Abstract
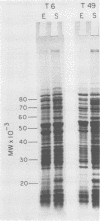
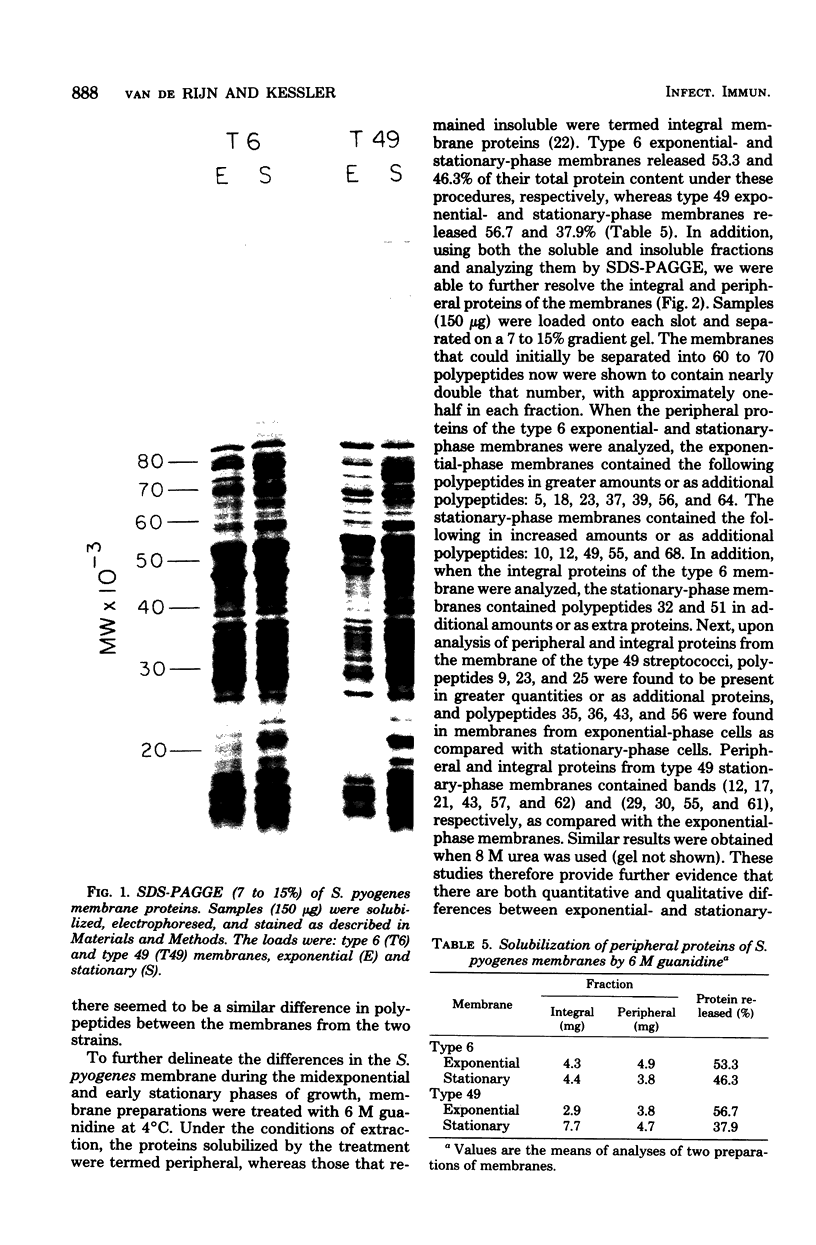
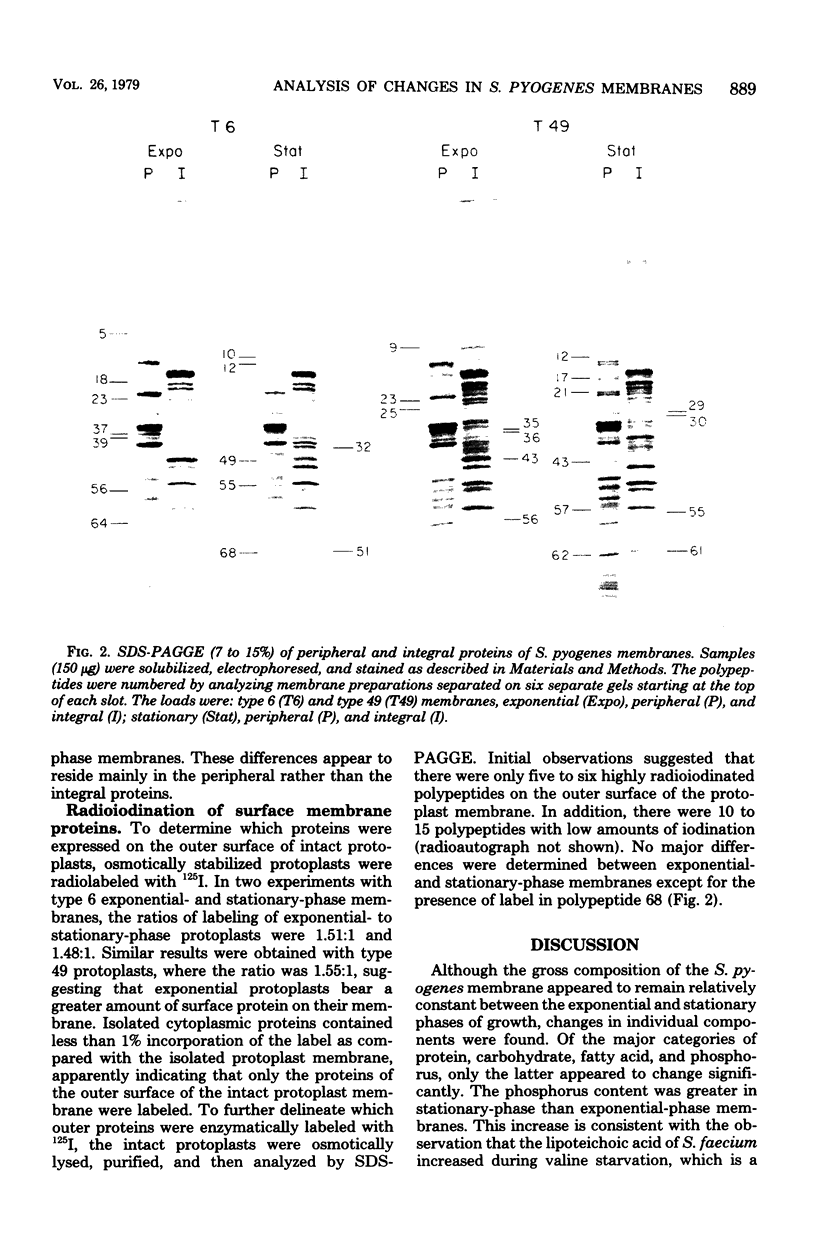
Changes in the structural components of the Streptococcus pyogenes membrane between exponential and early stationary phases of growth are reported. The overall protein composition ranged from 70 to 73% of the dry weight of the membranes, irrespective of the phase of growth from which they were isolated. Amino acid analyses of membranes isolated from streptococci in either the exponential or stationary phase of growth demonstrated that two amino acids, cysteine and tryptophan, were absent. Further analysis of the membrane proteins by sodium dodecyl sulfate-polyacrylamide gradient gel electrophoresis demonstrated that there were proteins unique to a particular phase of growth as well as differences in the amount of specific proteins from the various growth phases. In addition, membranes isolated from exponential-phase cultures contained a higher percentage of peripheral protein than did stationary-phase membranes. There also appeared to be an increase in the amount of outer surface proteins during this growth phase. The phosphorus content of the membranes increased during the stationary phase of growth, whereas the sugar composition remained constant. The only sugar found under various conditions of growth in any of the strains was glucose. Total fatty acid content and the mole percent composition of various fatty acids did not change in the different phases of growth. However, the mole percent composition of fatty acids in the membranes of various group A streptococci did differ between strains. Therefore, these results provide evidence that the composition of membranes of S. pyogenes does not remain constant throughout the growth phases of the culture.