Abstract
Metallothioneins (MTs) are a family of low molecular weight, cysteine-rich, metal-binding proteins that have a wide range of functions in cellular homeostasis and immunity. MTs can be induced by a variety of conditions including metals, glucocorticoids, endotoxin, acute phase cytokines, stress, and irradiation. In addition to their important immunomodulatory functions, MTs can protect essential cellular compartments from toxicants, serve as a reservoir of essential heavy metals, and regulate cellular redox potential. Many of the roles of MTs in the neuroinflammation, intestinal inflammation, and stress response have been investigated and were the subject of a session at the 6th International Congress on Stress Proteins in Biology and Medicine in Sheffield, UK. Like the rest of the cell stress response, there are therapeutic opportunities that arise from an understanding of MTs, and these proteins also provide potential insights into the world of the heat shock protein.
Keywords: Metallothionein, Stress, Immunity, Inflammation
Introduction
Exposure to stressful conditions produces a spectrum of cellular changes that includes de novo synthesis of a range of different stress response proteins. The most recognized of these proteins is the family of heat shock proteins (HSPs). Since their discovery, they have been shown to play important roles in cellular stress responses and in a broad range of normal homeostatic mechanisms. These HSPs held center stage at the 6th International Congress on Stress Proteins in Biology and Medicine in Sheffield, UK, but another stress response protein family was also the topic of a session at this meeting. This protein, metallothionein (MT), has structural, genetic, and biochemical features that are quite distinct from the HSP families, but MT shares some striking functional similarities with the HSPs. An understanding of MT is important in its own right but may also lend itself to a better understanding of how cells process and integrate stress responses, and to an appreciation of the clinical opportunities that manipulation of stress proteins may represent. The MT session explored the role MT plays in immune function, neuroprotection, inflammatory colitis, and infection.
Metallothionein is an unusual protein family. Members of this family are quite small (about 7 kDa) and very cysteine-rich (about 33 mol%). There are four main isoforms of MT (MT1 to 4) that are highly homologous structurally and are also quite conserved over long evolutionary distances. The protein is named for its ability to interact via its many thiols with divalent heavy metal cations such as mercury, cadmium, zinc, and copper. A variety of agents can influence the synthesis of the MT proteins, including the divalent heavy metals, reactive oxygen species, acute phase cytokines, interferon, glucocorticoids, calcium ionophores, and phorbol esters. While MT has traditionally been considered to be an intracellular protein that can be found in both the cytoplasm and nucleus, it is also found in a variety of extracellular spaces, despite the absence of a signal peptide. Molecular modeling of the MT proteins suggests that they have structural features that are reminiscent of those associated with proteins that are directed to extracellular compartments by signal peptides. Like the HSPs, the MTs have important roles to play in each of these compartments.
In unstressed cells, the zinc and copper, which are the essential metals normally associated with MT, are held in an intracellular reservoir for use by metalloenzymes, by transcription factors, and by other metalloproteins. In cells exposed to toxic divalent heavy metals, an MT isoform can sequester these elements and diminish the acute effects of the metals. MT can also act as a scavenger of reactive oxygen and nitrogen species and can regulate cellular redox potential. Outside the cell, MT can influence cellular behaviors such as proliferation and chemotaxis and thus potentially regulate cell behaviors by binding to membrane receptors.
In some of the original experiments done to examine the roles MT has in immune regulation, we immunized mice with ovalbumin in the presence of exogenous MT (Lynes et al. 1993). The results of these experiments suggested that extracellular MT could suppress the specific response to antigen challenge and that this suppression could be blocked by simultaneous administration of a monoclonal anti-MT antibody (Canpolat and Lynes 2001). Subsequent experiments showed that targeted disruptions of the host MT1 and MT2 genes could influence the progression of infection with Listeria monocytogenes (Emeny et al. 2009), as well as the vigor with which an immunized animal responds to challenge with a T-dependent antigen (Crowthers et al. 2000). Similarly, immune activity can be enhanced by antigen challenge in the presence of monoclonal anti-MT alone, suggesting that there is an endogenous extracellular pool of MT that influences immune activation (Lynes et al. 2006). Manipulation of MT expression influences the progression of autoimmune disease, as has been shown for collagen-induced arthritis (Youn et al. 2002) and diabetes (Yang and Cherian 1994).
Examination of the primary amino acid sequence of MT identified motifs that are reminiscent of sequences associated with some chemotactic cytokines. Moreover, the MT gene cluster is located in the midst of a pair of chemokine genes, suggesting a potential evolutionary relationship between these genes. As a consequence, we have explored the functional capacity of MT as a chemotactic factor. MT can initiate T cell chemotaxis, and this chemotactic response can be blocked by either monoclonal antibody or by cholera toxin or by pertussis toxin (Yin et al. 2005). The action of these toxins implies that MT interacts with a G protein-coupled receptor, but other reports have suggested other molecular candidates that might serve as MT receptors (Fitzgerald et al. 2007; Wolff et al. 2006).
