Abstract
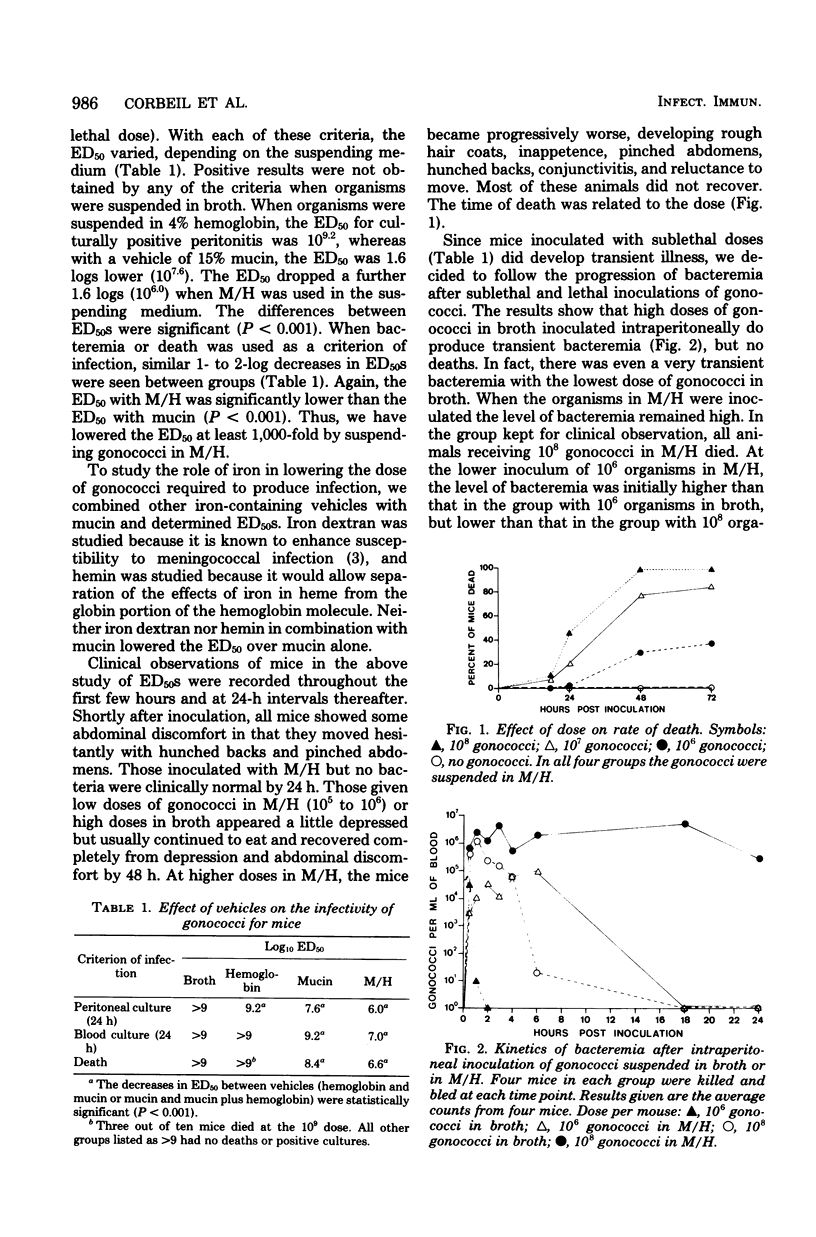
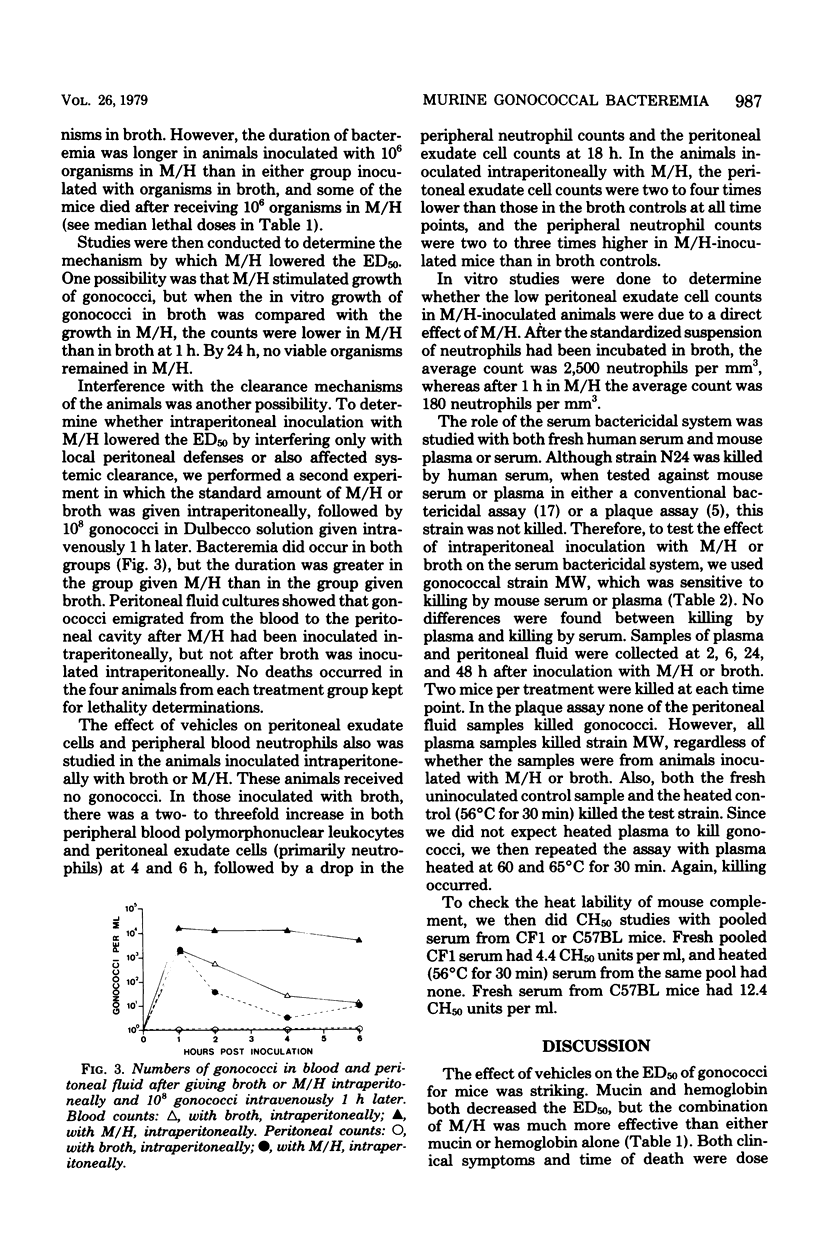
Gonococci do not readily cause disseminated infection in mice. To simulate some of the conditions leading to disseminated gonococcal infection in women, we suspended gonococci in mucin plus hemoglobin and studied the development of gonococcal bacteremia. The mucin-hemoglobin mixture was used because the menstruum appears to be involved in dissemination of gonococci from the genital tract during menstruation. Mice did not die after massive inocula of 109 gonococci given intraperitoneally in broth, but when gonococci were suspended in mucin (15%) alone, the 50% lethal dose was 108.4 and in 15% mucin plus 4% hemoglobin (M/H), the 50% lethal dose fell to 106.6. Sublethal doses produced local peritonitis and transient bacteremia. With larger inocula the local peritoneal infection progressed to fatal septicemia. Studies of the mechanism by which M/H lowered the 50% lethal dose showed that systemic clearance mechanisms were compromised, but not enough to account for the total decrease in the 50% lethal dose. If gonococci were given intravenously after intraperitoneal inoculation of M/H, sequestration of gonococci in the peritoneal cavity occurred, suggesting an effect on local peritoneal defenses. The effect on neutrophils appeared most significant, since numbers of neutrophils in the peritoneal fluid were decreased in the presence of M/H and neutrophils were destroyed by M/H in vitro. The serum bactericidal system was not affected. We conclude that M/H promotes gonococcal bacteremia by interference with