Abstract
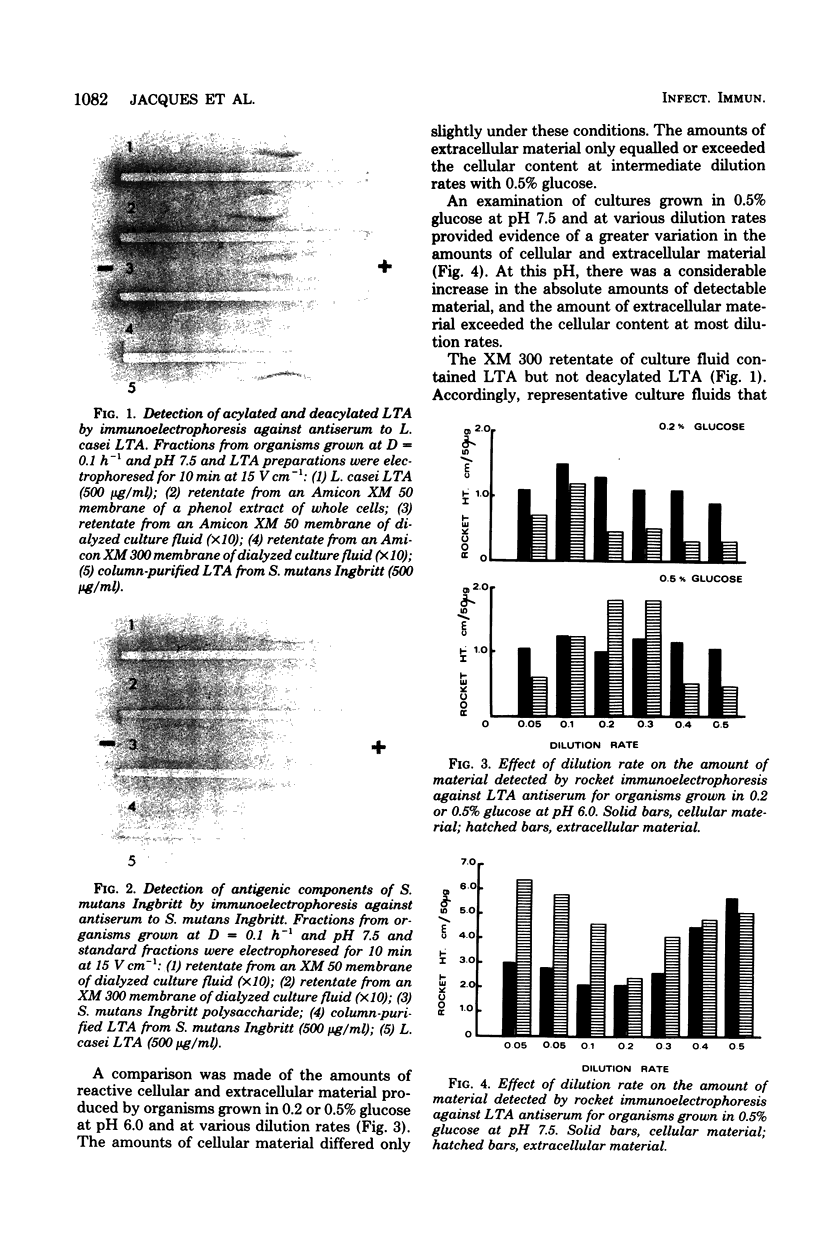
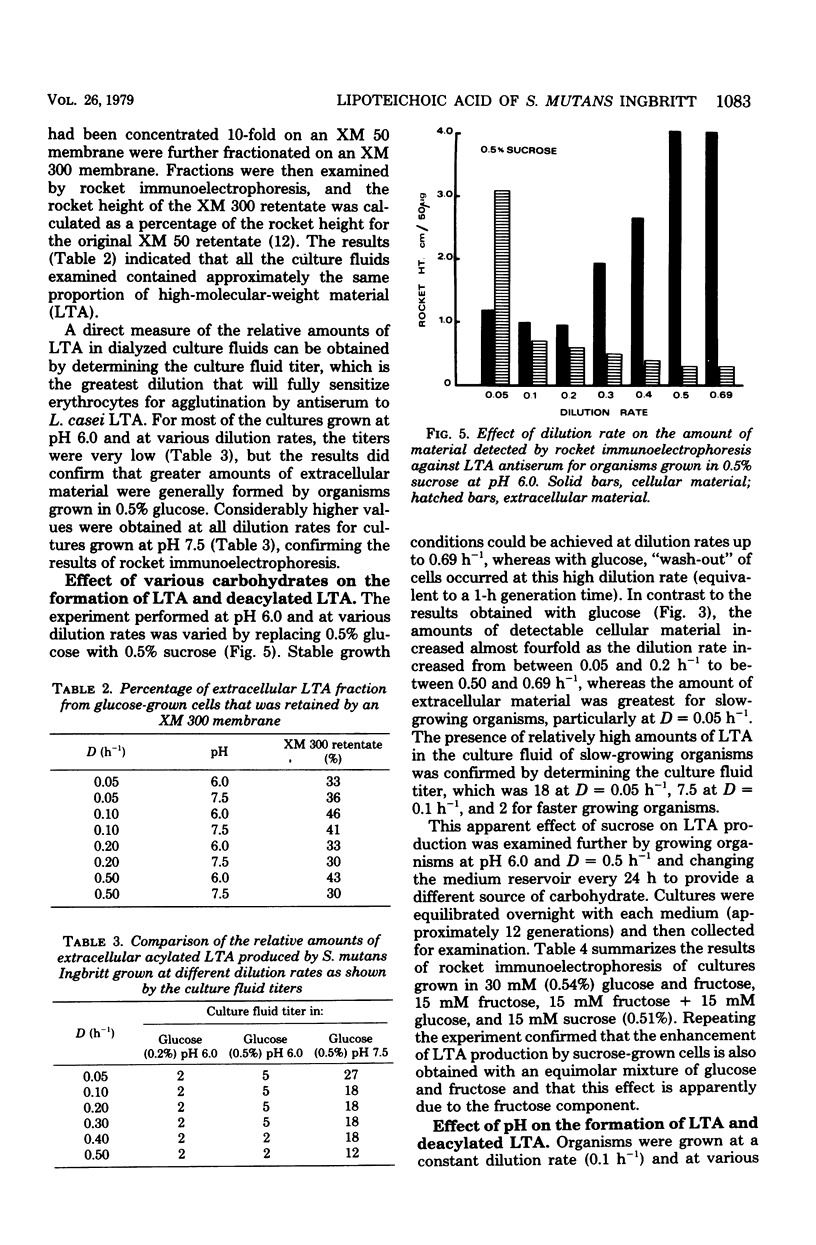
Streptococcus mutans Ingbritt was grown in a chemostat at defined dilution rates and pH values and under carbohydrate limitation. At a constant dilution rate of D = 0.1 h-1 and with either 0.5% glucose or 0.5% sucrose, the amounts of both cellular and extracellular lipoteichoic acid increased as the culture pH increased from 5.0 to 7.5. At a constant pH of 6.0, the amount of cellular lipoteichoic acid formed by cultures growing in 0.2% or 0.5% glucose was relatively constant over a range of dilution rates, although the amount of extracellular lipoteichoic acid formed in 0.2% glucose at intermediate dilution rates was less than that formed in 0.5% glucose. Organisms grown in 0.5% sucrose at pH 6.0 contained increasing amounts of cellular lipoteichoic acid as the dilution rate was increased. A comparison of the amounts of cellular lipoteichoic acid formed by organisms growing at D = 0.5 h-1 and pH 6.0 in glucose, sucrose, fructose, or mixtures of glucose and fructose in limiting amounts suggested that the enhanced production of lipoteichoic acic by sucrose-grown organisms was due to the fructose component. The culture fluids from both glucose- and sucrose-grown organisms contained detectable amounts of serotype c antigen, whereas glucose-grown cultures also contained significant amounts of an extracellular hexose-containing polymer.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of Streptococcus mutans strains in some selected areas of the world. Odontol Revy. 1972;23(4):401–410. [PubMed] [Google Scholar]
- Bratthall D. Immunodiffusion studies on the serological specificity of streptococci resembling Streptococcus mutans. Odontol Revy. 1969;20(3):231–243. [PubMed] [Google Scholar]
- Bratthall D., Pettersson B. M. Common and unique antigens of Streptococcus mutants. J Dent Res. 1976 Jan;55:A60–A64. doi: 10.1177/002203457605500123011. [DOI] [PubMed] [Google Scholar]
