Abstract
A relationship between obesity and type 2 diabetes is now generally well accepted. This relationship represents several major health hazards including morbid obesity and cardiovascular complications worldwide. Diabetes mellitus is a complex metabolic disorder characterized by impaired insulin release and insulin resistance. Lipids play an important physiological role in skeletal muscle, heart, liver and pancreas. Deregulation of fatty acid metabolism is the main culprit for developing insulin resistance and type 2 diabetes. A predominant predisposing factor to developing obesity, insulin resistance and type 2 diabetes is the permanent elevation of free fatty acids in plasma followed by impaired utilization of lipids by muscle. Diabetes-induced inflammation and oxidative stress have also vital role for development of insulin resistance in diabetic patients. The present review is intended to describe the correlation between lipids, obesity and insulin resistance based on current literature, in order to elucidate involved molecular mechanisms in depth.
Keywords: obesity, insulin resistance, lipoproteins, free fatty acids, molecular signaling
Introduction
Type 2 diabetes is a complex metabolic disorder characterized by a persistent high rise in blood glucose along with the modification of some biochemical processes, including lipolysis, lipid peroxidation, and dearth of production of glucose transporters, resulting from defects in insufficient insulin secretion, inefficient insulin function or both.1 Obesity is a condition in which there is excess storage of fat in the body leading to cardiovascular morbidities, insulin resistance and even the development of type 2 diabetes. In obese patients, adipose tissue releases a high amount of non-esterified fatty acids (NEFA), glycerol, hormones, and proinflammatory cytokines. These elements are involved in the development of insulin resistance. When insulin resistance is accompanied by dysfunction of pancreatic islet β-cells, the cells fail to control blood glucose level due to insulin release impairment. These abnormalities in β-cell function cause development of type 2 diabetes. High lipid level in blood has long been associated with type 2 diabetes. Patients with non-insulin-dependent diabetes mellitus (NIDDM), also known as type 2 diabetes mellitus, sometimes have abnormal lipid profiles. Type 2 diabetes is one of the free radical diseases, which increases complications with increased lipid peroxides.2 In this disease, oxidative stress is increased due to an increased production of oxygen-free radicals and inefficient performance of the antioxidant defense system.3 Increased oxygen-free radicals result in the lipid peroxidation of cellular lipids and play a vital role in atherosclerosis and microvascular complications in type 2 diabetes.4 Type 2 diabetes is related to significant cardiovascular morbidity and mortality by modulation of lipid profiles. Dyslipidemia, which occurs in approximately 50% of patients with type 2 diabetes, results in cardiovascular complications by elevated triglyceride (TG) levels, low high-density lipoprotein (HDL) cholesterol levels, and high rise of small, dense low-density lipoprotein (LDL) cholesterol.5 Dyslipidemia, therefore, may be characterized by raised small, dense LDL levels, elevated levels of TG, and low levels of HDL. Individually, the latter two factors increase the risk of cardiovascular diseases, and the combination of these is a risk factor for cardiovascular complications. This risk factor is at least as strong as that of having a high level of LDL cholesterol.6
Insulin resistance is a condition in which cells do not respond to the normal action of insulin secreted from the pancreatic β-cells. As a result, cellular uptake of glucose does not occur and blood glucose level is elevated. This leads, finally, to type 2 diabetes. Resistance to insulin has a direct link to the changes in lipid profiles in NIDDM, and usually it is associated with higher concentrations of TG, and lower concentrations of HDL.7 Insulin resistance develops a number of alterations in lipid metabolism and lipoprotein composition, which renders LDL cholesterol and other lipoproteins more pathogenic in patients with type 2 diabetes. Hypertriglyceridemia results in decreased HDL, which is also a main feature of plasma lipid alterations observed in type 2 diabetic patients.8 The low level of HDL, which exerts anti-atherogenic and antioxidative effects when present in sufficient amounts, is a key feature of NIDDM. Elevation in plasma TG level is observed when HDL level is reduced. This process is mediated by cholesterol ester transfer protein (CETP). Hypertriglyceridemia may be an increased hepatic secretion of very low-density lipoproteins (VLDL) and a delayed clearance of TG-rich lipoproteins, which may mainly be due to increased levels of substrates for TG production, and enhanced free fatty acids (FFA), and glucose levels. The latter could be secondary to decreased activity of lipoprotein lipase (LPL), a key enzyme for lipoprotein-TG.9 Thus, there is a strong association between type 2 diabetes and dyslipidemia. The prevalence of obesity worldwide and its increasing association with diabetes demand for a mechanistic understanding of obesity-related insulin resistance as a priority to understand this correlation in a defined way. In this review we will focus particularly on molecular aspects by which lipids/FFA promote insulin resistance to elucidate the strong association between obesity, insulin resistance and type 2 diabetes.
