Abstract
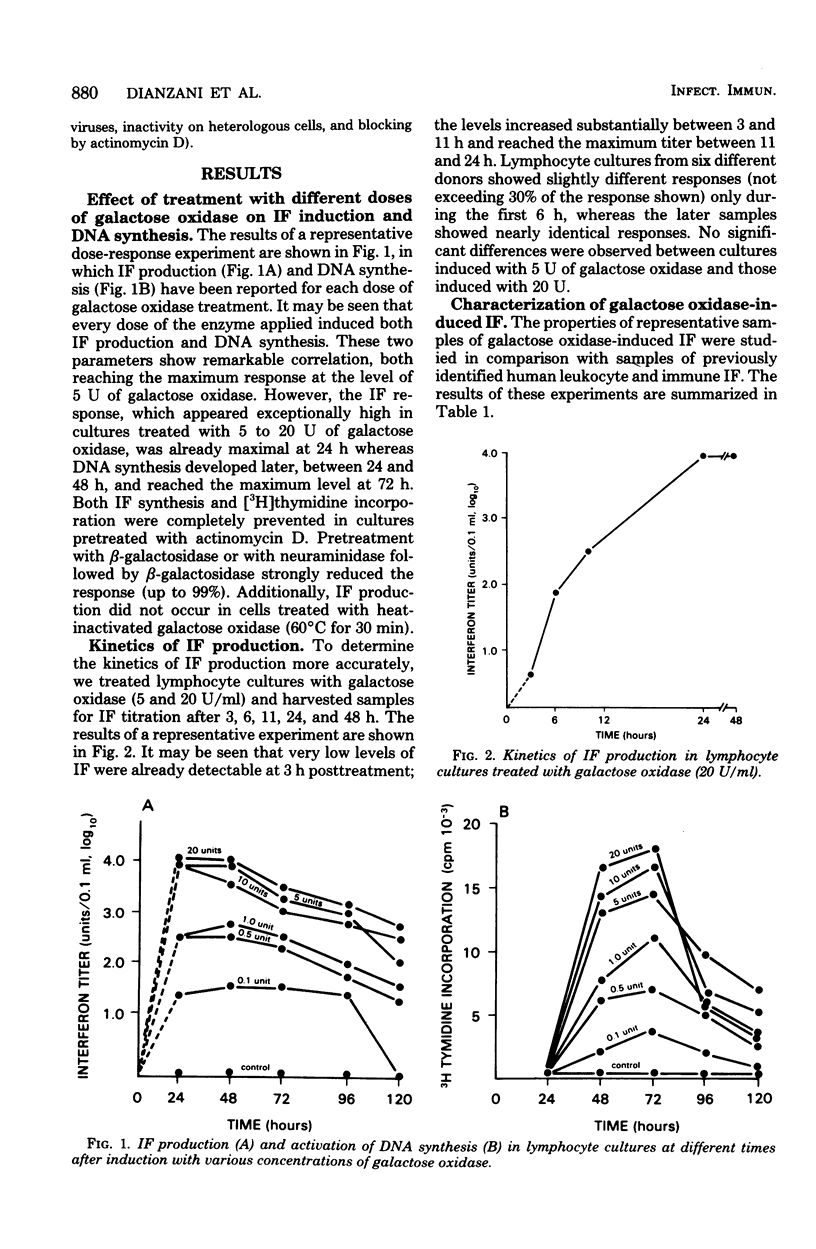
Human lymphocyte cultures produced large amounts of interferon after treatment with the enzyme galactose oxidase. Interferon production was detectable as early as 3 h after enzymatic treatment and reached a level of about 10(4) reference units 20 to 24 h later. Galactose oxidase-induced interferon appeared to be immune interferon on the basis of acid lability, lack of neutralization by antibody to leukocyte interferon, and slow kinetics of activation of the cellular antiviral state. Interferon production was inhibited to the same extent (99%) by pretreatment of the cells with beta-galactosidase or with neuraminidase followed by beta-galactosidase, suggesting that the critical event for activation of interferon production is the oxidation of exposed galactose residues on lymphocyte membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biniaminov M., Ramot B., Rosenthal E., Novogrodsky A. Galactose oxidase-induced blastogenesis of human lymphocytes and the effect of macrophages on the reaction. Clin Exp Immunol. 1975 Jan;19(1):93–98. [PMC free article] [PubMed] [Google Scholar]
- Blalock J. E., Langford M. P., Georgiades J., Stanton G. J. Nonsensitized lymphocytes produce leukocyte interferon when cultured with foreign cells. Cell Immunol. 1979 Mar 1;43(1):197–201. doi: 10.1016/0008-8749(79)90163-1. [DOI] [PubMed] [Google Scholar]
- Dianzani F., Salter L., Fleischmann W. R., Jr, Zucca M. Immune interferon activates cells more slowly than does virus-induced interferon. Proc Soc Exp Biol Med. 1978 Oct;159(1):94–97. doi: 10.3181/00379727-159-40291. [DOI] [PubMed] [Google Scholar]
- Dixon J. F., Parker J. W., O'Brien R. L. Transformation of human peripheral lymphocytes by galactose oxidase. J Immunol. 1976 Mar;116(3):575–578. [PubMed] [Google Scholar]
- Epstein L. B., Kreth H. W., Herzenberg L. A. Fluorescence-activated cell sorting of human T and B lymphocytes. II. Identification of the cell type responsible for interferon production and cell proliferation in response to mitogens. Cell Immunol. 1974 Jun;12(3):407–421. doi: 10.1016/0008-8749(74)90097-5. [DOI] [PubMed] [Google Scholar]
- Epstein L. B. Mitogen and antigen induction of interferon in vitro and in vivo. Tex Rep Biol Med. 1977;35:42–56. [PubMed] [Google Scholar]
