SUMMARY
Production of reactive oxygen species (ROS) increases with neuronal activity that accompanies synaptic development and function. Transcription-related factors and metabolic enzymes that are expressed in all tissues have been described to counteract neuronal ROS to prevent oxidative damage. Here, we describe the antioxidant gene LanCL1 that is prominently enriched in brain neurons. Its expression is developmentally regulated and induced by neuronal activity, neurotrophic factors implicated in neuronal plasticity and survival, and oxidative stress. Genetic deletion of LanCL1 causes enhanced accumulation of ROS in brain, and development-related lipid, protein, and DNA damage, mitochondrial dysfunction and apoptotic neurodegeneration. LanCL1 transgene protects neurons from ROS. LanCL1 protein purified from eukaryotic cells catalyzes the formation of thioether products similar to glutathione S-transferase. These studies reveal a neuron-specific glutathione defense mechanism that is essential for neuronal function and survival.
INTRODUCTION
Structural and functional plasticity of the developing nervous system are modulated by neuronal activity (Flavell and Greenberg, 2008). During this process, neurons are especially vulnerable to oxidative stress because neuronal activity increases oxygen utilization for energy production with the accompanying production of reactive oxygen species (ROS) (Coyle and Puttfarcken, 1993; Ikonomidou and Kaindl, 2011). Excessive ROS causes progressive oxidative damage to lipids, proteins and DNA in neurons (Finkel and Holbrook, 2000), impairs synaptic function (Massaad and Klann, 2011; Stranahan and Mattson, 2012), and is implicated in developmental-related neurodegenerative diseases including Alzheimer’s and Parkinson’s diseases (Andersen, 2004; Kondo et al., 2013). Antioxidant defense is evoked by neuronal activity to control ROS levels (Papadia et al., 2008; Soriano et al., 2011). Most antioxidant mechanisms are under the control of transcription factors PGC-1α and Nrf-2 (Crunkhorn, 2012; St-Pierre et al., 2006). These mechanisms appear widely conserved across species and cell types, and may be redundant since the genetic deletion of individual enzymes or even PGC-1α produces only modest phenotypic alterations without exogenous insult (Carlsson et al., 1995; Ho et al., 2004; Reaume et al., 1996; St-Pierre et al., 2006). Glutathione is a major effector of antioxidant defense by virtue of its ability to scavenge free radicals and participate in the reduction of hydrogen peroxide (H2O2) (Hayes and McLellan, 1999). The glutathione antioxidant defense mechanism involves multiple enzymes, including the glutathione-dependent enzymes, glutathione peroxidase (GPX) and glutathione S-transferase (GST). (Crunkhorn, 2012; St-Pierre et al., 2006)(Crunkhorn, 2012; St-Pierre et al., 2006)
LanCL1 (Lanthionine synthetase C-like protein 1, also known as P40 or GRP69A) (Bauer et al., 2000) is a mammalian member of the LanC-like protein superfamily encompassing a highly divergent group of peptide-modifying enzymes present in plants and bacteria (LanCs). Prokaryotic LanC is a zinc-containing enzyme that acts in concert with lantibiotic dehydratases to facilitate intramolecular conjugation of cysteine to serine or threonine residues, yielding macrocyclic thioether (Lanthionine) products with potent antimicrobial activity (Champak Chatterjee, 2005). Three LanC-like genes—LanCL1, LanCL2, and LanCL3 are present in human genome (Landlinger et al., 2006). Human LanCL1 protein binds zinc ion and glutathione and appears to play a regulatory role in axonal growth (Chung et al., 2007; Zhang et al., 2009). Here we report that LanCL1 is primarily expressed in brain neurons, is developmentally regulated, is induced by neuronal activity, and is essential for mitigating neuronal oxidative stress during normal postnatal development and in response to oxidative stresses. Additionally, LanCL1 transgene expression is protective against oxidative stress. Enzymatic assays demonstrate catalysis of glutathione conjugation to synthetic substrates similar to the glutathione S-transferase (Habig and Jakoby, 1981). These observations indicate that LanCL1 is part of glutathione antioxidant defense mechanism that is uniquely essential for neuronal function.
