Abstract
Background and Purpose
Whether the mammalian target of rapamycin (mTOR) pathway is protective against brain injury from stroke or is detrimental is controversial, and whether it is involved in the protective effects of ischemic postconditioning against stroke is unreported. Our study focuses on the protective role of mTOR against neuronal injury after stroke with and without ischemic postconditioning.
Methods
We used both an in vitro oxygen-glucose deprivation model with a mixed neuronal culture and hypoxic postconditioning, as well as an in vivo stroke model with ischemic postconditioning. Rapamycin, a specific pharmacological inhibitor of mTOR, and mTOR short hairpin RNA (shRNA) lentiviral vectors were used to inhibit mTOR activity. A lentiviral vector expressing S6K1, a downstream molecule of mTOR, was used to confirm the protective effects of mTOR. Infarct sizes were measured and protein levels were examined by Western blot.
Results
We report that stroke resulted in reduced levels of phosphorylated proteins in the mTOR pathway, including S6K1, S6, and 4EBP1, and that ischemic postconditioning increased these proteins. mTOR inhibition, both by the mTOR inhibitor rapamycin and by mTOR shRNA, worsened ischemic outcomes in vitro and in vivo, and abolished the protective effects of hypoxic postconditioning and ischemic postconditioning on neuronal death in vitro and brain injury size in vivo. Overexpression of S6K1 mediated by lentiviral vectors significantly attenuated brain infarction.
Conclusions
mTOR plays a crucial protective role in brain damage after stroke and contributes to the protective effects of ischemic postconditioning.
Keywords: ischemic postconditioning, stroke, mTOR, S6K1
Introduction
The protective effects of ischemic postconditioning (IPC)—the interruption of reperfusion after stroke—are well established in various stroke models,1 yet the underlying protective mechanisms are poorly understood.2 The mammalian target of rapamycin (mTOR) signaling pathway plays a central role in metabolism, cell growth, differentiation, development, and cell survival.3 A few studies have shown that brain injury induced by ischemia involves the mTOR pathway.4–9 Whether it is beneficial or detrimental to neuronal survival remains controversial,10 and its contribution to the neuroprotective effects of IPC has not been reported.
mTOR has a large molecular weight of ~ 289 kDa and belongs to the phosphatidylinositol 3-kinase (PI3K)-related kinase family11 (Figure 1A). Many triggers modulate mTOR activity, which is assessed by phosphorylation levels of mTOR. mTOR activity is inhibited by hypoxia, adenosine triphosphate depletion, and DNA damage, but activated by nutrients (amino acids) and growth factors. These inhibitory or stimulatory factors also modulate Akt, which is known to protect against ischemic brain injury,10 probably through activation of the mTOR cell signaling pathway. mTOR forms two complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), to regulate mTOR activity.11 mTORC1 function has been extensively studied, while mTORC2 function remains elusive. mTORC1 components include PRAS40, mTOR, and Raptor. The two most studied mTOR substrates are S6K1 and 4EBP1. S6K1 results in S6 phosphorylation, which leads to protein synthesis.12 mTOR phosphorylates 4EBP1, which is then released from eIF-4E, allowing the latter to promote protein synthesis and cell survival.3,11,13
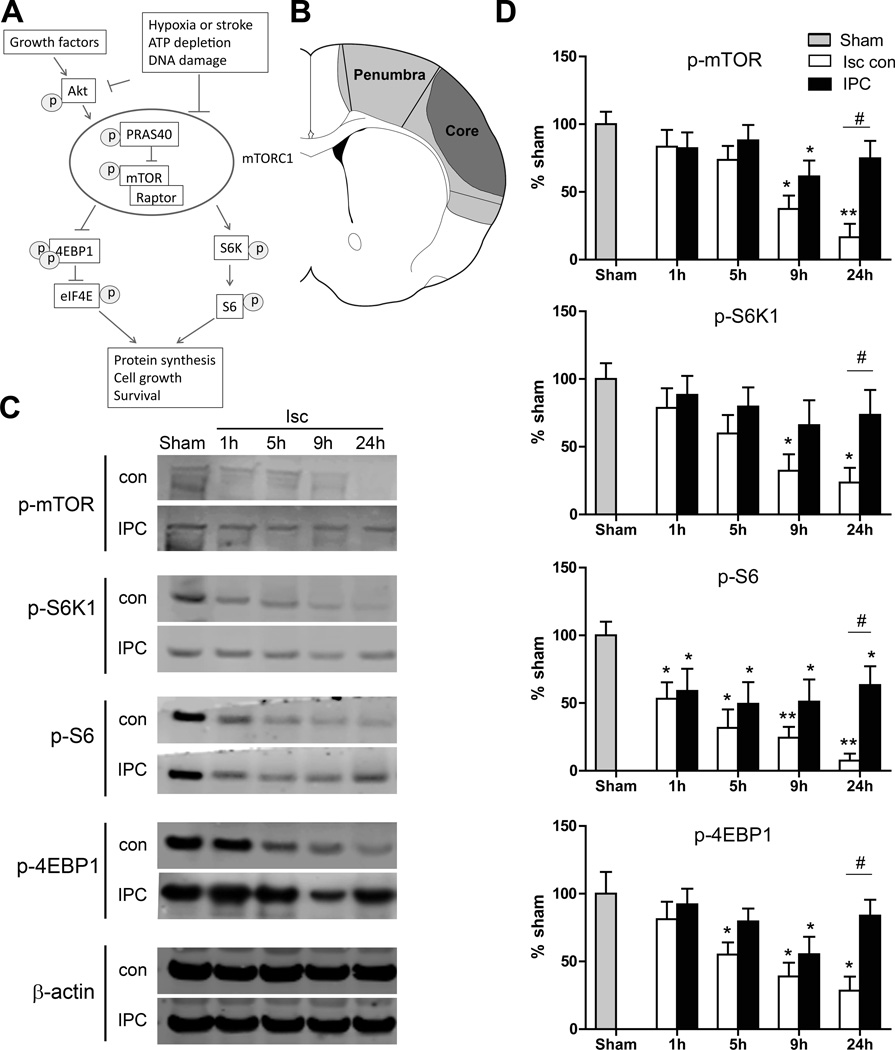
Figure 1.
