Abstract
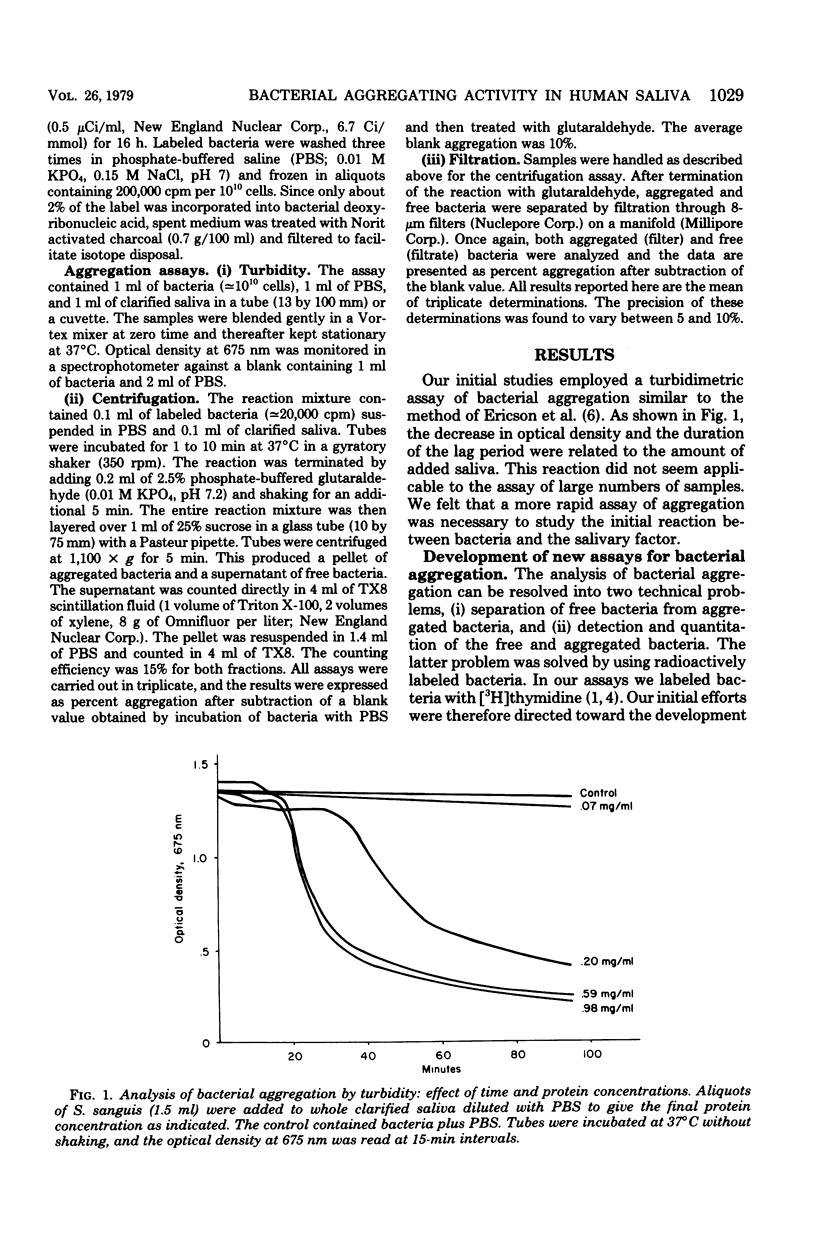
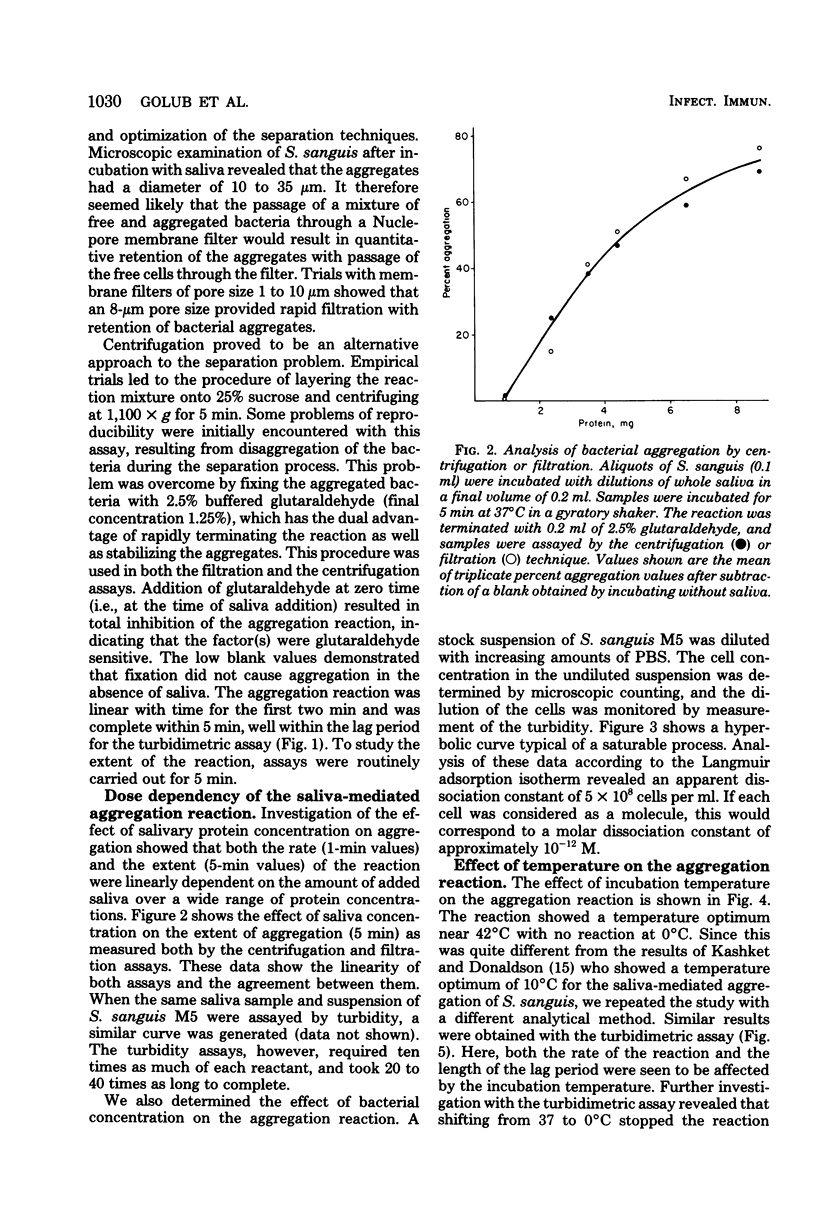
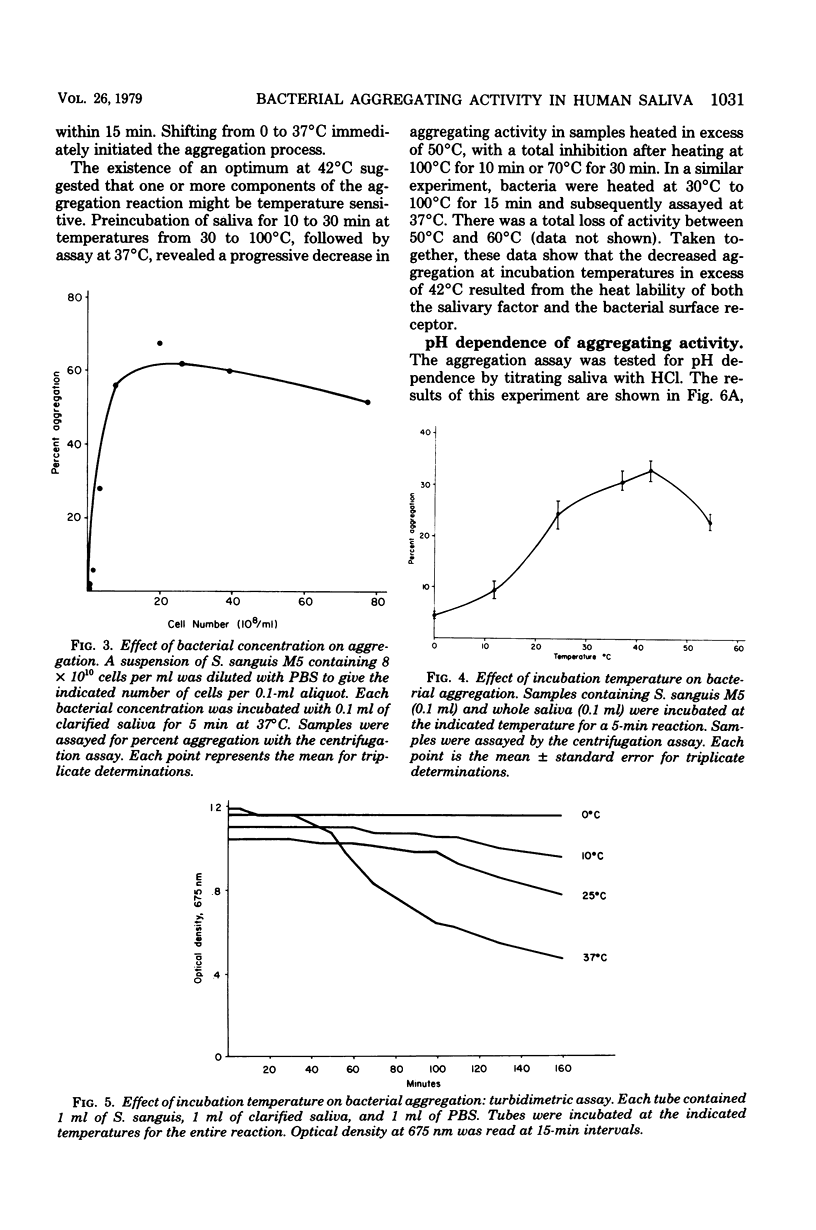
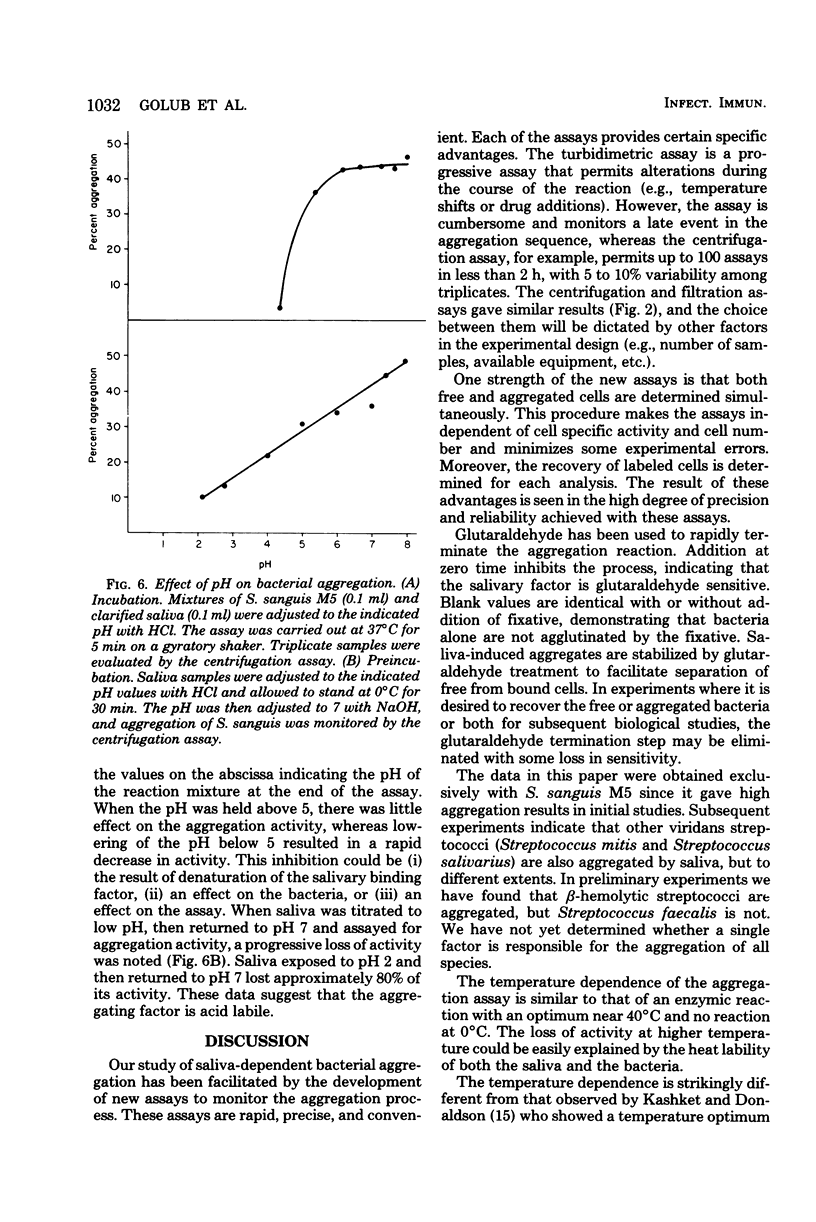
Two new assays for saliva-mediated aggregation of oral bacteria have been developed, based on the use of [3H]thymidine-labeled cells. One assay separates free cells from aggregated cells by centrifugation through sucrose, whereas the other utilizes membrane filters (8 micrometers, Nuclepore) to effect the separation. Comparison of these assays with the turbidity method reveals that they are faster (X20 to 40) and require 10 times less saliva and bacteria. The aggregation of Streptococcus sanguis M5, as determined with these assays, is complete in 5 min and is dose dependent on added cells and saliva. The reaction exhibits a temperature optimum of 42 degrees C with no reaction at 0 degrees C. If the pH is reduced to below 5, saliva-dependent aggregation is inhibited. The salivary factor(s) are heat labile, losing 100% of their activity after 100 degrees C, 10 min or 70 degrees C, 30 min.