RESULTS
LanCL1 expression is induced by neuronal activity and oxidative stress
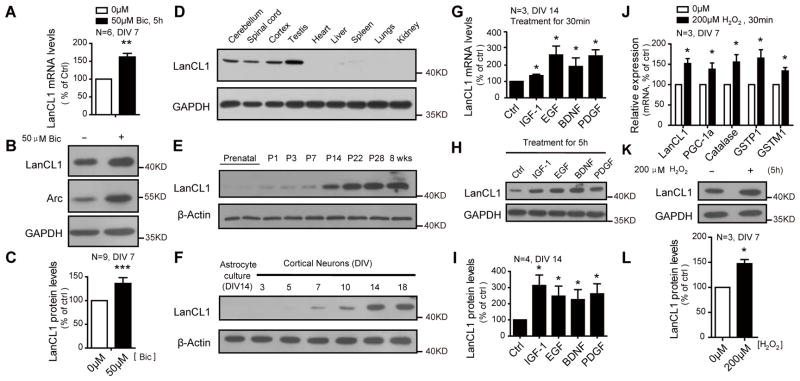
We identified LanCL1 based on its neuronal expression and rapid induction by activity. LanCL1 mRNA and protein are induced in vivo by maximal electroconvulsive seizure (MECS) (Figure S1B and S1C). The induced expression of LanCL1 by activity is also recapitulated in cortical neuron culture by addition of bicuculline that blocks the inhibitory action of GABA receptors (Ueno et al., 1997) (Figure 1A–1C). LanCL1 is primarily expressed in neural tissues and testis (Figure 1D and Figure S1A). The expression of LanCL1 protein in brain is developmentally regulated, increasing during the first postnatal month and remaining high in adult (Figure 1E). Expression in neuron cultures increases between DIV 7–14 (Figure 1F) and parallels the formation of synapses and spontaneous neuronal activity (Kamioka et al., 1996) This pattern of activity-regulated expression is typical of neuronal immediate early genes (Brakeman et al., 1997; Lyford et al., 1995). Consistent with a role for LanCL1 in an induced genomic program to activity, neurotrophic factors such as IGF-1, EGF, BDNF, and PDGF that modulate synaptic activity and protect neurons against oxidative stress(Cheng and Mattson, 1995; Skaper et al., 1998; Zhang et al., 1993) induce LanCL1 expression (Figure 1G–1I). Furthermore we found that LanCL1 is induced by agents that evoke oxidative stress including glutamate (Ratan and Baraban, 1995) and H2O2 (Figure 1J–1L and Figure S1D), and this induction occurs concurrently with the canonical oxidative stress response that includes PGC-1α and β and ROS-detoxifying enzymes copper/zinc superoxide dismutase (SOD1) and manganese SOD (SOD2) (St-Pierre et al., 2006).
Figure 1. Induction of LanCL1 expression by neurotrophic factors and oxidative stress inducing agents.
(A) qRT-PCR shows induction of LanCL1 mRNA in bicuculline-treated (Bic, 5h) cortical neurons (DIV7). Error bars indicate SEM. **p= 0.0016. n=6.
(B and C) Western blots and quantification show increased LanCL1 protein levels in bicuculline-treated (15h) cortical neurons (DIV 7). Error bars indicate SEM. *p<0.0001. n=9.
(D) Western blots show the expression pattern of LanCL1 in multiple organs.
(E and F) Western blots show the temporal expression pattern of LanCL1 in the postnatal mouse cortex and neuronal versus astrocyte cultures.
(G) qRT-PCR shows induction of LanCL1 mRNA in neurotrophic factors-treated (30min) cortical neurons (DIV14). Error bars indicate SEM. IGF-1 *p= 0.0491; EGF, *p= 0.0490; BDNF, *p= 0.0489; PDGF, *p= 0.0265. n=3.
(H and I) Western blots and quantification show increased LanCL1 protein levels in neurotrophic factors-treated (5h) cortical neurons (DIV 14). Error bars indicate SEM. IGF-1 *p=0.0403; EGF, *p=0.0292; BDNF, *p=0.0256; PDGF, *p=0.0224. n=4.
(J) qRT-PCR shows induction of LanCL1 mRNA along with oxidative defense genes in cortical neurons of DIV7 in response to H2O2 treatment (30min). The relative induction fold is normalized against non-treatment control. Error bars indicate SEM, LanCL1 *p=0.0258; PGC1-α, *p=0.0345; Catalase, *p=0.0465; GSTP1, *p=0.0456, GSTM1 *p=0.0276; n=3.