The effects of IPC on protein expression in the mTOR pathway. A, Diagram showing the major proteins in the mTOR cell signaling pathway. P indicates phosphorylation. B, Brain schematic showing the ischemic penumbra and core regions. The ischemic penumbra, which represents tissue spared by IPC, was dissected for Western blot analysis. The core is the area of infarct that received IPC. C, Representative protein bands of a Western blot for p-mTOR, p-S6K1, p-S6 and p-4EBP1 at 1, 5, 9, and 24 hours post-stroke. β-actin indicates equal protein loading. D, Relative optical densities of protein bands, normalized to those in the sham group and expressed as percentages. *, ** vs sham, P<0.05, 0.01, respectively; #P<0.05, between the two indicated groups. n=8/group. Isc con, ischemic control; IPC, ischemic postconditioning.
As we and others have shown, IPC improves glucose uptake, reduces free radical generation,14 inhibits inflammation,15,16 and promotes protein activity in the PI3K/Akt pathway17 and, as introduced above, these factors all modulate mTOR activity. We hypothesized that IPC protects the brain by modulating mTOR activity. In this study, we examined whether mTOR plays a crucial role in the protective effect of IPC against stroke.
Methods
Methods are detailed in the online-only Data Supplement. Animal experiments were conducted according to protocols approved by the Stanford Institutional Animal Care and Use Committee (IACUC) and NIH Guidelines for Care and Use of Laboratory Animals.
Construction of Lentiviral Vectors
As we were unable to clone the mTOR gene into the plasmid backbone, we instead constructed lentiviral vectors expressing S6K1 and enhanced green fluorescent protein (eGFP); S6K1 is a downstream protein of mTOR and an indicator of mTOR activity. The control vector was a lentiviral plasmid backbone with only eGFP inserted without the S6K1 sequence. In addition, we used lentiviral vectors containing mTOR short hairpin RNA (shRNA) to inhibit mTOR expression and a scrambled shRNA gene as a negative control for mTOR shRNA.
In Vitro Oxygen-Glucose Deprivation (OGD) Model and Studies
Primary mixed neuronal cultures were prepared from rat fetal brains and experiments were performed days 9 to 11 after preparation. OGD was induced for 6 hours in a hypoxic chamber and in vitro hypoxic postconditioning (HPC) was performed by 3 cycles of 15 minutes restoration of glucose and oxygen and 15 minutes OGD. Cell viability was quantified by measuring lactate dehydrogenase release 18 hours after OGD restoration using a previously described colorimetric assay.18 The effects of rapamycin, mTOR shRNA and S6K1 vectors on neuronal injury were studied.
Focal Cerebral Ischemia and IPC Model
Focal cerebral ischemia was generated by 30-minute occlusion of the bilateral common carotid arteries (CCAs) with permanent occlusion of the distal middle cerebral artery in male Sprague-Dawley rats (300–350 g) as described previously.14 Immediately after bilateral CCA release, IPC was performed by 30 seconds of CCA suture release followed by 10 seconds of occlusion, which was repeated 3 times, as described.14
For in vivo drug and lentiviral vector injections, the drug and viral vectors were coded to blind the surgeon performing both the virus injections and stroke models. Rapamycin (5 µl, 1 mM) was infused into the ventricular space ipsilateral to the ischemia using a microsyringe pump controller 1 hour prior to ischemia. Lentiviruses were injected by a 10-µl needle into the left cortex 5 days prior to ischemia. Infarct sizes were assessed 2 days after stroke using cresyl violet staining. The area of the infarcted cortex was measured by a person who was blinded to the animal’s condition, normalized to the contralateral cortex, and expressed as a percentage, as described previously.14
Protein Preparation and Western Blot
To confirm the efficacy of gene transfer of S6K1 and knockdown effects of mTOR shRNA, the mixed neuron cells were grown in 6-well plates and transfected with lentiviral vectors. The cells were harvested 48 hours after gene transfer, and proteins were extracted for Western blotting.
To study the effects of rapamycin and IPC on protein levels in the mTOR pathway, animals were sacrificed at 1, 5, 9, and 24 hours after stroke (n=8). A sham surgery group was used as the control. Brain tissues from the ischemic penumbra were harvested to detect the expression of proteins in the mTOR pathway by Western blot (Figure 1). However, to study the effects of gene transfer on protein expression, brain tissue 1 mm in diameter around the needle track was dissected for Western blotting.
Statistical Analysis
Graphpad Prism 5.0 software was used for statistical analyses. One-way or two-way ANOVA was used followed by the Fisher least significant difference post hoc test. Tests were considered significant at P values <0.05. Data are presented as mean±SEM.