While the most significant structural homologies are found between mammalian MTs, this protein family has members in species as diverse as humans and bacteria. SmtA is an MT expressed by a number of bacterial species (Morby et al. 1993), and it has been found to have the potential for immunomodulatory activities reminiscent of those established for eukaryotic MTs. As a consequence, bacterial MTs such as those expressed by Pseudomonas aeruginosa may serve as virulence factors and may represent a novel target for therapeutic intervention during Pseudomonas infection.
Roles of metallothioneins in animal models of neuroinflammation
Acute injury to the brain such as that caused by trauma and stroke causes waves of gene expression in an orderly manner: immediate-early genes, HSPs, cytokines, and adhesion molecules; a number of proteases and their inhibitors; and a final expression of remodeling and repair proteins (see (Allan and Rothwell 2001) for review). Interleukin-6 (IL-6) is one of the critical cytokines upregulated during brain injury and the associated inflammatory response, and its deficiency causes dramatic effects on the overall brain transcriptome (Poulsen et al. 2005). Among the proteins being influenced by IL-6, some are known to be neuroprotective, such as HSP105alpha (Yamagishi et al. 2002), HSP70 (Giffard and Yenari 2004), and MT1/2 (Penkowa et al. 1999). These MT isoforms are consistently found upregulated in neurodegenerative diseases such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis, and multiple sclerosis, as well as following acute and chronic brain injury where neuroinflammation and oxidative stress are occurring (Manso et al. 2011). Besides induction by neuroinflammatory conditions, Mt1 and Mt2 are also sensitive to psychological (immobilization) and other types of stress that are likely mediated by glucocorticoids (Gasull et al. 1994; Hidalgo et al. 1990).
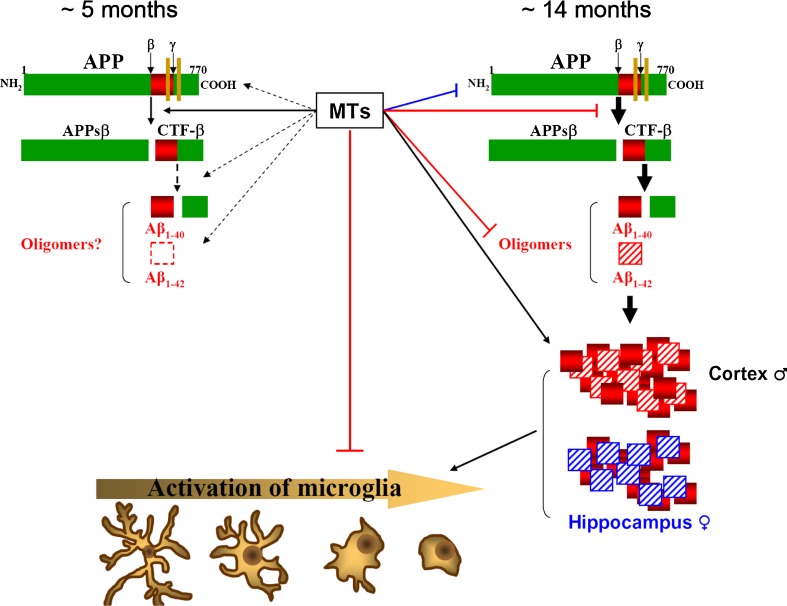
The discovery of human MT3, which suggested its involvement in the etiology of AD (Uchida et al. 1991), prompted an interest in the roles of MTs in the brain. AD is the most commonly diagnosed dementia worldwide, affecting about 40 % of people older than 80 years and is clearly on the rise (Selkoe 2012). Clinically defined by a slowly progressing loss of cognitive functions ultimately leading to dementia and death, the main neuropathological hallmarks of AD include extracellular senile plaques (mainly comprised of aggregated β-amyloid (Aβ) peptides) and intraneuronal neurofibrillary tangles (NFTs; hyperphosphorylated forms of the microtubule-binding protein tau) in the cortex and hippocampus, together with clear signs of neuroinflammation, metal dyshomeostasis, and oxidative stress (Kepp 2012; Selkoe 2012). To understand the putative role(s) of MTs on this devastating disease, the use of transgenic mouse models of AD will be critical (Wisniewski and Sigurdsson 2010). The Tg2576 mouse model is one of the most extensively studied models of amyloid deposition (Hsiao et al. 1996); Tg2576 mice develop amyloid plaques at 9–12 months and display inflammation, gliosis, oxidative stress, and impairment in cognitive tasks. Not surprisingly, Mt1 and Mt2 were upregulated in cells surrounding the amyloid plaques in these mice as well as in other models of AD, whereas MT3 expression was mostly unaffected (Carrasco et al. 2006). The role of these proteins has been assessed by crossing the Tg2576 mice with either Mt1 and Mt2 KO (Manso et al. 2012a) or Mt3 KO mice (Manso et al. 2012b) and by injecting Zn7-MT2A (Manso et al. 2011) and Zn7-MT3 (Manso et al. 2012b). While the exact mechanisms remain unknown, the results suggest that at an early age (∼5 months), MTs are rather detrimental, favoring the phenotype of the Tg2576 mice (amyloid cascade, mortality, behavioral alterations) perhaps by decreasing copper bioavailability which would increase the formation of Aβ trimers (Crouch et al. 2009) or prevent copper binding to prion protein and subsequent sensitization of NMDA receptors (You et al. 2012). At an advanced age (∼14 months), the effects of MT on the amyloid cascade are reduced, while at the same time, the formation of plaques is favored. Likely because of the latter, there are signs of increased reactivity of the resident immune cells in the brain, microglia, which is known as microgliosis (the morphology and physiology of these cells may change dramatically upon activation, going gradually from a ramified state in resting cells to a round morphology when fully activated). This response does not occur in all microglia but in those surrounding the plaques. Remarkably, this occurs despite the tendency of MTs to inhibit microgliosis in normal conditions. This has been summarized in Fig. 1.