(K and L) Western blots and quantification show increased LanCL1 protein level in H2O2-treated cortical neurons (DIV 7). Error bars indicate SEM, *p=0.0292, n=3.
See also Figure S1
Loss of LanCL1 causes development-dependent neuronal death and inflammation
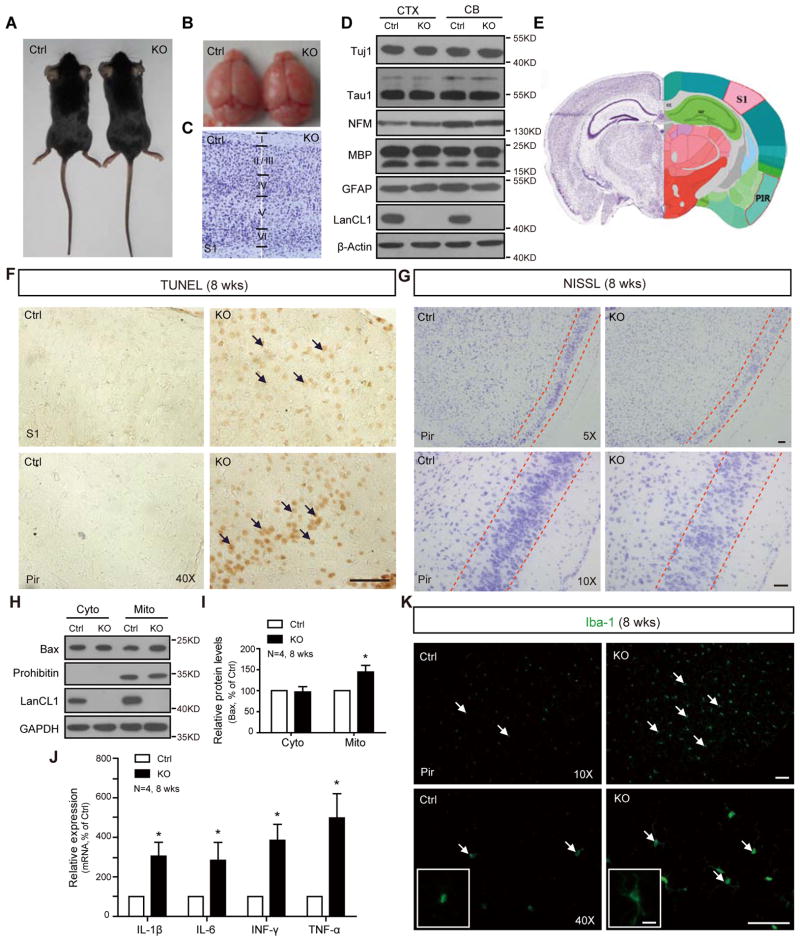
To examine the functional role of LanCL1 in the response to oxidative stress, we generated a LanCL1 knockout mouse (LanCL1−/−, ko) (Figure S2A–S2D). LanCL1−/− mice are born at an expected Mendelian ratio (Figure S2E), and display normal postnatal viability and growth. At 4 weeks of age, the gross brain morphology, cortical lamination, and expression of select neuronal and glial markers is comparable to WT mice (Figure 2A–2D). This indicates LanCL1 is not essential role for embryonic or early postnatal brain development. However, during later postnatal development, LanCL1−/− mice demonstrate prominent neuronal degeneration. Brains of 8- to 12-week LanCL1−/− mice display increased TUNEL positive staining in cerebral cortex and cerebellum (Figure 2F and data not shown). Apoptotic death is present in the entire cortex but is most prominent in neurons of layers II/III. Nissl staining reveals a loss of cortical neurons in the layers II/III (Figure 2G). BCL-2 family member Bax is increased in the mitochondria fraction from cortex (Figure 2H and 2I), consistent with increased apoptotic death (Rosse et al., 1998). Neuronal death in the brain of 8-week LanCL1−/− mice is accompanied by neuroinflammatory responses including increased levels of inflammatory cytokines IL1, IL6, TNF and INF (Figure 2J), and a 50% increase of activated microglia (p=0.0045, n=6) displaying an increased size of soma and number of cellular processes (Figure 2K).
Figure 2. Apoptotic neuronal death and inflammation in LanCL1 ko brains.
(A and B) Photographs show that the genetic deletion of LanCL1 does not affect the body or brain size (LanCL1 −/−, ko, hereafter).
(C) Nissl staining reveals cortical lamination.
(D) Western blots show the normal expression of axonal, astrocytes and myelination markers.
(E) Illustration (from Allen Brain) shows the brain zoning in mice as reference for (F) and (G).
(F) TUNEL staining shows an increase in apoptosis (brown) of the 8-week LanCL1 ko cortex (CTX, S1 & Pir). Bar, 50μm.
(G) Nissl staining reveals reduced density of cortical neurons in the 8-week LanCL1 ko cortex. Bar, 50μm.
(H) Subcellular fractionation shows an increase in Bax in the mitochondria of the 8-week LanCL1 ko cortex by western blotting. Prohibitin: the mitochondrial marker. Cyto: cytosol and Mito: mitochondria.
(I) Quantification of the increase in Bax in mitochondrial fraction of LanCL1 ko relative to wt control. Error bars indicate SEM, *p=0.0331, n=4.
(J) qRT-PCR shows the increase mRNA levels of inflammation factors in LanCL1 ko cortex. Error bars indicate SEM, IL-1 *p=0.04; IL-6, *p=0.0441; INF-γ, *p=0.0417; TNF-α, *p=0.0348; n=4
(K) Iba-1 staining shows an increase in the number of activated microglia with increased size of soma and number of cellular processes in the cortex of 8-week LanCL1 ko brains. Bar, 50μm. The insets show enlarged microglia. Bar, 10μm.