Results
IPC Attenuated Stroke-Induced Reductions in Protein Phosphorylation in the mTOR Pathway
We first characterized the effects of stroke and IPC on levels of phosphorylated mTOR, S6K1, S6, and 4EBP1proteins in the mTOR pathway (Figure 1). In the penumbra p-S6 levels were decreased as early as 1 hour after stroke, p-4EBP1 levels were decreased starting at 5 hours, and all 4 proteins including p-mTOR were significantly decreased 9 and 24 hours after stroke. With IPC, significant improvements were seen at 24 hours in all phosphorylated protein levels (Figure 1C and 1D). We also measured total protein levels, and they were relatively stable post-stroke compared with their phosphorylated counterparts, but most were still significantly reduced at 24 hours; IPC had no significant effect on their expression (Supplemental Figure I).
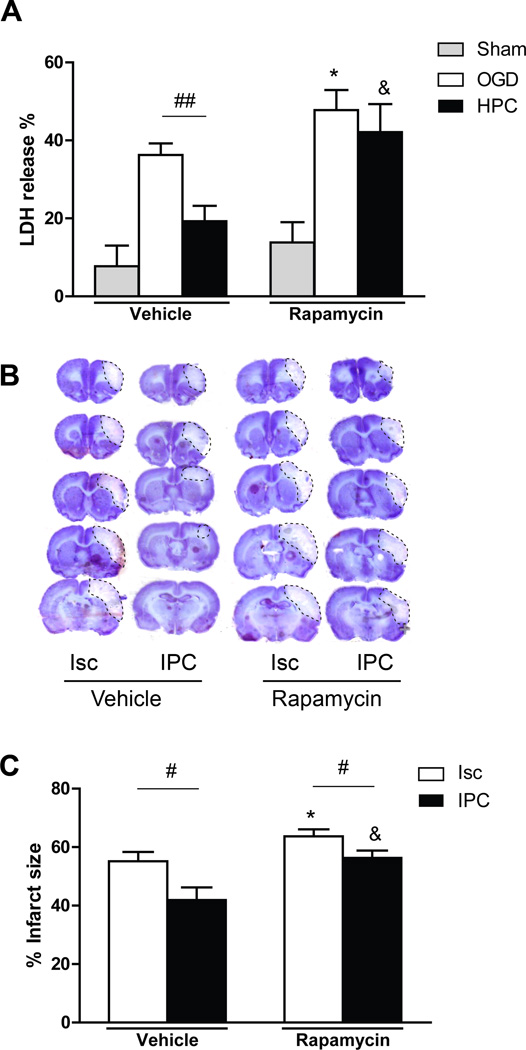
Rapamycin Worsened Infarction In Vivo and Inhibited Protection by IPC In Vivo and by HPC In Vitro
After demonstrating that IPC promoted mTOR activity and associated phosphorylated proteins in the mTOR pathway (Figure 1), we tested whether the specific mTOR inhibitor rapamycin could block its protective effects in vitro and in vivo. In vitro, rapamycin worsened cell death induced by OGD in a mixed neuronal culture (Figure 2A). HPC mitigated this cell death, but rapamycin abolished HPC’s protective effects (Figure 2A). The in vivo study confirmed that rapamycin worsens infarct sizes, with or without IPC (Figure 2B and 2C).
Figure 2.
Rapamycin blocked the protective post-stroke effects of HPC and IPC both in vitro and in vivo. A, Effects of rapamycin and HPC on neuronal death as measured by LDH release in vitro. LDH release was measured 18 hours post-OGD. All data from cultures with OGD were normalized to the values of control, non-OGD samples treated with vehicle that did not contain rapamycin. n=16/group. LDH = lactate dehydrogenase. OGD, oxygen glucose deprivation; HPC, hypoxic postconditioning. B, Representative coronal sections of ischemic brains stained by cresyl violet method. Infarct regions are traced. C, Average infarct sizes were measured from 5 coronal sections. n=8/group. *, & vs the vehicle of the indicated group, respectively, P<0.05; #, ##P<0.05, 0.01, respectively, between the two indicated groups.