- Campbell L. K., Knox K. W., Wicken A. J. Extractability of cell wall polysaccharide from lactobacilli and streptococci by autoclaving and by dilue acid. Infect Immun. 1978 Dec;22(3):842–851. doi: 10.1128/iai.22.3.842-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. Determination of maltase and isomaltase activities with a glucose-oxidase reagent. Biochem J. 1961 Sep;80:547–551. doi: 10.1042/bj0800547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneo-Moore L., Terleckyj B., Shockman G. D. Analysis of growth rate in sucrose-supplemented cultures of Streptococcus mutans. Infect Immun. 1975 Nov;12(5):1195–1205. doi: 10.1128/iai.12.5.1195-1205.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Baird J. K., Hunter J. R., Longyear V. M. Variations in surface polymers of Streptococcus mutans. J Dent Res. 1976 Apr;55(Spec No):C42–C49. doi: 10.1177/002203457605500323011. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Hunter J. R., Longyear V. M. Growth of Streptococcus mutans in a chemostat. Arch Oral Biol. 1974 Aug;19(8):659–664. doi: 10.1016/0003-9969(74)90134-4. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Jacques N. A., Hardy L., Knox K. W., Wicken A. J. Effect of growth conditions on the formation of extracellular lipoteichoic acid by Streptococcus mutans BHT. Infect Immun. 1979 Jul;25(1):75–84. doi: 10.1128/iai.25.1.75-84.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Jacques N. A., Campbell L. K., Wicken A. J., Hurst S. F., Bleiweis A. S. Phenotypic stability of the cell wall of Streptococcus mutans Ingbritt grown under various conditions. Infect Immun. 1979 Dec;26(3):1071–1078. doi: 10.1128/iai.26.3.1071-1078.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Markham J. L., Wicken A. J. Formation of cross-reacting antibodies against cellular and extracellular lipoteichoic acid of Streptococcus mutans BHT. Infect Immun. 1976 Mar;13(3):647–652. doi: 10.1128/iai.13.3.647-652.