Fig. 1.
The role of MTs in the formation of plaques in the brain. MTs have sex- and age-dependent effects on amyloidosis pathways throughout yet unknown mechanisms. At early ages, MTs seem to promote β-secretase activity directly (solid line) and eventually further processing of APP indirectly (dashed lines) and are thus detrimental. In contrast, at advanced ages, MTs decrease the amyloid cascade while promoting increased plaque formation (and concomitant microgliosis) and changing the Aβ1–40:Aβ1–42 ratios in cortex vs hippocampus in a sex-dependent manner
Metallothioneins as danger signals in intestinal inflammation
Inflammatory bowel diseases (IBDs), comprising Crohn’s Disease and ulcerative colitis, are chronic intestinal inflammatory pathologies of the gastrointestinal tract. They are typical diseases of the Western countries, usually affecting young adults (Cho 2008). Patients present recurrent symptoms of abdominal pain, bloody diarrhea, and weight loss. Severe ongoing mucosal inflammation can cause complications such as strictures and fistulae, often necessitating surgery. Although the exact etiology is unknown, it is widely accepted that IBD occurs in genetically predisposed individuals due to an excessive immune response to undefined luminal antigens, probably derived from the microbial flora. Decreased epithelial barrier function is a hallmark of IBD and results in an increased influx of bacteria into the lamina propia which subsequently drives the uncontrolled immune response (Blumberg 2009). Current immunosuppressive therapy is only effective in some patients, and loss of response is frequently observed. Consequently, the identification of innovative treatment strategies that provide clinical remission and mucosal healing is a primary goal in IBD research.
Intestinal homeostasis and epithelial barrier integrity is maintained by controlled renewal and differentiation of intestinal epithelial cells (IECs) (Gunther et al. 2012; Maloy and Powrie 2011). In patients with IBD, this equilibrium is disturbed, which is characterized by excessive IEC death and excessive infiltration and activation of immune cells. During cell death, endogenous signals are released that alert the immune system of damage by attracting and/or activating immune cells. These signals are called “danger signals” or “danger-associated molecular patterns” and represent an important part of the innate immune response. However, in cases of excessive cellular damage, danger signals may sustain immune activation and inflammation (Matzinger 1994; Siggers and Hackam 2011). Danger signals have been proposed to contribute to IBD (Mueller 2013a); for example, blocking high-mobility group box 1 resulted in partial suppression of the immune response and disease amelioration in murine IBD models (Yamasaki et al. 2009).