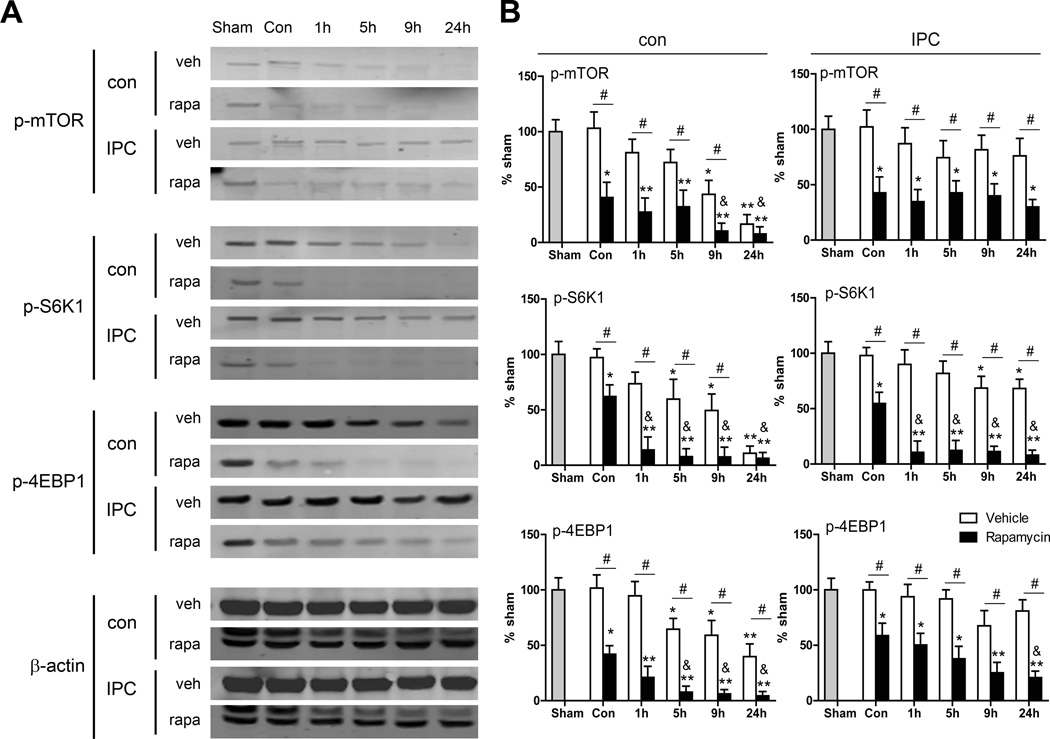
The effects of rapamycin on protein levels of p-mTOR, p-S6, and p-4EBP1 in animals without stroke and after stroke with or without IPC were examined (Figure 3). Rapamycin reduced these protein levels in non-ischemic brains. Rapamycin also inhibited these protein levels in ischemic brains without IPC in the penumbra from 1 to 24 hours. Further, rapamycin treatment significantly reduced these proteins at all time points in the ischemic penumbra with IPC (Figure 3A and 3B). Nevertheless, it did not alter total protein levels compared with vehicle (Supplemental Figure II).
Figure 3.
The effects of rapamycin on protein levels in the mTOR pathway. A, Representative protein bands of major proteins in the mTOR pathway. B, Quantified levels of each protein. *, ** vs. sham brain, P<0.05, 0.01, respectively; &, vs. con (non-ischemic control with rapamycin injection), P<0.05; #, between the two indicated groups, P<0.05, 0.01, respectively. n=8/group. Isc, ischemic; con, control; IPC, ischemic postconditioning; rapa, rapamycin.
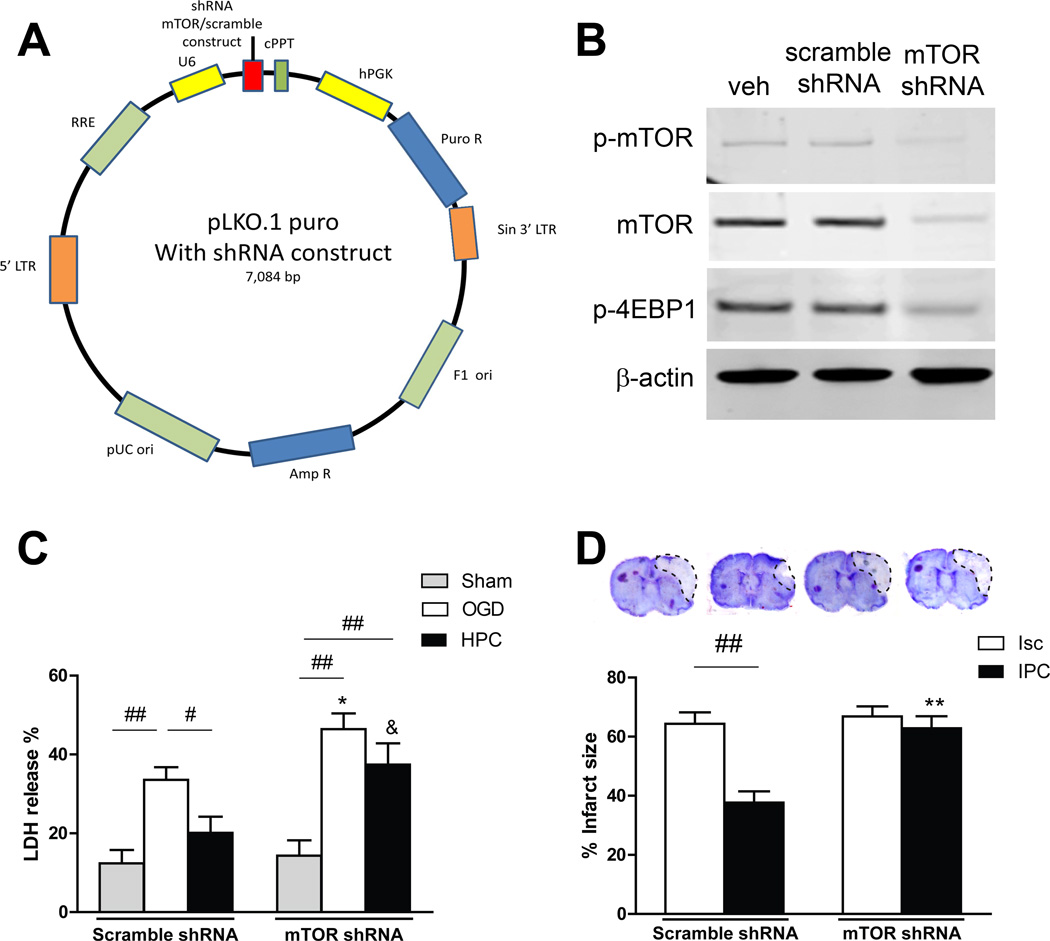
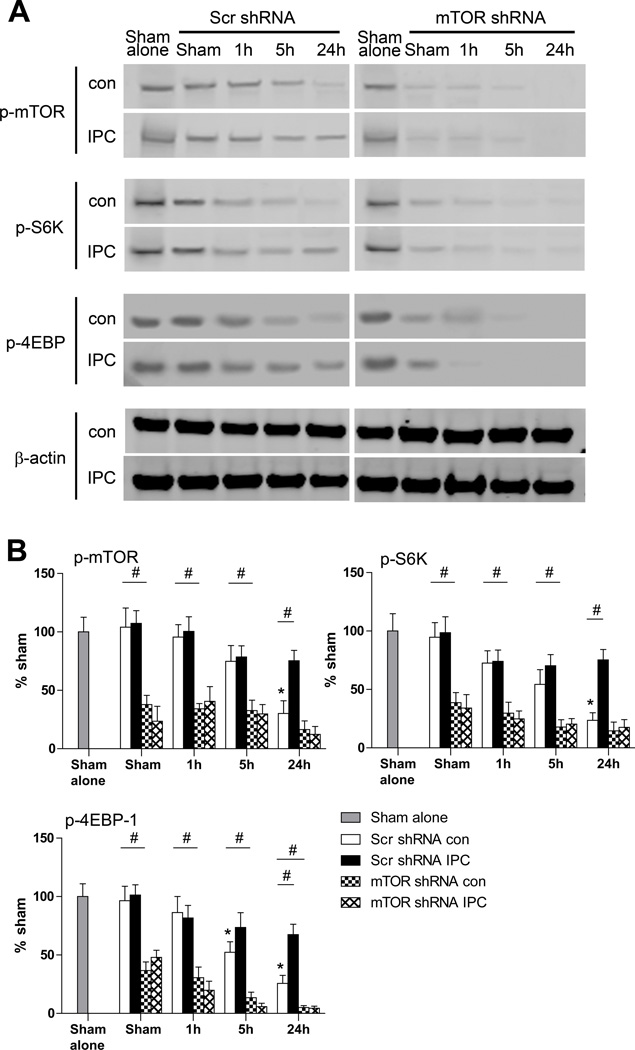
mTOR shRNA Abolished the Protective Effects of HPC In Vitro and IPC In Vivo
Rapamycin might generate systemic effects even when injected locally if it enters the blood circulation. We employed a lentiviral mTOR shRNA to further analyze the effects of mTOR inhibition on pathological and protective outcomes of stroke and IPC (Figure 4A). Western blot results indicate that mTOR shRNA transfected in cell culture successfully blocked protein expression of p-mTOR and its downstream proteins, p-4EBP1 (Figure 4B). Like rapamycin, transfection of mTOR shRNA worsened neuronal stroke damage both in vitro and in vivo, and abolished the protective effects of HPC in vitro and IPC in vivo (Figure 4C and 4D).
Figure 4.