See also Figure S2
LanCL1 is required for mitigating oxidative stress under physiological conditions
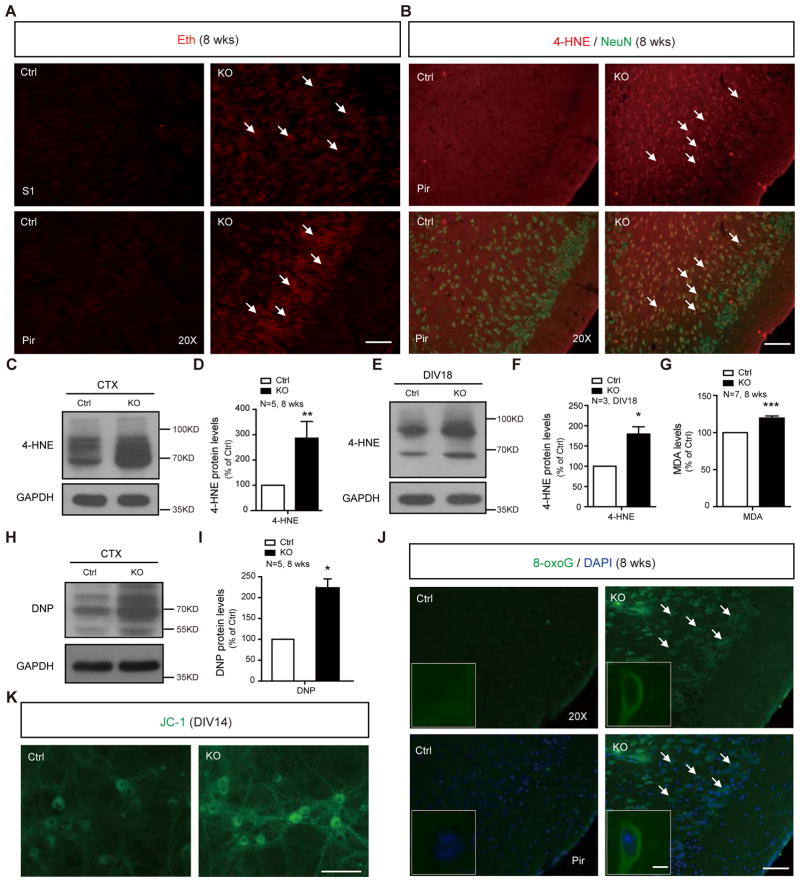
We suspected that the neuronal death in LanCL1−/− mice is a result of progressive oxidative damage. In the 4-week LanCL1−/− mice, multiple antioxidant defense genes such as PGC-1α, PGC-1β, SOD1, SOD2, Catalase, Cyt-C and ANT are upregulated in the cortex (Figure S3A) and cerebellum (Data not shown), indicative of oxidative stress (St-Pierre et al., 2006). At this stage, no apparent oxidative damage or neuronal death is evident (Figure S3B and data not shown). By 8 to 12 weeks, LanCL1−/− cortex shows an accumulation of ROS and lipid peroxidation as indicated by fluorescent ethidium labeling (Brennan et al., 2009) (Figure 3A) and 4-Hydroxy-2-Nonenal (4-HNE) western blot (Figure 3C, 3D and Figure S3D), and redox imbalance as indicated by a decrease of the NADPH/NADP ratio (Mugoni et al., 2013) (Figure S3C). All these changes indicate development-dependent oxidative damage in LanCL1−/− brains. Double labeling with 4-HNE and neuronal marker (NeuN) revealed a widespread increase in 4-HNE in cortical neurons of 8-week LanCL1−/− mice (Figure 3B). Increased 4-HNE levels are also detected in cultured LanCL1−/− neurons (Figure 3E, 3F and Figure S3E). The increase in 4-HNE is accompanied by an increase in malondialdehyde (MDA) (Figure 3G), implicating lipid peroxidation (Sharma et al., 2004). Oxidative carbonylation of proteins assayed by blot with anti-dinitrophenol (DNP) antibody (Nystrom, 2005) is increased more than 2 fold (Figure 3H, 3I and Figure S3F). Increased immunoreactivity of 8-Oxoguanine (8-oxoG) indicates free radical oxidative damage to DNA (Figure 3J).
Figure 3. Oxidative damages and ROS accumulation in LanCL1 ko brains.
(A) Representative images of ethidium fluorescence (Eth, red) show the accumulation of reactive oxygen species (ROS) in the cortex of 8-week LanCL1 ko brain. Bar:50μm.
(B) Immunostaining with anti-4-Hydroxynonenal (4-HNE, red) and anti-NeuN antibody (green) shows an increase in 4-HNE positive neurons in the 8-week LanCL1 ko cortex. Bar, 50μm.
(C and D) Western blots and quantification show an increase in the level of 4-HNE in the cortex of the 8-week LanCL1 ko brain. Error bars indicate SEM, **p=0.0033, n=5.
(E and F) Western blots and quantification show increase in the level of 4-HNE in LanCL1 cortical culture. Error bars indicate SEM, *p=0.0236, n=3.
(G) Quantification shows increase of malondialdehyde (MDA) in cortex of 8-week LanCL1 ko. Error bars indicate SEM, ***p=0.0003, n=7.
(H and I) Western blots with DNP antibody and quantification show increase of DNP levels in the cortex of 8-week LanCL1 ko cortex. Error bars indicate SEM, *p=0.0176, n=5.
(J) Immunostaining with 8-oxoG antibody shows an increase in 8-oxoG positive cells (green) in the 8-week LanCL1 ko cortex. Bar, 50μm. The insets show enlarged neurons. Bar, 5μm.
(K) JC-1 staining shows an increase in the fluorescence intensity of monomeric form of JC-1 (green) in LanCL1 ko cultures (DIV14). Bar, 50μm.