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- FREIMER E. H. Studies on L forms and protoplasts of group A streptococci. II. Chemical and immunological properties of the cell membrane. J Exp Med. 1963 Mar 1;117:377–399. doi: 10.1084/jem.117.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedel B. A., Jackson R. W. Activation of the alternative complement pathway by a streptococcal lipoteichoic acid. Infect Immun. 1978 Oct;22(1):286–287. doi: 10.1128/iai.22.1.286-287.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillit H. M., Read S. E., Sherman R. L., Zabriskie J. B., Van de Rijn I. Cellular reactivity to altered glomerular basement membrane in glomerulonephritis. N Engl J Med. 1978 Apr 20;298(16):861–868. doi: 10.1056/NEJM197804202981601. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Bernheimer A. W. Purification and physical properties of group C streptococcal phage-associated lysin. J Exp Med. 1971 May 1;133(5):1105–1117. doi: 10.1084/jem.133.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby G., van de Rijn I., Zabriskie J. B., Abdin Z. H., Williams R. C., Jr Antibodies reacting with cytoplasm of subthalamic and caudate nuclei neurons in chorea and acute rheumatic fever. J Exp Med. 1976 Oct 1;144(4):1094–1110. doi: 10.1084/jem.144.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., van de Rijn I. Quantitative immunoelectrophoretic analysis of Streptococcus pyogenes membrane. Infect Immun. 1979 Dec;26(3):892–902. doi: 10.1128/iai.26.3.892-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARKOWITZ A. S., LANGE C. F., Jr STREPTOCOCCAL RELATED GLOMERULONEPHRITIS. I. ISOLATION, IMMUNOCHEMISTRY AND COMPARATIVE CHEMISTRY OF SOLUBLE FRACTIONS FROM TYPE 12 NEPHRITOGENIC STREPTOCOCCI AND HUMAN GLOMERULI. J Immunol. 1964 Apr;92:565–575. [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreary D. K., Kossa W. C., Ramachandran S., Kurtz R. R. A novel and rapid method for the preparation of methyl esters for gas chromatography: application to the determination of the fatty acids of edible fats and oils. J Chromatogr Sci. 1978 Aug 10;16(8):329–331. doi: 10.1093/chromsci/16.8.329. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Panos C., Cohen M., Fagan G. Lipid alterations after cell wall inhibition. Fatty acid content of Streptococcus pyogenes and derived L-form. Biochemistry. 1966 May;5(5):1461–1468. doi: 10.1021/bi00869a003. [DOI] [PubMed] [Google Scholar]
- Panos C., Fagan G., Zarkadas C. G. Comparative electrophoretic and amino acid analyses of isolated membranes from Streptococcus pyogenes and stabilized L-form. J Bacteriol. 1972 Oct;112(1):285–290. doi: 10.1128/jb.112.1.285-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnebli H. P., Abrams A. Membrane adenosine triphosphatase from Streptococcus faecalis. Preparation and homogeneity. J Biol Chem. 1970 Mar 10;245(5):1115–1121. [PubMed] [Google Scholar]
- Stollerman G. H. Rheumatogenic and nephritogenic streptococci. Circulation. 1971 Jun;43(6):915–921. doi: 10.1161/01.cir.43.6.915. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Tauber J. W., Polley M. J., Zabriskie J. B. Nonspecific complement activation by streptococcal structures. II. Properdin-independent initiation of the alternate pathway. J Exp Med. 1976 Jun 1;143(6):1352–1366. doi: 10.1084/jem.143.6.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Freimer E. H. An immunological relationship between the group. A streptococcus and mammalian muscle. J Exp Med. 1966 Oct 1;124(4):661–678. doi: 10.1084/jem.124.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Zabriskie J. B., McCarty M. Group A streptococcal antigens cross-reactive with myocardium. Purification of heart-reactive antibody and isolation and characterization of the streptococcal antigen. J Exp Med. 1977 Aug 1;146(2):579–599. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]