Extracellular MTs are a novel class of danger signals because they are able to attract leukocytes and influence the immune response (Laukens et al. 2009; Lynes et al. 2006; Yin et al. 2005). Increased serum MT levels are found in response to stress and MT detection at inflammation sites suggests the release of MTs in response to cellular damage (Armario et al. 1987; Chung and West 2004; Espejo et al. 2005; Inoue et al. 2005; Penkowa et al. 2005; Wesselkamper et al. 2006). Previous research focusing on the role of MTs in IBD primarily considered the intracellular roles of the protein. Results reported in the literature on MT expression in IBD and results of studies exploring the role of MTs in experimental IBD models are discrepant and inconclusive (for overview, see (Waeytens et al. 2009) and (Laukens et al. 2009)). We, and others, have shown that MTs are induced in the colon during the initiation of intestinal inflammation in experimental IBD models (Al-Gindan et al. 2009; Devisscher et al. 2011). In the healthy colon, MTs are predominantly expressed in the epithelium, whereas during active colitis, high immunoreactivity is found in the inflammatory infiltrate. We recently reported that genetic deletion of the Mt1 and Mt2 genes in mice reduced the severity of acute and chronic colitis (Devisscher et al. 2014). MT knockout mice showed a higher survival rate, less weight loss, and less colon shortening in an acute colitis model. The attenuated colonic inflammation in MT knockout mice was characterized by reduced leukocyte infiltration compared to wild-type mice. Interestingly, the same benefit was achieved using a monoclonal anti-MT antibody, suggesting that the presence of extracellular MTs participate in disease progression. Using small animal imaging, we indirectly showed that MTs are released in the colon during colitis. After intravenous administration of indium-labeled anti-MT antibodies, high signals were found in the colon during active colitis, whereas this was not the case in healthy animals. Because MTs are mainly expressed in IECs, we focused on the release of MTs from IECs in vitro. Attempts to induce MT secretion using various inflammatory triggers and other stressors involved in IBD were not successful. Released MTs could only be detected in the case of IEC death and plasma membrane damage. These endogenously released MTs were able to attract leukocytes, and this attraction could be abolished by the addition of anti-MT antibodies. Taken together, these data suggest that MTs act as danger signals during colitis and represent a novel target for reducing leukocyte infiltration and inflammation in IBD patients.
Metallothionein alters the stress associated with listeriosis and cold restraint
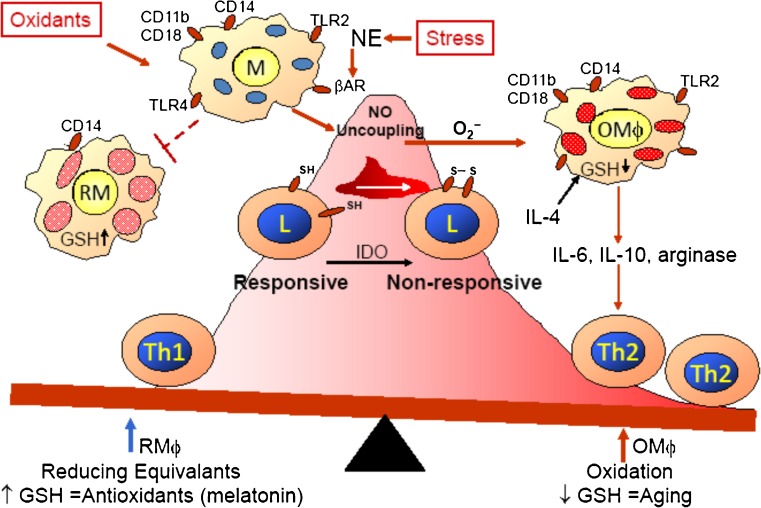
Exogenous (environment stresses) and endogenous (genetic and epigenetic) parameters have combined influences on health. Environmental stresses include biological (pathogens), chemical, and physical/psychological stresses, and these different types of stresses alone or in various combinations can affect an individual through a multitude of different pathways, involving many different organ systems. Our studies of environmental stressors have focused on interactive neuroendocrine and immune circuits. Both chemical exposure to lead (Pb) (Kishikawa et al. 1997; Tian and Lawrence 1995) and physical/psychological stress of cold restraint (Emeny et al. 2007, 2009) can suppress host defenses against bacterial infection (listeriosis) due, in part, to enhancing inflammation and oxidative stress. The increased incidence of infections in the elderly are posited to relate to the accumulation of lifetime environmental exposures, the consequential or accompanying decline of reducing equivalents, such as glutathione and other cellular thiols, and the manner by which the exogenous and endogenous factors converge to generate the exposome (Wild 2005). Interestingly, cold-restraint suppression of host defenses is due to sympathetic nervous system release of norepinephrine (Cao et al. 2002), which induces loss of cellular thiols of immune cells leading to reduced immunity. Inflammation and oxidative stress are mechanistically connected with aging (Pacifici and Davies 1991) and with the decline of regulated immune functions (Chinn et al. 2012; Lavrovsky et al. 2000), “immuno-aging.” Evidence of an imbalanced immune system, inflammation, and oxidative stress with aging is apparent with increased incidence of infections, cancers, and autoimmune diseases. “Immuno-aging” is often associated with cardiovascular disease, type 2 diabetes, neuroendocrine immune dysfunction, and