See also Figure S3
Mitochondria are particularly vulnerable to oxidative stress (Yakes and Van Houten, 1997). Mitochondria of LanCL1−/− cortical neurons appear impaired based on the JC-1 assay (Smiley et al., 1991) with a 50% increase in the intensity of green fluorescence (Figure 3K, p=0.0275, n=3), and a 40% decrease in the ratio of red/green fluorescence intensity (p=0.0031, n=3), suggesting impairment of the electrochemical gradient across inner mitochondrial membrane. The mitochondrial impairment is also indicated by reduced expression of mitochondrial related genes involved in mitochondrial energy metabolism (Figure S3G). These data suggest that LanCL1 is required for mitigating oxidative stress generated under physiological condition, and that oxidative damage is the cause of development-dependent neuronal death in LanCL1−/− mouse
LanCL1 catalyzes the formation of thioether products
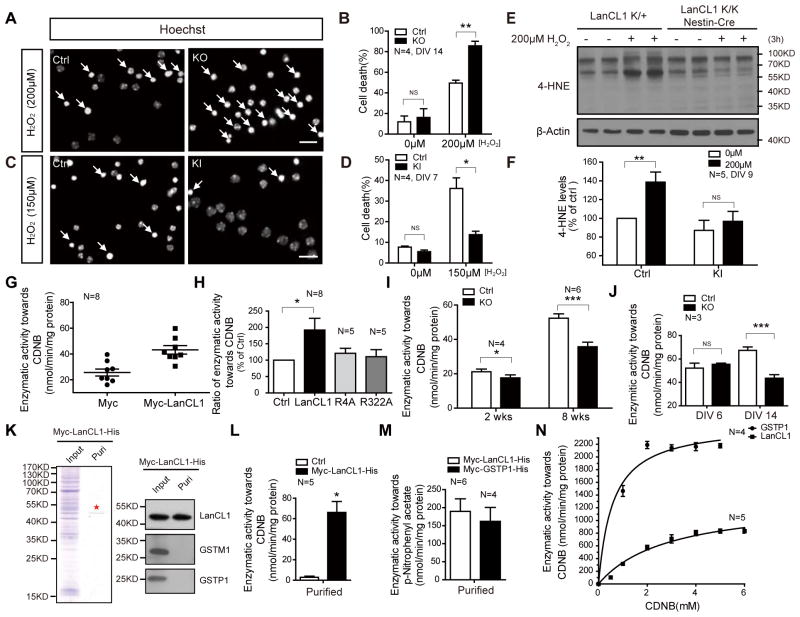
The role of LanCL1 in mitigating neuronal oxidative stress suggests a neuronal protective effect against stressors. To assess its cellular protective effect, we treated HeLa cells expressing GFP-tagged LanCL1 or GFP with H2O2. LanCL1 expressing cells show reduced apoptosis (PI/Hoechst staining; Figure S4A and S4B) and higher cell viability (CCK-8 assay; Figure S4C). To test its role in neuronal protection, we cultured cortical neurons from WT or LanCL1−/− mice and treated them with oxidative stress-inducing agents H2O2 or NMDA-type glutamate antagonist MK-801 (Papadia et al., 2008). LanCL1−/− neurons exhibit increased cell death (Figure 4A, 4B and Figure S4D, S4E). We also determined that the cellular protective effect of neurotrophic factors EGF and BDNF against H2O2 is impaired in cultured cortical neurons from LanCL1−/− mice (Figure S4F, S4G). To further confirm its neuronal protective effect, we generated a conditional LanCL1 transgenic mouse by insertion of a floxed expression construct into the Rosa 26 locus. V5-tagged LanCL1 is conditionally expressed in the brain upon crossing with Nestin-Cre driver mice (Figure S4H–S4K). Cortical neurons derived from transgenic LanCL1 mice express a higher level of LanCL1 protein and are relatively resistant to H2O2 induced neuronal death (Figure S4K and Figure 4C, 4D). Furthermore, accumulation of 4-HNE induced by H2O2 treatment is significantly reduced in neuronal cultures of LanCL1 transgenic mice (Figure 4E, 4F), demonstrating a role for LanCL1 in mitigating neuronal oxidative stress.
Figure 4. LanCL1 possesses catalytic activity for thioether formation and protects cells against oxidative stress.
(A–D) Hoechst staining shows that deletion of LanCL1 increased neuronal death induced by H2O2 (12 hour treatment, DIV14, Ctrl: LanCL1 +/+, KO: LanCL1 −/−) (A, B), and LanCL1 transgene reduced neuronal death (12 hour treatment, DIV7, Ctrl: LanCL1 K/K, KI: LanCL1 K/K Nestin-Cre) (C, D). The data represent the mean ± SEM from four independent experiments, with a total number of 2,000 neurons analyzed for each group. Bar, 50μm. Error bars indicate SEM, *p= 0.0135, **p= 0.0069. n=4.
(E and F) Western blots and quantification show that LanCL1 KI culture neurons are more resistant to H2O2-induced 4-HNE accumulation (DIV9, Ctrl: LanCL1 K/+, KI: LanCL1 K/K Nestin-Cre). Error bars indicate SEM, **p= 0.0030, NS p=0.5079. n=5.
(G and H) Quantifications show increased GST activity in LanCL1-overexpressing HeLa cells compared with ctrl cells or LanCL1 point mutants that lack glutathione binding. Ctrl: Myc; LanCL1: Myc-LanCL1; R4A: Myc-LanCL1(R4A); R322A: Myc-LanCL1(R322A). Error bars indicate SEM, *p=0.0189, n=8.
(I) Quantification shows a correlation between the reduction in GST activity in LanCL1 ko cortex with LanCL1 expression level in 2-weeks to 8-week mice. Error bars indicate SEM. *p=0.0118 for 2-week sample, n=4; ***p=0.0004 for 8-week sample, n=6.
(J) Quantification shows a correlation of a reduction in GST activity in LanCL1 ko culture neurons with its protein level in DIV6 to DIV14 cultures. Error bars indicate SEM. ***p=0.0002, n=3.
(K) Coomassie brilliant blue staining and western blots show the affinity purified polyhistidine tagged LanCL1 preparation from HeLa cells. GSTM1 and GSTP1 were depleted from purified LanCL1. ★ indicates the LanCL1 band. Puri: purification.
