+CD138+ plasma cells expressing intracellular IgG from individual mice (right). * p<0.05; ****p<0.0001 by Kruskall-Wallis test with Dunn’s multiple comparison post-test (n=10 mice/group). Horizontal lines depict median values.
Discussion
Monoclonal antibody-based therapies targeting IL-6 and its receptor have been in development for the last two decades as therapies for autoimmune diseases and cancer. Tocilizumab (Hoffman-La Roche), the first of these drugs, targets both membrane bound and soluble IL-6R and has been approved in the US for treatment of RA. The first clinical trial of Tocilizumab in SLE patients had limited success. Although the biologic was effective in reducing disease activity, autoantibody levels and circulating plasma cells, patients developed a problematic neutropenia, leaving them susceptible to infection (38). This antibody is currently being evaluated in a Phase 2 trial for the treatment of primary Sjogren’s syndrome (39). This and additional drugs targeting this pathway are being evaluated for efficacy in treating systemic juvenile idiopathic arthritis, cutaneous and systemic lupus, systemic sclerosis, Crohn’s disease and psoriatic arthritis, amongst others (24). Understanding the mechanisms of action of these therapies is critical for developing approaches to harness the beneficial effects of these drugs while minimizing unwanted side-effects and can also lead to new insights into disease pathogenesis.
Prior studies noted that IL-6 deficiency (40), IL-6R blockade in combination with anti-CD4 (41) and IL-6 inhibition (42), could relieve nephritis and dsDNA antibodies in MRLlpr/lpr or NZBWF1 mouse models of lupus. The capacity of IL-6 deficiency or inhibition to curtail pathology in these models and in the present study can be attributed not only to direct actions of IL-6 but also to indirect actions mediated through other processes regulated by IL-6. In the present study, we also found dramatic correction of these phenotypes in the SY model, with normalization of elevated IgG antibodies to nuclear antigens, dsDNA and histones, as well as elimination of glomerulonephritis and restoration of renal function. We further describe novel disease features of SY mice, including inflammation of the lungs, focal lymphocytic infiltrates of the salivary glands, and production of autoantibodies to La/SS-B, and furthermore demonstrate abrogation of these features by IL-6 deficiency. We did not detect defects in saliva production in these mice, which were evaluated at still relatively young ages of 5–8 months, whereas in humans Sjogren’s onset is typically in the fifth or sixth decade. Further evaluation of this model for reduced exocrine gland function may be warranted. Nevertheless, our observations provide support for the concept of targeting the IL-6 pathway in Sjogren’s Syndrome, which is characterized by the presence of IgG antibodies to Ro/SS-A and La/SS-B antigens and focal lymphocytic infiltrates in the salivary glands and can involve lungs. Our results also suggest that this therapy might be useful for treatment of rheumatic lung disease in general.
Class II HLA associations (43) and the class-switched and somatically-hypermutated nature of autoantibodies in SLE and Sjogren’s Syndrome, point to a critical role for T helper lymphocytes in these disorders. In fact, Lawson et al. demonstrated that αβ T cells are essential for disease in the Yaa-driven BXSB lupus mouse model (37). The presence of activated T lymphocytes within kidneys of SY mice (23) further supports the hypothesis that effector T lymphocytes participate directly in end-organ damage. Prior studies of IL-6 deficiency or blockade in mouse models of lupus have focused primarily on the pathology of nephritis and the associated autoantibodies and B cell phenotypes, with only a limited assessment of the T cell compartment. Experimental evidence has shown that IL-6 has a role in promoting Th17 and Tfh differentiation, thus leading to our hypothesis that ablation of this cytokine would modify accumulation of T helper cells with these phenotypes in SY mice. Furthermore, Serada et al. showed that treatment with an anti-IL-6R antibody in EAE prevented the accumulation of myelin-specific Th17 and Th1 cells, indicating a role for IL-6 in promoting both subsets (44). Additionally, recent work demonstrated the importance of IL-6 acting on T cells in Sjogren’s Syndrome; specifically, epithelial cells from patient salivary glands induced the differentiation of Tfh cells in vitro, and this required IL-6. However, the authors showed that IL-6 is not important for the production of IL-21 from these cells (19). In our study we assessed the cytokine production potential of CD4+ T cells in spleen and kidney. We observed that SY kidneys were primarily infiltrated with CD4+ T cells with capacity for IFN-γ production, though cells capable of making either IL-21 or IL-17 were also present, but at lower numbers. Unlike in SY kidneys, CD4+ T cells with IL-17 production potential were not elevated in SY spleens. IL-12 is produced by myeloid cells and is a canonical driver of IFN-γ-producing Th1 T cell polarization; additionally, variants in the IL12A gene were shown to associate with Sjogren’s syndrome (45). We observed that serum IL-12 levels were normalized by IL-6 deficiency. This is consistent with our prior observation that IL-6 deficiency corrects monocytosis in this model (9). Because both IFN-γ- and IL-17-secreting T cells have been documented in kidneys of human lupus nephritis patients, our observations in SY and SY6KO mice further support a critical role for IL6-mediated T cell pathogenesis in lupus nephritis (46–50).