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer R., Gill K., Slade H. D. Chemical composition of Streptococcus mutans type c antigen: comparison to type a, b, and d antigens. J Dent Res. 1976 Jan;55:A109–A115. doi: 10.1177/002203457605500103011. [DOI] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- Rölla G. Formation of dental integuments--some basic chemical considerations. Swed Dent J. 1977;1(6):241–251. [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in Streptococcus mutans NCTC 10449. Infect Immun. 1979 Jun;24(3):821–828. doi: 10.1128/iai.24.3.821-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson L. A., Little W., Hageage G. J. Application of fluorescent antibody methods in the analysis of plaque samples. J Dent Res. 1976 Jan;55:A80–A86. doi: 10.1177/002203457605500126011. [DOI] [PubMed] [Google Scholar]
- Tinanoff N., Tanzer J. M., Freedman M. L. In vitro colonization of Streptococcus mutans on enamel. Infect Immun. 1978 Sep;21(3):1010–1019. doi: 10.1128/iai.21.3.1010-1019.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel E. Determination of fructose and fructose-yielding carbohydrates with cold anthrone. Anal Biochem. 1967 Apr;19(1):193–194. doi: 10.1016/0003-2697(67)90152-2. [DOI] [PubMed] [Google Scholar]
- Van Handel E. Microseparation of glycogen, sugars, and lipids. Anal Biochem. 1965 May;11(2):266–271. doi: 10.1016/0003-2697(65)90014-x. [DOI] [PubMed] [Google Scholar]
- Wetherell J. R., Jr, Bleiweis A. S. Antigens of Streptococcus mutans: characterization of a polysaccharide antigen from walls of strain GS-5. Infect Immun. 1975 Dec;12(6):1341–1348. doi: 10.1128/iai.12.6.1341-1348.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Gibbens J. W., Knox K. W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973 Jan;113(1):365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Characterization of group N streptococcus lipoteichoic acid. Infect Immun. 1975 May;11(5):973–981. doi: 10.1128/iai.11.5.973-981.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- van Houte J., Saxton C. A. Cell wall thickening and intracellular polysaccharide in microorganisms of the dental plaque. Caries Res. 1971;5(1):30–43. doi: 10.1159/000259730. [DOI] [PubMed] [Google Scholar]