cell senescence (Dandona et al. 2004; Mueller 2013b; Vasto et al. 2010; Wong et al. 2012). Since MT has been connected with both the association of cellular senescence and aging (Mocchegiani et al. 2012) and cold exposure and cardiovascular disease (Zhang et al. 2012), we have researched the effects of MT expression on mouse defenses against a L. monocytogenes (LM) infection and have assessed whether high or low MT expression affects the loss of host defenses when mice are stressed by cold restraint. It was not surprising to demonstrate that higher amounts of MT improved host defenses against LM (Emeny et al. 2009) since MT acts as an antioxidant; MT can lessen DNA damage and maintain the glutathione level even in stressed mice (Higashimoto et al. 2013), and MT lessens the oxidative stress and inflammation damage that occurs during streptozotocin-induced diabetes (Tachibana et al. 2014). Maintenance of glutathione is necessary to assist inducible nitric oxide synthase activity (MacMicking et al. 1997; Murata et al. 2002) and prevent nitric oxide (NO) uncoupling, which leads to greater production of the reactive oxygen species superoxide and damage to the mitochondria. The unexpected result was that a MT deficiency improved host defenses against LM; this might be because the intracellular condition is less hospitable due to greater oxidative stress leading to apoptosis and/or increase of lipid peroxides and peroxynitrite that would damage both the host cells and the LM. LM infection increases the expression of MT (∼4-fold) in the spleen and liver of wild-type C57BL/6 (B6-WT) mice, the two organs with the largest amounts of LM. After cold-restraint stress and LM infection, the MT levels still increase in B6-WT mice but to 75–85 % of the level without cold restraint. B6 mice with the MT transgene (B6-MTTGN), which have a constituently high MT level, also have increased MT levels after LM infection (4-fold in spleen; 10-fold in liver), and unlike the B6-WT mice, cold restraint plus LM caused a further 15–20 % increase. Cold restraint did not inhibit host defenses of B6-MTTGN mice; whereas, mice lacking MT (B6-MTKO), which had improved host defenses compared to WT-B6 mice, lost their protection after cold restraint, especially in the liver. The beneficial influences of MTKO and MTTGN on LM infection do not appear to relate to differential cytokine levels; however, LM-infected B6-WT mice had greater loss of body weight than infected B6-MTTGN or B6-MTKO mice. The B6-MTKO mice also had higher levels of IL-6, a cytokine that increases with stress and sickness behavior. Very different mechanisms are likely responsible for the improved host defenses of the MTKO and MTTGN mice. The elevated levels of MT in the B6-MTTGN mice may improve Th1-mediated immunity due to availability of glutathione. Glutathione levels have been suggested to differentially influence the development of Th1 and Th2 cells (Murata et al. 2002). Th1 activity, prevention of NO uncoupling, and maintenance of cellular thiols are suggested to be linked to the type of immunity needed for defense against an intracellular pathogen such as LM (Fig. 2).
Fig. 2.
Hypothetical scheme of stress-induced modulation of immunity. Biological, chemical, and physical/psychological stresses can independently or in combination increase oxidative stress leading to suppression of type 1 immunity, which is needed to defend against intracellular pathogens
Summary
MT represents a family of proteins with critical roles to play in normal cellular homeostasis and in the cellular responses to stressors. Like the HSPs, these roles span a wide spectrum of cellular types and physiological processes. Manipulations of the proteins’ synthesis or distribution may have critical importance in the therapeutic manipulation of a wide spectrum of diseases and should inform our understanding of how organisms manage stressful conditions.
Acknowledgments
MAL acknowledges the funding from the NIEHS (5R01ES007408). JH acknowledges the support of SAF2008-00435 and SAF2011-23272. LD is supported by a BOF grant from Ghent University (01D20510), and DL acknowledges the support of a FWO grant (1298213N).
References
- Al-Gindan Y, Shawarby M, Noto A, Taylor CG. Intestinal inflammation in rats induces metallothionein in colonic submucosa. J Clin Biochem Nutr. 2009;44(2):131–141. doi: 10.3164/jcbn.08-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2(10):734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Armario A, Hidalgo J, Bas J, Restrepo C, Dingman A, Garvey JS. Age-dependent effects of acute and chronic intermittent stresses on serum metallothionein. Physiol Behav. 1987;39(2):277–279. doi: 10.1016/0031-9384(87)90022-9. [DOI] [PubMed] [Google Scholar]