Although Subramanian et al. demonstrated a Tfh gene signature in SY mice (21), evidence for elevated T cell expression of CXCR5, a canonical Tfh marker that directs these cells to the germinal center, has been minimal in this model (21, 23). In the present study, cell surface expression of CXCR4, CXCR5 and downregulation of PSGL-1, a surrogate marker for Bcl-6 expression (33, 34), was characterized on CD4+ T cells in the spleen to determine whether Tfh or their extra-follicular counterparts, Texfh, are more prominent in SY mice. Based on these analyses, we have successfully demonstrated that CXCR4-expressing Texfh, but not Tfh, are elevated in this model. The T cell profile we observed in the SY mice was similar to observations made in the genetically unrelated MRLlpr/lpr lupus model (33), where Texfh cells predominate and promote IgG production through an IL-21-dependent mechanism. Together, these data suggest that CXCR4-expressing T cells with an apparent Tfh gene signature are likely to be important players in the lupus phenotype.
This work further implicates Texfh cells in the pathogenesis of murine lupus and provides novel rationale for targeting the IL-6 pathway in SLE, Sjogren’s syndrome and rheumatic lung disease.
Acknowledgments
The experiments described in this work were supported by funding to ADF and CGH and were completed while JSM was affiliated with OMRF and OUHSC. The authors thank the OMRF Imaging Core Facility for expert preparation, staining and imaging of tissue sections, Beverly Hurt for assistance with figure preparation and Kathryn Bryant for clerical assistance. This publication was made possible by grants R01 AI048097 (ADF), P50 AR060804 (ADF) and T32 AI007633 (CGH) from the NIH. The contents are the sole responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Contributions: JSM, CGH and ADF wrote the manuscript. JSM, SAM, CGH, KMC and ADF designed the experiments. JSM, SAM, CGH, AWC, ZP, CL and ADF performed experiments and analysis.
References
- 1.Manson JJ, Rahman A. Systemic lupus erythematosus. Orphanet J Rare Dis. 2006;1:6. doi: 10.1186/1750-1172-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175(6):3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 3.Munger MA, Johnson B, Amber IJ, Callahan KS, Gilbert EM. Circulating concentrations of proinflammatory cytokines in mild or moderate heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1996;77(9):723–727. doi: 10.1016/s0002-9149(97)89206-5. [DOI] [PubMed] [Google Scholar]
- 4.Horii Y, Iwano M, Hirata E, Shiiki M, Fujii Y, Dohi K, et al. Role of interleukin-6 in the progression of mesangial proliferative glomerulonephritis. Kidney Int Suppl. 1993;39:S71–S5. [PubMed] [Google Scholar]
- 5.Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(19):7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattori E, Della Rocca C, Costa P, Giorgio M, Dente B, Pozzi L, et al. Development of progressive kidney damage and myeloma kidney in interleukin-6 transgenic mice. Blood. 1994;83(9):2570–2579. [PubMed] [Google Scholar]
- 7.Stein B, Kung Sutherland MS. IL-6 as a drug discovery target. Drug Discov Today. 1998;3(5):202–213. [Google Scholar]
- 8.Kitamura H, Kawata H, Takahashi F, Higuchi Y, Furuichi T, Ohkawa H. Bone marrow neutrophilia and suppressed bone turnover in human interleukin-6 transgenic mice. A cellular relationship among hematopoietic cells, osteoblasts, and osteoclasts mediated by stromal cells in bone marrow. Am J Pathol. 1995;147(6):1682–1692. [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda K, Malykhin A, Teague-Weber BN, Sun XH, Farris AD, Coggeshall KM. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6.Sle1.Yaa animals. Blood. 2009;113(19):4534–4540. doi: 10.1182/blood-2008-12-192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13(5):339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oates JC, Gilkeson GS. Mediators of injury in lupus nephritis. Curr Opin Rheumatol. 2002;14(5):498–503. doi: 10.1097/00002281-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Yu CL, Sun KH, Tsai CY, Hsieh SC, Yu HS. Anti-dsDNA antibody up-regulates interleukin 6, but not cyclo-oxygenase, gene expression in glomerular mesangial cells: a marker of immune-mediated renal damage? Inflamm Res. 2001;50(1):12–18. doi: 10.1007/s000110050718. [DOI] [PubMed] [Google Scholar]
- 13.Takemura T, Yoshioka K, Murakami K, Akano N, Okada M, Aya N, et al. Cellular localization of inflammatory cytokines in human glomerulonephritis. Virchows Arch. 1994;424(5):459–464. doi: 10.1007/BF00191429. [DOI] [PubMed] [Google Scholar]
- 14.Iwano M, Dohi K, Hirata E, Horii Y, Shiiki H, Ishikawa H. Induction of interleukin 6 synthesis in mouse glomeruli and cultured mesangial cells. Nephron. 1992;62(1):58–65. doi: 10.1159/000186996. [DOI] [PubMed] [Google Scholar]