- Green J. A., Cooperband S. R., Kibrick S. Immune specific induction of interferon production in cultures of human blood lymphocytes. Science. 1969 Jun 20;164(3886):1415–1417. doi: 10.1126/science.164.3886.1415. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Stanton G. J., Baron S. Relative ability of mitogens to stimulate production of interferon by lymphoid cells and to induce suppression of the in vitro immune response. Proc Soc Exp Biol Med. 1977 Jan;154(1):138–141. [PubMed] [Google Scholar]
- Monahan T. M., Abell C. W. The requirement for cell surface galactosyl residues during oxidative activation of normal and chronic lymphocytic leukemia lymphocytes. Exp Cell Res. 1978 Oct 1;116(1):47–53. doi: 10.1016/0014-4827(78)90063-0. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Induction of lymphocyte transformation by sequential treatment with neuraminidase and galactose oxidase. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1824–1827. doi: 10.1073/pnas.70.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne L. C., Georgiades J. A., Johnson H. M. Large-scale production and partial purification of mouse immune interferon. Infect Immun. 1979 Jan;23(1):80–86. doi: 10.1128/iai.23.1.80-86.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L., Merigan T. C. Role of T lymphocytes in cellular immune responses during herpes simplex virus infection in humans. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3957–3961. doi: 10.1073/pnas.75.8.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Lief F. S. Cell-mediated cytotoxicity against virus-infected target cells in humans. I. Characterization of the effector lymphocyte. J Immunol. 1978 Aug;121(2):526–531. [PubMed] [Google Scholar]
- Trinchieri G., Santoli D., Knowles B. B. Tumour cell lines induce interferon in human lymphocytes. Nature. 1977 Dec 15;270(5638):611–613. doi: 10.1038/270611a0. [DOI] [PubMed] [Google Scholar]
- Wietzerbin J., Stephanos S., Falcoff R., Falcoff E. Some properties of interferons induced by stimulants of T and B lymphocytes. Tex Rep Biol Med. 1977;35:205–211. [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Production and properties of migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. J Immunol. 1973 Dec;111(6):1914–1922. [PubMed] [Google Scholar]