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Ability of Streptococcus mutans and a glucosyltransferase-defective mutant to colonize rodents and attach to hydroxyapatite surfaces. Infect Immun. 1978 Aug;21(2):681–684. doi: 10.1128/iai.21.2.681-684.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson T., Pruitt K., Wedel H. The reaction of salivary substances with bacteria. J Oral Pathol. 1975 Dec;4(6):307–323. doi: 10.1111/j.1600-0714.1975.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Ericson T., Sandham J., Magnusson I. Sedimentation method for studies of adsorption of microorganisms onto apatite surfaces in vitro. Caries Res. 1975;9(5):325–332. doi: 10.1159/000260165. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Hamada S., Torii M. Effect of sucrose in culture media on the location of glucosyltransferase of Streptococcus mutans and cell adherence to glass surfaces. Infect Immun. 1978 Jun;20(3):592–599. doi: 10.1128/iai.20.3.592-599.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- Kashket S., Donaldson C. G. Saliva-induced aggregation of oral streptococci. J Bacteriol. 1972 Dec;112(3):1127–1133. doi: 10.1128/jb.112.3.1127-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo W., Sato M., Ozawa H. Haemagglutinating activity of Leptotrichia buccalis cells and their adherence to saliva-coated enamel powder. Arch Oral Biol. 1976;21(6):363–369. doi: 10.1016/s0003-9969(76)80004-0. [DOI] [PubMed] [Google Scholar]
- Kondo W., Sato M., Sato N. Properties of the human salivary aggregating factor for Leptotrichia buccalis cells. Arch Oral Biol. 1978;23(6):453–458. doi: 10.1016/0003-9969(78)90076-6. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H., Ingersoll L. Molecular basis for the different sucrose-dependent adherence properties of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1977 Aug;17(2):330–337. doi: 10.1128/iai.17.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Competitive binding among oral strptococci to hydroxyapatite. J Dent Res. 1977 Feb;56(2):157–165. doi: 10.1177/00220345770560021001. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Ericson T. Effect of salivary agglutinins of reactions between hydroxyapatite and a serotype c strain of Streptococcus mutans. Caries Res. 1976;10(4):273–286. doi: 10.1159/000260208. [DOI] [PubMed] [Google Scholar]
- Mandel I. D. Nonimmunologic aspects of caries resistance. J Dent Res. 1976 Apr;55(Spec No):C22–C31. doi: 10.1177/002203457605500316011. [DOI] [PubMed] [Google Scholar]
- McGaughey C., Field B. D., Stowell E. C. Effects of salivary proteins on the adsorption of cariogenic streptococci by hydroxyapatite. J Dent Res. 1971 Jul-Aug;50(4):917–922. doi: 10.1177/00220345710500042201. [DOI] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo S. Z., Schotzko N. K., Folke L. E. Use of hydroxyapatite-coasted glass beads for preclinical testing of potential antiplaque agents. Appl Environ Microbiol. 1976 Sep;32(3):428–432. doi: 10.1128/aem.32.3.428-432.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