- Blumberg RS. Inflammation in the intestinal tract: pathogenesis and treatment. Dig Dis. 2009;27(4):455–464. doi: 10.1159/000235851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canpolat E, Lynes MA. In vivo manipulation of endogenous metallothionein with a monoclonal antibody enhances a T-dependent humoral immune response. Toxicol Sci. 2001;62(1):61–70. doi: 10.1093/toxsci/62.1.61. [DOI] [PubMed] [Google Scholar]
- Cao L, Filipov NM, Lawrence DA. Sympathetic nervous system plays a major role in acute cold/restraint stress inhibition of host resistance to Listeria monocytogenes. J Neuroimmunol. 2002;125(1–2):94–102. doi: 10.1016/S0165-5728(02)00039-5. [DOI] [PubMed] [Google Scholar]
- Carrasco J, Adlard P, Cotman C, Quintana A, Penkowa M, Xu F, Van Nostrand WE, Hidalgo J. Metallothionein-I and -III expression in animal models of Alzheimer disease. Neuroscience. 2006;143(4):911–922. doi: 10.1016/j.neuroscience.2006.08.054. [DOI] [PubMed] [Google Scholar]
- Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24(5):309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH. Inflammatory bowel disease: genetic and epidemiologic considerations. World J Gastroenterol. 2008;14(3):338–347. doi: 10.3748/wjg.14.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung RS, West AK. A role for extracellular metallothioneins in CNS injury and repair. Neuroscience. 2004;123(3):595–599. doi: 10.1016/j.neuroscience.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Crouch PJ, Hung LW, Adlard PA, Cortes M, Lal V, Filiz G, Perez KA, Nurjono M, Caragounis A, Du T, Laughton K, Volitakis I, Bush AI, Li QX, Masters CL, Cappai R, Cherny RA, Donnelly PS, White AR, Barnham KJ. Increasing Cu bioavailability inhibits Abeta oligomers and tau phosphorylation. Proc Natl Acad Sci U S A. 2009;106(2):381–386. doi: 10.1073/pnas.0809057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowthers KC, Kline V, Giardina C, Lynes MA. Augmented humoral immune function in metallothionein-null mice. Toxicol Appl Pharmacol. 2000;166(3):161–172. doi: 10.1006/taap.2000.8961. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Devisscher L, Hindryckx P, Olievier K, Peeters H, De Vos M, Laukens D. Inverse correlation between metallothioneins and hypoxia-inducible factor 1 alpha in colonocytes and experimental colitis. Biochem Biophys Res Commun. 2011;416(3–4):307–312. doi: 10.1016/j.bbrc.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Devisscher L HP, Lynes M, Waeytens A, Cuvelier C, De Vos F, Vanhove C, De Vos M, Laukens D (2014) Role of metallothioneins as danger signals in the pathogenesis of colitis. J Pathol. doi:10.1002/path.4330 [DOI] [PubMed]
- Emeny RT, Gao D, Lawrence DA. Beta1-adrenergic receptors on immune cells impair innate defenses against listeria. J Immunol. 2007;178(8):4876–4884. doi: 10.4049/jimmunol.178.8.4876. [DOI] [PubMed] [Google Scholar]
- Emeny RT, Marusov G, Lawrence DA, Pederson-Lane J, Yin X, Lynes MA. Manipulations of metallothionein gene dose accelerate the response to listeria monocytogenes. Chem Biol Interact. 2009;181(2):243–253. doi: 10.1016/j.cbi.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Espejo C, Penkowa M, Demestre M, Montalban X, Martinez-Caceres EM. Time-course expression of CNS inflammatory, neurodegenerative tissue repair markers and metallothioneins during experimental autoimmune encephalomyelitis. Neuroscience. 2005;132(4):1135–1149. doi: 10.1016/j.neuroscience.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Nairn P, Bartlett CA, Chung RS, West AK, Beazley LD. Metallothionein-IIA promotes neurite growth via the megalin receptor. Exp Brain Res. 2007;183(2):171–180. doi: 10.1007/s00221-007-1032-y. [DOI] [PubMed] [Google Scholar]
- Gasull T, Giralt M, Hernandez J, Martinez P, Bremner I, Hidalgo J. Regulation of metallothionein concentrations in rat brain: effect of glucocorticoids, zinc, copper, and endotoxin. Am J Physiol. 1994;266(5 Pt 1):E760–767. doi: 10.1152/ajpendo.1994.266.5.E760. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16(1):53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- Gunther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2012;62(7):1062–1071. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- Hidalgo J, Borras M, Garvey JS, Armario A. Liver, brain, and heart metallothionein induction by stress. J Neurochem. 1990;55(2):651–654. doi: 10.1111/j.1471-4159.1990.tb04182.x. [DOI] [PubMed] [Google Scholar]