(L) Affinity purified LanCL1 catalyzes conjugation of the glutathione to 1-Chloro-2, 4-dinitrobenzene (CDNB). GSH: 0.5mM; CDNB, 0.5mM. Error bars indicate SEM, *p=0.0243, n=5.
(M) Affinity purified LanCL1 and GSTP1 catalyze conjugation of the glutathione to p-Nitrophenyl acetate. GSH: 0.5mM; p-Nitrophenyl acetate: 0.2mM. Error bars indicate SEM. LanCL1, n=6; GSTP1, N=4.
(N) Kinetics assay of affinity purified LanCL1 and GSTP1. GSH was 5mM with varying CDNB concentration from 0mM to 6mM. Error bars indicate SEM. LanCL1, n=5; GSTP1, N=4.
See also Figure S4
How does LanCL1 exert its neuronal protective effect? Since LanCL1 binds GSH, we wondered if LanCL1 is part of the glutathione mediated antioxidant defense mechanism. In mammalian cells, GSTs catalyze the reaction of glutathione with a wide range of electrophilic compounds to form thioethers (Hayes and Pulford, 1995), and play a role in mitigating oxidative stress. Similar with GSTs, prokaryotic LanC protein catalyzes the formation of thioether products (Champak Chatterjee, 2005). The antioxidant effect of LanCL1 together with its ability to bind glutathione (Zhang et al., 2009) led to the prediction that mammalian LanCL1 may catalyze the formation of thioether products like GST proteins. Expression of myc-tagged LanCL1 in HeLa cells increased GST activity towards 1-chloro-2,4-dinitrobenzene (CDNB) (Figure 4G), a common reporter substrate for GST activity (John D. Hayes, 1995). LanCL1 point mutants (R4A and R322A) that fail to bind glutathione (Zhang et al., 2009) failed to increase GST activity and showed reduced cellular protection (Figure 4H and Figure S4L, S4M). The finding that LanCL1 contributes to GST activity predicts that loss of LanCL1 will reduce GST activity in the brain of LanCL1−/− mouse. Accordingly, we assayed the GST activity of the extracts from 2-week and 8-week cortex of LanCL1−/− mice and noted a modest reduction in GST activity (16.99±3.99%, n=4, p=0.0118) at 2 weeks, and a more pronounced reduction (31.65±3.69%, n=6, p=0.0004) at 8 weeks. This suggests that LanCL1 contributes to the developmental increase of GST activity and parallels the expression profile of LanCL1 in the cortex of WT mice (Figure 4I, 1E). A similar development-dependent reduction of GST activity in DIV 14 but not DIV 6 LanCL1−/− neuronal cultures parallels the normal developmental expression of LanCL1 protein. (Figures 4J, 1F). The major brain GST enzymes GSTM1 and GSTP1 (Mitchell et al., 1997) are expressed at WT levels in LanCL1−/− brain (Figure S4N).
To assess if LanCL1 itself possesses enzymatic activity to catalyze the formation of thioether productions like GSTs, we expressed His-tagged LanCL1 and purified it from HeLa cells using Nickel affinity chromatography. A negative control expressed myc-tagged LanCL1 that lacked the His tag (Figure 4K left panel and Figure S4P). Coomassie staining shows that LanCL1 protein was not co-purified with GSTM1 or GSTP1 and appeared as a single band (Figure 4K, right panel). We assayed the enzymatic activity of purified LanCL1 protein preparation towards two different substrates for GST; CDNB and p-Nitrophenyl acetate (Habig and Jakoby, 1981). The LanCL1 preparation exhibited GST enzymatic activity towards both substrates. (Figure 4L, 4M). Enzymatic kinetics assays revealed a Vmax and Km of 1087nmol/min/mg protein and 1.93mM (CDNB), respectively (Figure 4N and Figure S4S). In the same assay, the activity of LanCL1 was measured in parallel with a canonical GST that is highly expressed in brain, GSTP1 (Figure S4Q and S4R) (Czerwinski et al., 1996). LanCL1 preparation catalysis was ~45% (Vmax) of GSTP1 (Figure S4S). These data indicate that recombinant LanCL1 functions to catalyze thioether formation, and support the conclusion that reduced GST activity in LanCL1−/− brain is a direct consequence of loss of LanCL1.
DISCUSSION
The present study identifies LanCL1 as an antioxidant defense gene that is part of the glutathione defense pathway. LanCL1 appears essential for a normal cellular response to stress in developing neurons. Loss of LanCL1 causes oxidative stress followed by development-dependent neuronal death. Antioxidant defense genes are upregulated in the 4-week LanCL1−/− mice (before the onset of neuronal death) (Figure S3A), and this is followed within the next 4–8 weeks by progressive oxidative damage to lipids, proteins, DNAs and mitochondria, and apoptotic cell death. Conversely, increased LanCL1 expression confers neuronal resistance to oxidative stress as neuronal survival is significantly improved with reduced lipid oxidation under H2O2 treatment (Figure 4E, 4F). In further support of a direct role of LanCL1 in mitigating oxidative stress, we demonstrate catalytic activity of purified recombinant LanCL1 in the formation of thioether products using both CDNB and p-Nitrophenyl acetate as substrates (Figure 4L, 4M).