Fatty Liver Disease, Obesity and Type 2 Diabetes
Non-alcoholic fatty liver disease (NAFLD) leads to obesity and type 2 diabetes.10,11 Hepatic TG content in NAFLD is directly related to the free fatty acid uptake by liver. FFA are released from subcutaneous adipocytes. FFA enter systemic circulation and are transported to the liver through the hepatic artery and portal vein. Additional FFA are released by lipolysis of visceral adipose tissue TG.12 Free fatty acid release rate is higher in case of obese patients than lean.13 Elevated hepatic lipase and hepatic lipoprotein lipase with postprandial lipemia and increased concentrations of FFA have been found in NAFLD patients, which may be responsible for the high concentration of postprandial incorporation of dietary fatty acids.14 Intra-hepatic TG (IHTG) in obese patients is associated with type 2 diabetes.15 Interestingly, liver also produces FFA by de novo synthesis through a series of complex cystosolic polymerization reactions.16 Hepatic fatty acid de novo synthesis is regulated particularly in response to insulin and glucose.16 Transcription factors, sterol regulatory element binding proteins or SREBPs and carbohydrate responding element binding proteins or ChREBPs are activated, which induce nearly all genes involved in de novo lipogenesis (DNL).16 In cases of NAFLD in humans, hepatic expression of several genes involved in DNL additionally increase insulin resistance in skeletal muscles. This may promote IHTG accumulation by diverting ingested carbohydrate (from high carbohydrate meal) away from storage and lower de novo fatty acid synthesis.17 DNL plays an important role to control total IHTG synthesis and VLDL-TG secretion is much higher in NAFLD patients in comparison to normal individuals.16 After taking food, cholesterol is absorbed into gastrointestinal cells and gets mixed with nascent chylomicrons, hepatic uptake of which provides sources of FFA in the liver. Lipoproteins are mostly produced in the liver. Lipid molecules are always in plasma circulation as lipoprotein complexes. Triglycerides are stored in the hepatic tissue as lipid droplets, secreted and circulated in the form of VLDL in the blood. Then VLDL is converted to intermediate density lipoprotein (IDL), and subsequently to LDL with higher cholesterol content, by the action of LPL. In cases where excess energy is needed, glucose is converted to fatty acids, and then to TG.6 After that, LDL circulates in the blood, reaches hepatocytes and binds with LDL receptor. In patients with insulin resistance, hepatic lipid homeostasis is maintained by the increased secretion of VLDL. The plasma NEFA-pool contributes the majority of the fatty acids that flow to the liver in the fasted state, which is the major source of fatty acids secreted by the liver in the form of VLDL.18 Insulin resistance, therefore, has a direct association with elevated lipid and lipoprotein concentrations, elevated VLDL production, and increased plasma LDL level.11 LDL molecules stay longer in the circulation, as LDL receptors show a lower affinity for these smaller particles.19 By low activity of LPL, or by high level of apolipoprotein C-3 or APOC-3, an inhibitor of LPL, a high lipid level occurs in the blood.9 As compared to steatosis, the steatohepatitis mostly links to abnormal VLDL synthesis and secretion.20 Thus, liver has distinct role in the development of insulin resistance.
Role of FFA in the Development of Insulin Resistance
A rise in FFA concentration has important physiological consequences; for example, during pregnancy, an elevated level of FFA induces insulin resistance and so valuable glucose is conserved for the developing foetus.21 Thus, FFA plays both a physiological and a pathological role in the body. But excess FFA in blood may cause serious metabolic syndrome. Over 80% of people with type 2 diabetes are obese and insulin resistant.22 Obesity and insulin resistance may be linked with some mediators including FFA, tumor necrosis factor-α (TNF-α), leptin and adinopectin. Adipocytes are claimed to be a site of insulin resistance.23 Insulin resistance increases lipolysis, leading to increased concentration of circulating FFA and to the development of insulin resistance in skeletal muscles and the liver.24 Thus, there is a strong association between increased plasma FFA, intramyocellular lipid accumulation and insulin resistance.22 Although there are many studies suggesting strong link of FFA and insulin resistance, the mechanism is not fully understood and more research is required to find the exact molecular basis.