- Higashimoto M, Isoyama N, Ishibashi S, Ogawa N, Takiguchi M, Suzuki S, Ohnishi Y, Sato M. Preventive effects of metallothionein against DNA and lipid metabolic damages in dyslipidemic mice under repeated mild stress. J Med Investig. 2013;60(3–4):240–248. doi: 10.2152/jmi.60.240. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Yanagisawa R, Sakurai M, Ichinose T, Sadakane K, Hiyoshi K, Sato M, Shimada A, Inoue M, Yoshikawa T. Role of metallothionein in antigen-related airway inflammation. Exp Biol Med (Maywood) 2005;230(1):75–81. doi: 10.1177/153537020523000110. [DOI] [PubMed] [Google Scholar]
- Kepp KP. Bioinorganic chemistry of Alzheimer’s disease. Chem Rev. 2012;112:5193–5239. doi: 10.1021/cr300009x. [DOI] [PubMed] [Google Scholar]
- Kishikawa H, Song R, Lawrence DA. Interleukin-12 promotes enhanced resistance to Listeria monocytogenes infection of lead-exposed mice. Toxicol Appl Pharmacol. 1997;147(2):180–189. doi: 10.1006/taap.1997.8308. [DOI] [PubMed] [Google Scholar]
- Laukens D, Waeytens A, De Bleser P, Cuvelier C, De Vos M. Human metallothionein expression under normal and pathological conditions: mechanisms of gene regulation based on in silico promoter analysis. Crit Rev Eukaryot Gene Expr. 2009;19(4):301–317. doi: 10.1615/CritRevEukarGeneExpr.v19.i4.40. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y, Chatterjee B, Clark RA, Roy AK. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp Gerontol. 2000;35(5):521–532. doi: 10.1016/S0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- Lynes MA, Borghesi LA, Youn J, Olson EA. Immunomodulatory activities of extracellular metallothionein. I. Metallothionein effects on antibody production. Toxicology. 1993;85(2–3):161–177. doi: 10.1016/0300-483X(93)90040-Y. [DOI] [PubMed] [Google Scholar]
- Lynes MA, Zaffuto K, Unfricht DW, Marusov G, Samson JS, Yin X. The physiological roles of extracellular metallothionein. Exp Biol Med (Maywood) 2006;231(9):1548–1554. doi: 10.1177/153537020623100915. [DOI] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- Manso Y, Adlard PA, Carrasco J, Vasak M, Hidalgo J. Metallothionein and brain inflammation. J Biol Inorg Chem. 2011;16(7):1103–1113. doi: 10.1007/s00775-011-0802-y. [DOI] [PubMed] [Google Scholar]
- Manso Y, Carrasco J, Comes G, Adlard PA, Bush AI, Hidalgo J. Characterization of the role of the antioxidant proteins metallothioneins 1 and 2 in an animal model of Alzheimer’s disease. Cell Mol Life Sci. 2012;69(21):3665–3681. doi: 10.1007/s00018-012-1045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manso Y, Carrasco J, Comes G, Meloni G, Adlard PA, Bush AI, Vasak M, Hidalgo J. Characterization of the role of metallothionein-3 in an animal model of Alzheimer’s disease. Cell Mol Life Sci. 2012;69(21):3683–3700. doi: 10.1007/s00018-012-1047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Costarelli L, Basso A, Giacconi R, Piacenza F, Malavolta M. Metallothioneins, ageing and cellular senescence: a future therapeutic target. Curr Pharm Des. 2012;19(9):1753–1764. [PubMed] [Google Scholar]
- Morby AP, Turner JS, Huckle JW, Robinson NJ. SmtB is a metal-dependent repressor of the cyanobacterial metallothionein gene smtA: identification of a Zn inhibited DNA-protein complex. Nucleic Acids Res. 1993;21(4):921–925. doi: 10.1093/nar/21.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C. Danger-associated molecular patterns and inflammatory bowel disease: is there a connection? Dig Dis. 2013;30(Suppl 3):40–46. doi: 10.1159/000342600. [DOI] [PubMed] [Google Scholar]
- Mueller K. Inflammation’s Yin-Yang. Science. 2013;339(6116):155. doi: 10.1126/science.339.6116.155. [DOI] [PubMed] [Google Scholar]
- Murata Y, Shimamura T, Hamuro J. The polarization of T(h)1/T(h)2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int Immunol. 2002;14(2):201–212. doi: 10.1093/intimm/14.2.201. [DOI] [PubMed] [Google Scholar]
- Pacifici RE, Davies KJ. Protein, lipid and DNA repair systems in oxidative stress: the free-radical theory of aging revisited. Gerontology. 1991;37(1–3):166–180. doi: 10.1159/000213257. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Carrasco J, Giralt M, Moos T, Hidalgo J. CNS wound healing is severely depressed in metallothionein I- and II-deficient mice. J Neurosci. 1999;19(7):2535–2545. doi: 10.1523/JNEUROSCI.19-07-02535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa M, Florit S, Giralt M, Quintana A, Molinero A, Carrasco J, Hidalgo J. Metallothionein reduces central nervous system inflammation, neurodegeneration, and cell death following kainic acid-induced epileptic seizures. J Neurosci Res. 2005;79(4):522–534. doi: 10.1002/jnr.20387. [DOI] [PubMed] [Google Scholar]