Detrimental effects of the mTOR shRNA lentiviral vector in stroke. A, Schematic backbone of lentiviral vector of pLKO.1 puro with shRNA construct. B, Effects of gene transfer in primary neuronal cultures on protein expression of p-mTOR and p-4EBP1 in the mTOR pathway. mTOR shRNA was confirmed to reduce protein levels of p-mTOR and p-4EBP1. Veh, vehicle. C, Effects of mTOR shRNA gene transfer on neuronal death in the OGD model measured by LDH release. shRNA exacerbated neuronal death in cell culture that underwent OGD, and abolished the protective effect of HPC. * vs OGD group with scramble shRNA, P<0.05; & vs post group with scramble shRNA, P<0.05; #, ##, P<0.05, 0.01, between indicated two groups, respectively. n=16/group. LDH = lactate dehydrogenase. OGD, oxygen-glucose deprivation; HPC, hypoxic postconditioning. D, The effects of mTOR shRNA on infarct sizes in ischemic brains with or without IPC. Top: Representative coronal brain sections for infarction stained by cresyl violet. Bottom: Bar graphs show infarct sizes. mTOR shRNA enlarged infarction in animals treated with control stroke and abolished the protective effects of IPC. ** vs scramble shRNA, ischemic group, P<0.01; ##, P<0.01, between indicated two groups. n=8/group. Isc, ischemic; IPC, ischemic postconditioning.
We further examined the in vivo effects of mTOR shRNA on major proteins expressed in the mTOR pathway, and confirmed that it reduced protein levels of p-mTOR, p-S6K1 and p-4EBP1 in ischemic brains both with and without IPC (Figure 5). We confirmed that mTOR shRNA inhibited total protein levels of mTOR (Supplemental Figure III).
Figure 5.
The effects of mTOR shRNA on protein levels in the mTOR pathway. A, Representative protein bands in the mTOR pathway measured by Western blot. Brain tissues from penumbra were collected and analyzed at 1, 5, 9, and 24 h post-stroke with or without IPC. B, Bar graphs show quantified levels of each protein. *, vs. sham brain, P<0.05; #, P<0.05, between the two indicated groups. n=8/group. Scr, scramble, con, control; IPC, ischemic postconditioning.