The catalytic activity of LanCL1 resembles that of GST proteins, however, LanCL1 is not a member of GST superfamily. LanCL1 does not share sequence or structural similarity with canonical GSTs (Sheehan et al., 2001; Zhang et al., 2009). An earlier study reported that LanCL1 interacts with cystathionine β-synthase (CBS), a trans-sulfuration enzyme that functions to increase glutathione synthesis (Zhong et al., 2012). Interestingly, transient knockdown of LanCL1 in cultured neurons resulted in elevated CBS activity and modest protection of neurons from oxidative stress (Zhong et al., 2012). We noted an increase in CBS activity in the brain extracts of LanCL1−/− mice (data not shown) and increased glutathione levels in LanCL1−/− cortex (Figure S4O), however this presumed homeostatic adaptation of glutathione cannot compensate for loss of LanCL1 in LanCL1−/− brain or cultured neurons.
The LanC family is evolutionarily ancient. While further enzymology is required, it appears that the ability to form thioethers is retained yet used for widely different purposes in prokaryotic cells and neurons. LanCL1 and LanCL2 are prominently expressed in brain, and provide precedent that antioxidant mechanisms can be cell-type specific. This contrasts with canonical antioxidant genes that are ubiquitously expressed in all cell types (Muller et al., 2007). Selective expression in neurons may be understood to result from the special demands of neurons for protection from ROS. LanCL1 deletion alone is sufficient to cause wide-spread and prominent neuronal death. This again contrasts with canonical antioxidant defense genes whose contribution to oxidative defense is revealed only upon challenge by exogenous oxidative stressors. It is possible that LanCL1 is uniquely effective for detoxification of critical substrates and for S-glutathionylation of critical proteins (Dalle-Donne et al., 2009). Our study indicates that ROS generated during normal developmental physiology are toxic in the absence of sufficient oxidative defense. The LanCL family will be important to integrate into understandings of synaptic physiology, stress response, and the selective vulnerability of neurons in aging and neurodegenerative diseases.
EXPERIMENTAL PROCEDURES
Generation of LanCL1 knockout mice
The targeting construct, in which exon 4 flanked by loxP sites, was made by modifying a BAC clone using recombineering. Homozygous LanCL1 mutants (ko) were generated by intercrossing of heterozygous mutants (LanCL1+/−). More details and the validation of the knockouts are described in the Supplemental Experimental Procedures.
Oxidative stress and cell death analyses in LanCL1 knockout mice
ROS levels were quantitated in LanCL1 wt/ko brain with Ethidium (Eth, Invitrogen). NADPH/NADP ratio assay was performed with NADP/NADPH assay kit (BioVision). Oxidative damage was detected by immunofluorescence on brain section with anti-4-HNE antibody (Abcam) and anti-8-oxoguanine antibody (Millipore). Western blots with anti-dinitrophenol (DNP) antibody (Millipore) were performed to detect carbonylation proteins. Cell apoptosis assay was performed with in situ cell death kit (Roche). More details could be found in the Supplemental Experimental Procedures
Western Blotting, RNA extraction and PCR methods, Induction of LanCL1, in vitro cell death assay, and affinity purification of polyhistidine-tagged LanCL1 protein
Please see Supplemental Experimental Procedures for detail description.
Glutathione S-transferase activity assay and enzymatic kinetics assay
The activity of the glutathione S-transferase was measured with the standard protocol described by Habig et al. The enzymatic kinetic assay was performed by fixing the GSH concentration at 5mM, and varying CDNB concentration from 0mM to 6mM. The kinetic data were analyzed by GraphPad Prism with Kcat analysis, the Vmax and the Km values for CDNB were determined from this analysis.
Statistical analysis
Data represent the mean and standard error of the mean (SEM). Student’s t test (one-tailed for western blot, ratio quantification and qRT-PCR, two-tailed for the others) was performed for all statistical significance analysis using GraphPad Prism software. *p<0.05, **p<0.01
Supplementary Material
HIGHLIGHTS.
LanCL1 expression is developmentally regulated and induced by neuronal activity.
Loss of LanCL1 causes progressive oxidative damage and neuronal death.
LanCL1 transgene protects neurons from oxidative stress.
LanCL1 catalyzes the formation of thioether products.
Acknowledgments
M.N.C., L.Z., Y.C., Y.Z. generated LanCL1 mouse models with the help of the Transgenic Facility of Johns Hopkins University. This work was supported by grants from National 973 Basic Research Program of China (2009CB941400 to B.X.) and National Institutes of Health (5P50DA000266-42 to P.F.W.), and in part by the National Basic Research Program of China (No. 2010CB529900) and the National Natural Science Foundation of China (No. 81123003).