- Poulsen CB, Penkowa M, Borup R, Nielsen FC, Caceres M, Quintana A, Molinero A, Carrasco J, Giralt M, Hidalgo J. Brain response to traumatic brain injury in wild-type and interleukin-6 knockout mice: a microarray analysis. J Neurochem. 2005;92(2):417–432. doi: 10.1111/j.1471-4159.2004.02877.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Preventing Alzheimer’s disease. Science. 2012;337(6101):1488–1492. doi: 10.1126/science.1228541. [DOI] [PubMed] [Google Scholar]
- Siggers RH, Hackam DJ. The role of innate immune-stimulated epithelial apoptosis during gastrointestinal inflammatory diseases. Cell Mol Life Sci. 2011;68(22):3623–3634. doi: 10.1007/s00018-011-0821-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana H, Ogawa D, Sogawa N, Asanuma M, Miyazaki I, Terami N, Hatanaka T, Horiguchi CS, Nakatsuka A, Eguchi J, Wada J, Yamada H, Takei K, Makino H. Metallothionein deficiency exacerbates diabetic nephropathy in streptozotocin-induced diabetic mice. Am J Physiol Ren Physiol. 2014;306(1):F105–115. doi: 10.1152/ajprenal.00034.2013. [DOI] [PubMed] [Google Scholar]
- Tian L, Lawrence DA. Lead inhibits nitric oxide production in vitro by murine splenic macrophages. Toxicol Appl Pharmacol. 1995;132(1):156–163. doi: 10.1006/taap.1995.1096. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M. The growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like protein. Neuron. 1991;7(2):337–347. doi: 10.1016/0896-6273(91)90272-2. [DOI] [PubMed] [Google Scholar]
- Vasto SSG, Bulati M, Candore G, Castiglia L, Colonna-Romano G, Lio D, Nuzzo D, Pellicano M, Rizzo C, Ferrara N, Caruso C. Biomarkers of aging. Front Biosci. 2010;2:392–402. doi: 10.2741/s72. [DOI] [PubMed] [Google Scholar]
- Waeytens A, De Vos M, Laukens D. Evidence for a potential role of metallothioneins in inflammatory bowel diseases. Mediat Inflamm. 2009;2009:729172. doi: 10.1155/2009/729172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselkamper SC, McDowell SA, Medvedovic M, Dalton TP, Deshmukh HS, Sartor MA, Case LM, Henning LN, Borchers MT, Tomlinson CR, Prows DR, Leikauf GD. The role of metallothionein in the pathogenesis of acute lung injury. Am J Respir Cell Mol Biol. 2006;34(1):73–82. doi: 10.1165/rcmb.2005-0248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- Wisniewski T, Sigurdsson EM. Murine models of Alzheimer’s disease and their use in developing immunotherapies. Biochim Biophys Acta. 2010;1802(10):847–859. doi: 10.1016/j.bbadis.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff NA, Abouhamed M, Verroust PJ, Thevenod F. Megalin-dependent internalization of cadmium-metallothionein and cytotoxicity in cultured renal proximal tubule cells. J Pharmacol Exp Ther. 2006;318(2):782–791. doi: 10.1124/jpet.106.102574. [DOI] [PubMed] [Google Scholar]
- Wong BW, Meredith A, Lin D, McManus BM. The biological role of inflammation in atherosclerosis. Can J Cardiol. 2012;28(6):631–641. doi: 10.1016/j.cjca.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Ishihara K, Saito Y, Hatayama T. Hsp105alpha enhances stress-induced apoptosis but not necrosis in mouse embryonal f9 cells. J Biochem. 2002;132(2):271–278. doi: 10.1093/oxfordjournals.jbchem.a003221. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Mitsuyama K, Masuda J, Kuwaki K, Takedatsu H, Sugiyama G, Yamada S, Sata M. Roles of high-mobility group box 1 in murine experimental colitis. Mol Med Rep. 2009;2(1):23–27. doi: 10.3892/mmr_00000056. [DOI] [PubMed] [Google Scholar]
- Yang J, Cherian MG. Protective effects of metallothionein on streptozotocin-induced diabetes in rats. Life Sci. 1994;55(1):43–51. doi: 10.1016/0024-3205(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Yin X, Knecht DA, Lynes MA. Metallothionein mediates leukocyte chemotaxis. BMC Immunol. 2005;6:21. doi: 10.1186/1471-2172-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Tsutsui S, Hameed S, Kannanayakal TJ, Chen L, Xia P, Engbers JD, Lipton SA, Stys PK, Zamponi GW. Abeta neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 2012;109(5):1737–1742. doi: 10.1073/pnas.1110789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J, Hwang SH, Ryoo ZY, Lynes MA, Paik DJ, Chung HS, Kim HY. Metallothionein suppresses collagen-induced arthritis via induction of TGF-beta and down-regulation of proinflammatory mediators. Clin Exp Immunol. 2002;129(2):232–239. doi: 10.1046/j.1365-2249.2002.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hu N, Hua Y, Richmond KL, Dong F, Ren J. Cardiac overexpression of metallothionein rescues cold exposure-induced myocardial contractile dysfunction through attenuation of cardiac fibrosis despite cardiomyocyte mechanical anomalies. Free Radic Biol Med. 2012 doi: 10.1016/j.freeradbiomed.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]