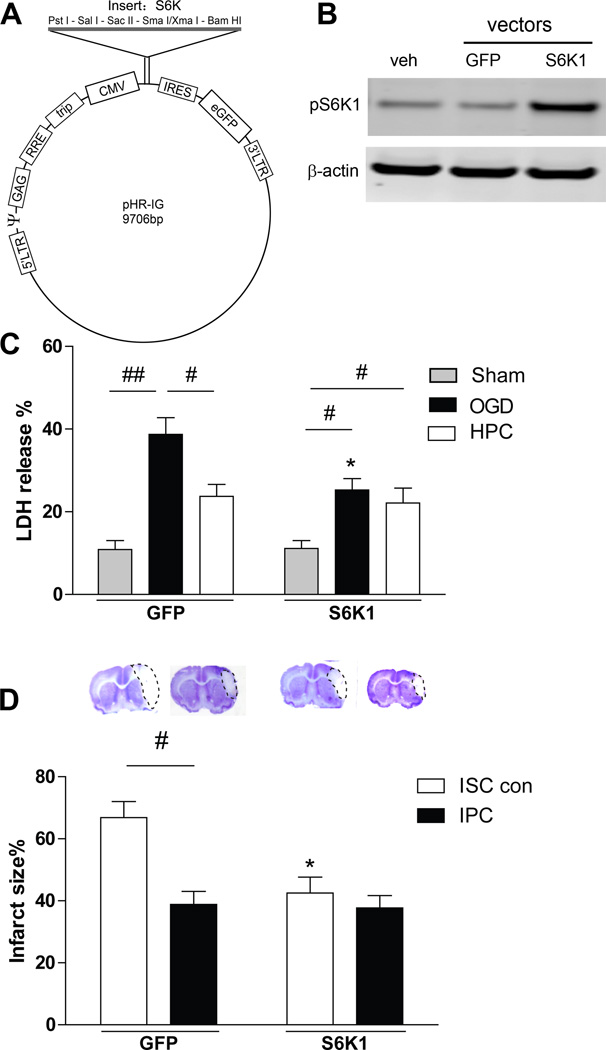
S6K Gene Transfer Protected Against Brain Injury
After confirming that mTOR inhibition worsened brain injury, we intended to study if gene transfer of an mTOR lentiviral vector alone would inhibit stroke-induced brain injury. When we were unable to construct an mTOR vector, likely as a result of its large molecular weight, we instead constructed a lentiviral vector of S6K1 (Figure 6A), one of the most studied proteins downstream of mTOR. We confirmed that gene transfer of the S6K1 vector resulted in overexpression of p-S6K1 and S6K1 (Figure 6B, Supplemental Figure IV). Both in vitro and in vivo studies demonstrated that S6K1 gene transfer inhibited neuronal death and brain injury, but there were no synergistic effects with HPC in vitro or with IPC in vivo (Figure 6C and 6D). It is likely that IPC or S6K1 was protective to a threshold beyond which neuronal injury could not be further reduced.
Figure 6.
S6K1 lentiviral vector construction and in vitro and in vivo effects. A, Schematic backbone of lentiviral vector of pHR’tripCMV-RES-eGFP with S6K1 construction. B, Western blot confirmation of the effects of gene transfer in primary neuronal cultures on protein expression of p-S6K1. Gene transfer resulted in robust increases in protein expression of p-S6K1. Veh, vehicle. C, The effects of S6K1 gene transfer on neuronal death in the OGD model measured by LDH release. * vs the OGD with green fluorescent protein control vectors, P<0.05. #, ##, P<0.05, 0.01, respectively, between two indicated groups. n=16/group. D, Effects of S6K1 gene transfer on infarct sizes in ischemic brains with or without IPC. Representative coronal sections of ischemic brains and statistical results are shown. * vs ischemic control stroke (ISC con), P<0.05; #, P<0.05, between two indicated groups. n=8/group.
Discussion
The mTOR pathway is involved in brain injury induced by ischemia, however whether it is beneficial or detrimental to the ischemic brain remains controversial. There is evidence that several neuroprotectants upregulate mTOR activity, including the antioxidants melatonin and estradiol,19,20 and the anti-inflammatory agent silibinin.7 In addition, rapamycin treatment increased cleaved caspase protein levels and promoted neuronal death after hypoxia-ischemia.21 Furthermore, S6K1 gene deletion in cultured astrocytes resulted in increased apoptosis, and S6K1 inhibition increased mortality and infarction in mice.9 In contrast, studies have also reported that mTOR is detrimental to neuronal survival. Ghiglieri et al22 showed that the mTOR inhibitor rapamycin prevented induction of post-ischemic long-term potentiation in an in vitro OGD model in brain slices, and attenuated ischemic cell damage. Wang et al6 showed that rapamycin protected against neuronal injury by autophage induction after stroke. In neonatal hypoxic ischemia, Balduini et al23 also found that mTOR/S6K1 inhibition by rapamycin reduced neuronal injury via autophage induction.