Footnotes
Author Contributions
M.N.C. initiated this project and provided the foundation of this work. C.H. and D.J.P. did the bulk of biochemical and cellular experiments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Bauer H, Mayer H, Marchler-Bauer A, Salzer U, Prohaska R. Characterization of p40/GPR69A as a peripheral membrane protein related to the lantibiotic synthetase component C. Biochemical and biophysical research communications. 2000;275:69–74. doi: 10.1006/bbrc.2000.3260. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nature neuroscience. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champak Chatterjee MP, Xie Lili, van der Donk Wilfred A. Biosynthesis and Mode of Action of Lantibiotics. Chem Rev. 2005;105:633–683. doi: 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. PDGFs protect hippocampal neurons against energy deprivation and oxidative injury: evidence for induction of antioxidant pathways. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15:7095–7104. doi: 10.1523/JNEUROSCI.15-11-07095.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CH, Kurien BT, Mehta P, Mhatre M, Mou S, Pye QN, Stewart C, West M, Williamson KS, Post J, et al. Identification of lanthionine synthase C-like protein-1 as a prominent glutathione binding protein expressed in the mammalian central nervous system. Biochemistry. 2007;46:3262–3269. doi: 10.1021/bi061888s. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Crunkhorn S. Deal watch: Abbott boosts investment in NRF2 activators for reducing oxidative stress. Nature reviews Drug discovery. 2012;11:96. doi: 10.1038/nrd3655. [DOI] [PubMed] [Google Scholar]
- Czerwinski M, Gibbs JP, Slattery JT. Busulfan conjugation by glutathione S-transferases alpha, mu, and pi. Drug metabolism and disposition: the biological fate of chemicals. 1996;24:1015–1019. [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends in biochemical sciences. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods in enzymology. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free radical research. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Critical reviews in biochemistry and molecular biology. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Ho YS, Xiong Y, Ma W, Spector A, Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. The Journal of biological chemistry. 2004;279:32804–32812. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Kaindl AM. Neuronal death and oxidative stress in the developing brain. Antioxidants & redox signaling. 2011;14:1535–1550. doi: 10.1089/ars.2010.3581. [DOI] [PubMed] [Google Scholar]
- John D, Hayes DJP. The Glutathione S-Transferase Supergene Family: Regulation of GST and the Contribution of the lsoenzymes to Cancer Chemoprotection and Drug Resistance. Critical Reviews in Biochernisty and Molecular Biology. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Kamioka H, Maeda E, Jimbo Y, Robinson HP, Kawana A. Spontaneous periodic synchronized bursting during formation of mature patterns of connections in cortical cultures. Neurosci Lett. 1996;206:109–112. doi: 10.1016/s0304-3940(96)12448-4. [DOI] [PubMed] [Google Scholar]
- Kondo T, Asai M, Tsukita K, Kutoku Y, Ohsawa Y, Sunada Y, Imamura K, Egawa N, Yahata N, Okita K, et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Abeta and differential drug responsiveness. Cell stem cell. 2013;12:487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Landlinger C, Salzer U, Prohaska R. Myristoylation of human LanC-like protein 2 (LANCL2) is essential for the interaction with the plasma membrane and the increase in cellular sensitivity to adriamycin. Biochimica et biophysica acta. 2006;1758:1759–1767. doi: 10.1016/j.bbamem.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxidants & redox signaling. 2011;14:2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AE, Morin D, Lakritz J, Jones AD. Quantitative profiling of tissue- and gender-related expression of glutathione S-transferase isoenzymes in the mouse. The Biochemical journal. 1997;325(Pt 1):207–216. doi: 10.1042/bj3250207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugoni V, Postel R, Catanzaro V, De Luca E, Turco E, Digilio G, Silengo L, Murphy MP, Medana C, Stainier DY, et al. Ubiad1 is an antioxidant enzyme that regulates eNOS activity by CoQ10 synthesis. Cell. 2013;152:504–518. doi: 10.1016/j.cell.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. The EMBO journal. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratan RR, Baraban JM. Apoptotic death in an in vitro model of neuronal oxidative stress. Clin Exp Pharmacol Physiol. 1995;22:309–310. doi: 10.1111/j.1440-1681.1995.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nature genetics. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxidants & redox signaling. 2004;6:289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. The Biochemical journal. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Floreani M, Negro A, Facci L, Giusti P. Neurotrophins rescue cerebellar granule neurons from oxidative stress-mediated apoptotic death: selective involvement of phosphatidylinositol 3-kinase and the mitogen-activated protein kinase pathway. Journal of neurochemistry. 1998;70:1859–1868. doi: 10.1046/j.1471-4159.1998.70051859.x. [DOI] [PubMed] [Google Scholar]
- Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano FX, Leveille F, Papadia S, Bell KF, Puddifoot C, Hardingham GE. Neuronal activity controls the antagonistic balance between peroxisome proliferator-activated receptor-gamma coactivator-1alpha and silencing mediator of retinoic acid and thyroid hormone receptors in regulating antioxidant defenses. Antioxidants & redox signaling. 2011;14:1425–1436. doi: 10.1089/ars.2010.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain ageing. Nature reviews Neuroscience. 2012;13:209–216. doi: 10.1038/nrn3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang L, Liu Y, Xu J, Zhu G, Cang H, Li X, Bartlam M, Hensley K, Li G, et al. Structure of human lanthionine synthetase C-like protein 1 and its interaction with Eps8 and glutathione. Genes Dev. 2009;23:1387–1392. doi: 10.1101/gad.1789209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tatsuno T, Carney JM, Mattson MP. Basic FGF, NGF, and IGFs protect hippocampal and cortical neurons against iron-induced degeneration. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1993;13:378–388. doi: 10.1038/jcbfm.1993.51. [DOI] [PubMed] [Google Scholar]
- Zhong WX, Wang YB, Peng L, Ge XZ, Zhang J, Liu SS, Zhang XN, Xu ZH, Chen Z, Luo JH. Lanthionine synthetase C-like protein 1 interacts with and inhibits cystathionine beta-synthase: a target for neuronal antioxidant defense. The Journal of biological chemistry. 2012;287:34189–34201. doi: 10.1074/jbc.M112.383646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.