- 15.Sun KH, Yu CL, Tang SJ, Sun GH. Monoclonal anti-double-stranded DNA autoantibody stimulates the expression and release of IL-1beta, IL-6, IL-8, IL-10 and TNF-alpha from normal human mononuclear cells involving in the lupus pathogenesis. Immunology. 2000;99(3):352–360. doi: 10.1046/j.1365-2567.2000.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 17.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29(1):138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science (New York, NY. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong YZ, Nititham J, Taylor K, Miceli-Richard C, Sordet C, Wachsmann D, et al. Differentiation of follicular helper T cells by salivary gland epithelial cells in primary Sjogren's syndrome. J Autoimmun. 2014 doi: 10.1016/j.jaut.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(12):6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(26):9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science (New York, NY. 2006;312(5780):1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 23.Fairhurst AM, Hwang SH, Wang A, Tian XH, Boudreaux C, Zhou XJ, et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. European journal of immunology. 2008;38(7):1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Shirota Y, Yarboro C, Fischer R, Pham TH, Lipsky P, Illei GG. Impact of anti-interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Annals of the rheumatic diseases. 2013;72(1):118–128. doi: 10.1136/annrheumdis-2012-201310. [DOI] [PubMed] [Google Scholar]
- 26.Mavragani CP, Moutsopoulos HM. Sjogren's Syndrome. Annu Rev Pathol. 2013 doi: 10.1146/annurev-pathol-012513-104728. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Wahren-Herlenius M, Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382(9894):819–831. doi: 10.1016/S0140-6736(13)60954-X. [DOI] [PubMed] [Google Scholar]
- 28.Peri Y, Agmon-Levin N, Theodor E, Shoenfeld Y. Sjogren's syndrome, the old and the new. Best Pract Res Clin Rheumatol. 2012;26(1):105–117. doi: 10.1016/j.berh.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Roescher N, Tak PP, Illei GG. Cytokines in Sjogren's syndrome. Oral Dis. 2009;15(8):519–526. doi: 10.1111/j.1601-0825.2009.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimoto K, Tanaka M, Kojima M, Setoyama Y, Kameda H, Suzuki K, et al. Regulatory mechanisms for the production of BAFF and IL-6 are impaired in monocytes of patients of primary Sjogren's syndrome. Arthritis Res Ther. 2011;13(5):R170. doi: 10.1186/ar3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson CP, Brayer J, Yamachika S, Esch TR, Peck AB, Stewart CA, et al. Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjogren's syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(13):7538–7543. doi: 10.1073/pnas.95.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekine H, Watanabe H, Gilkeson GS. Enrichment of anti-glomerular antigen antibody-producing cells in the kidneys of MRL/MpJ-Fas(lpr) mice. J Immunol. 2004;172(6):3913–3921. doi: 10.4049/jimmunol.172.6.3913. [DOI] [PubMed] [Google Scholar]
- 33.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. The Journal of experimental medicine. 2008;205(12):2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185(1):313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293(5537):2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 36.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189(11):1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawson BR, Koundouris SI, Barnhouse M, Dummer W, Baccala R, Kono DH, et al. The role of alpha beta+ T cells and homeostatic T cell proliferation in Y-chromosome-associated murine lupus. J Immunol. 2001;167(4):2354–2360. doi: 10.4049/jimmunol.167.4.2354. [DOI] [PubMed] [Google Scholar]
- 38.Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62(2):542–552. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. ClinicalTrials.gov[Internet] [cited 2014 Mar 10];Efficacy of Tocilizumab in Primary Sjogren's Syndrome (ETAP) 2013 [about 4 screens]
- 40.Cash H, Relle M, Menke J, Brochhausen C, Jones SA, Topley N, et al. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol. 2010;37(1):60–70. doi: 10.3899/jrheum.090194. [DOI] [PubMed] [Google Scholar]
- 41.Mihara M, Takagi N, Takeda Y, Ohsugi Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clinical and experimental immunology. 1998;112(3):397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang B, Gardner DB, Griswold DE, Bugelski PJ, Song XY. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology. 2006;119(3):296–305. doi: 10.1111/j.1365-2567.2006.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reveille JD. The genetic basis of autoantibody production. Autoimmun Rev. 2006;5(6):389–398. doi: 10.1016/j.autrev.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105(26):9041–9046. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren's syndrome. Nat Genet. 2013;45(11):1284–1292. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Apostolidis SA, Crispin JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus. 2011;20(2):120–124. doi: 10.1177/0961203310389100. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Ito S, Chino Y, Goto D, Matsumoto I, Murata H, et al. Laser microdissection-based analysis of cytokine balance in the kidneys of patients with lupus nephritis. Clinical and experimental immunology. 2010;159(1):1–10. doi: 10.1111/j.1365-2249.2009.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181(12):8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tucci M, Lombardi L, Richards HB, Dammacco F, Silvestris F. Overexpression of interleukin-12 and T helper 1 predominance in lupus nephritis. Clinical and experimental immunology. 2008;154(2):247–254. doi: 10.1111/j.1365-2249.2008.03758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masutani K, Akahoshi M, Tsuruya K, Tokumoto M, Ninomiya T, Kohsaka T, et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 2001;44(9):2097–2106. doi: 10.1002/1529-0131(200109)44:9<2097::AID-ART360>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]