Here we first demonstrated that levels of multiple phosphorylated proteins in the mTOR pathway were reduced soon after stroke onset in the ischemic brain, including mTOR, S6K1, S6, and 4EBP1, suggesting that protein degradation in the mTOR pathway precedes final brain injury. Our study includes a detailed characterization of several post-stroke protein levels in the mTOR pathway, whereas previous studies often investigated only one or two proteins at a single time point in the acute post-stroke phase.7,8 Shi et al8 reported decreased levels of p-mTOR after stroke, but this was based on one time point measured at 24 hours. Pastor et al9 showed that protein levels of p-S6K1 and p-S6 were reduced 4 to 48 hours after OGD, however, no in vivo experiments were performed. Moreover, these results conflict with other reports showing that p-S6K1 increased at day 17 or day 4 after stroke4 and that p-mTOR protein expression increased at 2 to 24 hours in developing rat brains with hypoxia-ischemia.21 We systemically measured phosphorylation of major proteins in the mTOR pathway after stroke, both with and without IPC, and showed reduced-phosphorylation of p-mTOR, p-S6K1, p-S6, and p-4EBP1 at multiple time points. This was attenuated by IPC, suggesting that IPC improved mTOR activity and corresponded to its protective effects. Because we and others have previously shown that Akt plays a critical role in IPC,1,17 and that Akt promotes mTOR activity,24 we cannot exclude the possibility that IPC may improve mTOR activity via enhancement of Akt activity and that Akt may be a significant factor in the role of mTOR in IPC. Nevertheless, more experiments are required to prove this hypothesis.
We also showed for the first time that both the specific mTOR inhibitor rapamycin and mTOR shRNA enlarged infarction, and that S6K1 gene transfer reduced neuronal death in vitro and inhibited infarction in vivo, suggesting a neuroprotective role for mTOR in stroke. In most previous studies rapamycin was the sole mTOR inhibitor used to address the effects of the mTOR pathway in stroke,5,6,25–27 and both beneficial5,19,20 and detrimental6,22 effects of the mTOR pathway have been reported. In contrast, we used multiple approaches to show that both mTOR shRNA and rapamycin promoted neuronal death in vitro as well as enlarged infarction in a controlled stroke in vivo, and attenuated the protective effects of IPC both in vitro and in vivo. In addition, we showed that S6K1 gene transfer reduced infarction. Taken together, our studies support a neuroprotective role for the mTOR pathway in stroke.
Although we have provided strong evidence that mTOR activity supports neuronal survival after stroke both with and without IPC, how it achieves such protection requires further investigation. IPC may reduce acute infarction by blocking apoptosis through mTOR activation, as IPC inhibits apoptosis after stroke1 and mTOR blocks apoptosis.28 Estradiol administration has been shown to inhibit apoptosis in the post-stroke ischemic cortex by promoting the mTOR/p70S6K1 signaling pathway.19,20 Deletion of PTEN, a tumor suppressor protein, blocked neuronal apoptosis by enhancing mTOR phosphorylation.8 Furthermore, S6K1 gene deletion impaired Bcl-2-associated death promoter (BAD) phosphorylation and decreased Bcl-2 and Bcl-xL expression in astrocytes.10 BAD phosphorylation interacts with protein 14-3-3-sequestering pro-apoptotic proteins, and both Bcl-2 and Bcl-xL are anti-apoptotic proteins that maintain mitochondrial function. Reduced p-BAD, Bcl-2, and Bcl-xL result in mitochondria-mediated apoptosis.10 Alternatively, the mTOR pathway may also generate neuroprotection by promoting protein synthesis, which contributes to the protective effects of IPC. We have shown that 9 to 24 hours after stroke IPC, protein levels of p-eIF4E and p-S6K1 improve, which are critical for protein synthesis.29 Brain injury induced by stroke has been associated with protein synthesis inhibition,30 which is regulated by mTOR, p-eIF4E, and p-S6K1 activity. IPC may enhance protein synthesis, promoting compensation for protein loss after stroke, synaptic plasticity, and injury repair.
In conclusion, we have employed for the first time multiple approaches to inhibit and promote mTOR activity in the setting of stroke in vitro and in vivo and have provided strong evidence that mTOR activity contributes to neuronal survival in stroke both with and without IPC or HPC. These results indicate that mTOR is a potential target for neuroprotection in stroke treatment.
Supplementary Material
Acknowledgments
The authors thank Elizabeth Hoyte for figure preparation and Cindy H. Samos for manuscript editing.
Sources of Funding
This study was supported by AHA grant in aid 10GRNT4200024 and 1R01NS064136-01 (H.Z.).
Footnotes
Disclosures
None.