phagocytosis and intracellular killing of gonococci. The model simulates the disseminated gonococcal infection cases in women which follow pelvic inflammatory disease in its progression from local peritonitis to transient or lethal bacteremia and in factors (mucin and hemoglobin) which enhance infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arko R. J. Neisseria gonorrhoeae: experimental infection of laboratory animals. Science. 1972 Sep 29;177(4055):1200–1201. doi: 10.1126/science.177.4055.1200. [DOI] [PubMed] [Google Scholar]
- CINADER B., DUBISKI S., WARDLAW A. C. DISTRIBUTION, INHERITANCE, AND PROPERTIES OF AN ANTIGEN, MUB1, AND ITS RELATION TO HEMOLYTIC COMPLEMENT. J Exp Med. 1964 Nov 1;120:897–924. doi: 10.1084/jem.120.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver G. A., Kenny C. P., Lavergne G. Iron as a replacement for mucin in the establishment of meningococcal infection in mice. Can J Microbiol. 1976 Jun;22(6):832–838. doi: 10.1139/m76-120. [DOI] [PubMed] [Google Scholar]
- Corbeil L. B., Wunderlich A. C., Ito J. I., McCutchan J. A. Plaque assay for measuring serum bactericidal activity against gonococci. J Clin Microbiol. 1978 Nov;8(5):618–620. doi: 10.1128/jcm.8.5.618-620.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbach D. A. Acute pelvic inflammatory disease: etiology, risk factors and pathogenesis. Clin Obstet Gynecol. 1976 Mar;19(1):147–169. doi: 10.1097/00003081-197603000-00013. [DOI] [PubMed] [Google Scholar]
- Handsfield H. H. Disseminated gonococcal infection. Clin Obstet Gynecol. 1975 Mar;18(1):131–142. doi: 10.1097/00003081-197503000-00012. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye D., Gill F. A., Hook E. W. Factors influencing host resistance to Salmonella infections: the effects of hemolysis and erythrophagocytosis. Am J Med Sci. 1967 Aug;254(2):205–215. doi: 10.1097/00000441-196708000-00011. [DOI] [PubMed] [Google Scholar]
- Keefer C. S., Spink W. W. STUDIES OF GONOCOCCAL INFECTION. IV. THE EFFECT OF MUCIN ON THE BACTERIOLYTIC POWER OF WHOLE BLOOD AND IMMUNE SERUM. J Clin Invest. 1938 Jan;17(1):23–30. doi: 10.1172/JCI100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. A., Levine S., Braude A. I. Influence of colony type on susceptibility of gonococci to killing by human serum. J Immunol. 1976 Jun;116(6):1652–1655. [PubMed] [Google Scholar]
- Mitsui Y., Matsumura K., Kondo C., Takashima R. The role of mucin on experimental Pseudomonas keratitis in rabbits. Invest Ophthalmol. 1976 Mar;15(3):208–210. [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. The critical role of iron in host-bacterial interactions. J Clin Invest. 1978 Jun;61(6):1428–1440. doi: 10.1172/JCI109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TACHIBANA D. K., ULRICH M., ROSENBERG L. T. THE INHERITANCE OF HEMOLYTIC COMPLEMENT ACTIVITY IN CF-1 MICE. J Immunol. 1963 Aug;91:230–232. [PubMed] [Google Scholar]
- Walton E., Gladstone G. P. Factors affecting the susceptibility of staphylococci to killing by the cationic proteins from rabbit polymorphonuclear leucocytes: the effects of alteration of cellular energetics and of various iron compounds. Br J Exp Pathol. 1976 Oct;57(5):560–570. [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