References
- 1.Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:873–885. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao H. Hurdles to clear before clinical translation of ischemic postconditioning against stroke. Translational stroke research. 2013;4:63–70. doi: 10.1007/s12975-012-0243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jewell JL, Guan KL. Nutrient signaling to mtor and cell growth. Trends in biochemical sciences. 2013;38:233–242. doi: 10.1016/j.tibs.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L, Jia J, Zhou X, Xiao Y, Wang Y, Mao X, et al. Delayed administration of a pten inhibitor bpv improves functional recovery after experimental stroke. Neuroscience. 2013;231:272–281. doi: 10.1016/j.neuroscience.2012.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu F, Wu J, Nicholson AD, Echeverry R, Haile WB, Catano M, et al. Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:9848–9858. doi: 10.1523/JNEUROSCI.1241-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy. 2012;8:77–87. doi: 10.4161/auto.8.1.18274. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Wang Z, Zhang X, Zhang X, Dong L, Xing Y, et al. Protection by silibinin against experimental ischemic stroke: Up-regulated pakt, pmtor, hif-1alpha and bcl-2, down-regulated bax, nf-kappab expression. Neuroscience letters. 2012;529:45–50. doi: 10.1016/j.neulet.2012.08.078. [DOI] [PubMed] [Google Scholar]
- 8.Shi GD, OuYang YP, Shi JG, Liu Y, Yuan W, Jia LS. Pten deletion prevents ischemic brain injury by activating the mtor signaling pathway. Biochemical and biophysical research communications. 2011;404:941–945. doi: 10.1016/j.bbrc.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 9.Pastor MD, Garcia-Yebenes I, Fradejas N, Perez-Ortiz JM, Mora-Lee S, Tranque P, et al. Mtor/s6 kinase pathway contributes to astrocyte survival during ischemia. The Journal of biological chemistry. 2009;284:22067–22078. doi: 10.1074/jbc.M109.033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Qu Y, Tang B, Xiong T, Mu D. Role of mammalian target of rapamycin in hypoxic or ischemic brain injury: Potential neuroprotection and limitations. Reviews in the neurosciences. 2012;23:279–287. doi: 10.1515/revneuro-2012-0001. [DOI] [PubMed] [Google Scholar]
- 11.Laplante M, Sabatini DM. Mtor signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruvinsky I, Meyuhas O. Ribosomal protein s6 phosphorylation: From protein synthesis to cell size. Trends in biochemical sciences. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Johnson SC, Rabinovitch PS, Kaeberlein M. Mtor is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: Ischemic postconditioning reduces infarct size after focal ischemia in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- 15.Feng R, Li S, Li F. Toll-like receptor 4 is involved in ischemic tolerance of postconditioning in hippocampus of tree shrews to thrombotic cerebral ischemia. Brain research. 2011;1384:118–127. doi: 10.1016/j.brainres.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Kong Y, Rogers MR, Qin X. Effective neuroprotection by ischemic postconditioning is associated with a decreased expression of rgma and inflammation mediators in ischemic rats. Neurochem Res. 2013;38:815–825. doi: 10.1007/s11064-013-0984-5. [DOI] [PubMed] [Google Scholar]
- 17.Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, et al. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: Ischemic postconditioning. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:232–241. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- 18.Dugan LL, Bruno VM, Amagasu SM, Giffard RG. Glia modulate the response of murine cortical neurons to excitotoxicity: Glia exacerbate ampa neurotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:4545–4555. doi: 10.1523/JNEUROSCI.15-06-04545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh PO. Melatonin prevents ischemic brain injury through activation of the mtor/p70s6 kinase signaling pathway. Neuroscience letters. 2008;444:74–78. doi: 10.1016/j.neulet.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Koh PO, Cho JH, Won CK, Lee HJ, Sung JH, Kim MO. Estradiol attenuates the focal cerebral ischemic injury through mtor/p70s6 kinase signaling pathway. Neuroscience letters. 2008;436:62–66. doi: 10.1016/j.neulet.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Xiong T, Qu Y, Zhao F, Ferriero D, Mu D. Mtor activates hypoxia-inducible factor-1alpha and inhibits neuronal apoptosis in the developing rat brain during the early phase after hypoxia-ischemia. Neuroscience letters. 2012;507:118–123. doi: 10.1016/j.neulet.2011.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghiglieri V, Pendolino V, Bagetta V, Sgobio C, Calabresi P, Picconi B. Mtor inhibitor rapamycin suppresses striatal post-ischemic ltp. Experimental neurology. 2010;226:328–331. doi: 10.1016/j.expneurol.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Balduini W, Carloni S, Buonocore G. Autophagy in hypoxia-ischemia induced brain injury. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(Suppl 1):30–34. doi: 10.3109/14767058.2012.663176. [DOI] [PubMed] [Google Scholar]
- 24.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of pi3k inhibitors: Lessons learned from early clinical trials. Nature reviews. Clinical oncology. 2013;10:143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 25.Koh PO. Gingko biloba extract (egb 761) prevents cerebral ischemia-induced p70s6 kinase and s6 phosphorylation. The American journal of Chinese medicine. 2010;38:727–734. doi: 10.1142/S0192415X10008196. [DOI] [PubMed] [Google Scholar]
- 26.Lu DY, Liou HC, Tang CH, Fu WM. Hypoxia-induced inos expression in microglia is regulated by the pi3-kinase/akt/mtor signaling pathway and activation of hypoxia inducible factor-1alpha. Biochemical pharmacology. 2006;72:992–1000. doi: 10.1016/j.bcp.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 27.Shang J, Deguchi K, Yamashita T, Ohta Y, Zhang H, Morimoto N, et al. Antiapoptotic and antiautophagic effects of glial cell line-derived neurotrophic factor and hepatocyte growth factor after transient middle cerebral artery occlusion in rats. Journal of neuroscience research. 2010;88:2197–2206. doi: 10.1002/jnr.22373. [DOI] [PubMed] [Google Scholar]
- 28.Huang S, Shu L, Easton J, Harwood FC, Germain GS, Ichijo H, et al. Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. The Journal of biological chemistry. 2004;279:36490–36496. doi: 10.1074/jbc.M401208200. [DOI] [PubMed] [Google Scholar]
- 29.Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. Eif4e--from translation to transformation. Oncogene. 2004;23:3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 30.Paschen W. Shutdown of translation: Lethal or protective? Unfolded protein response versus apoptosis. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:773–779. doi: 10.1097/01.WCB.0000075009.47474.F9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.