Molecular Pathophysiology of Diabetes
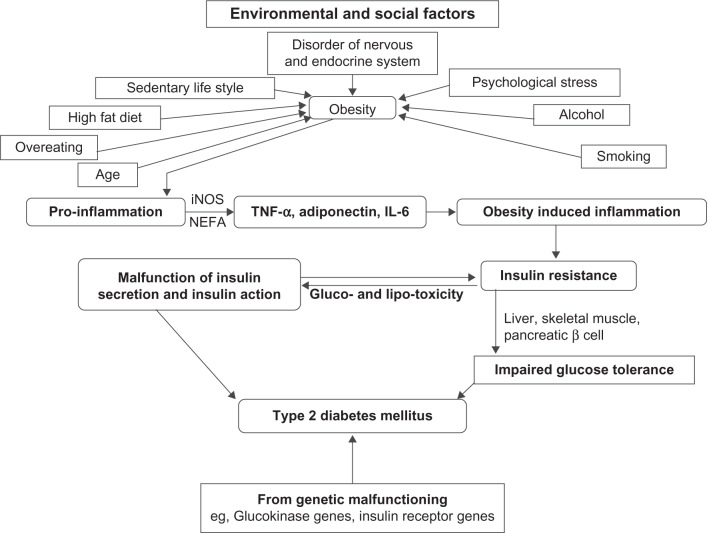
For many years, scientists have been trying to understand pathophysiology at the molecular level to control the elevated blood glucose in type 2 diabetes.25 Type 2 diabetes is reaching an epidemic stage with the increased prevalence of obese people.26 The development of type 2 diabetes results from an interaction of a patient’s genetic background27 with social and environmental factors (Fig. 1). Disorders of the nervous and endocrine systems, a sedentary life-style, psychological stress, a high fat diet, over-eating, age, smoking and alcohol intake are some of the causes which pave the way to obesity, leading to diabetes. Alteration in communication among some vital organs such as the liver, skeletal muscles, pancreas, fat tissue, gastrointestinal tract and brain may result in an elevated blood glucose level, leading to type 2 diabetes. Since most patients with type 2 diabetes are overweight or obese, the role of fatty acid cannot be ignored in the development of diabetes. An interaction of NEFA with glucose metabolism has been described.28 The correlation between obesity and insulin resistance may be assumed to be a “cause and effect” relationship, since clinical and preclinical studies indicate that weight loss/gain correlates closely with increasing/decreasing insulin sensitivity.29 The major influence of fat distribution, especially the negative influence of intra-abdominal or visceral fat depot, is now largely established.30 Hyperglycemia has been claimed to be a prerequi site for lipotoxicity to occur, and therefore the term glucolipotoxicity, rather than lipotoxicity, is more appropriate to describe the deleterious effects of lipids on β-cell function.31 The role of obesity in the pathophysiology of type 2 diabetes and insulin resistance has been attested to in several studies.32,33 Although the cause of inflammation in obesity has not yet been elucidated fully, various metabolic and immune pathways are claimed to be closely involved. The role of immune cells in promoting inflammation in obesity has been confirmed in humans.34 Obesity initially develops pro-inflammation starting from metabolic cells (adipocyte, hepatocyte, or myocyte) and eventually recruits immune cells with the release of inflammatory cytokines such as TNF-α, interleukin (IL)-6, and adiponectin. Secretion of leptin, TNF-α, resistin, adiponectin, inducible nitric oxide synthase (iNOS) and an elevated plasma NEFA level gradually leads to obesity-induced inflammation that may interfere with glucose metabolism and insulin sensitivity and produce type 2 diabetes.35 The enzyme iNOS is a key inflammatory mediator in obesity and causes insulin resistance in the skeletal muscles. It inhibits secretion of adiponectin from adipocytes and impairs insulin secretion in the liver. Elevated iNOS in blood vessels causes vascular dysfunction in obesity.36 Macrophage accumulation in adipose tissues in obese patients shares the expression of multiple genes causing adipose tissue inflammation in obesity.37 Similarly, some genetic modifications such as the glucokinase gene, insulin receptor substrate-I (IRS-I), mitochondrial genes, and so on alter insulin secretion or function, leading to type 2 diabetes.
Figure 1.
Combined impact of genetic makeup, environmental and social factors in the process of development of type 2 diabetes associated with obesity through impaired insulin secretion and insulin action, explaining the progression from insulin resistance to an impaired glucose tolerance test (IGT) and type 2 diabetes.
Obesity to Insulin Resistance in Type 2 Diabetes
Here we have projected three important pathways that are directly associated with the development of insulin resistance.
mTOR pathway promotes insulin resistance and type 2 diabetes by nutrient sensing
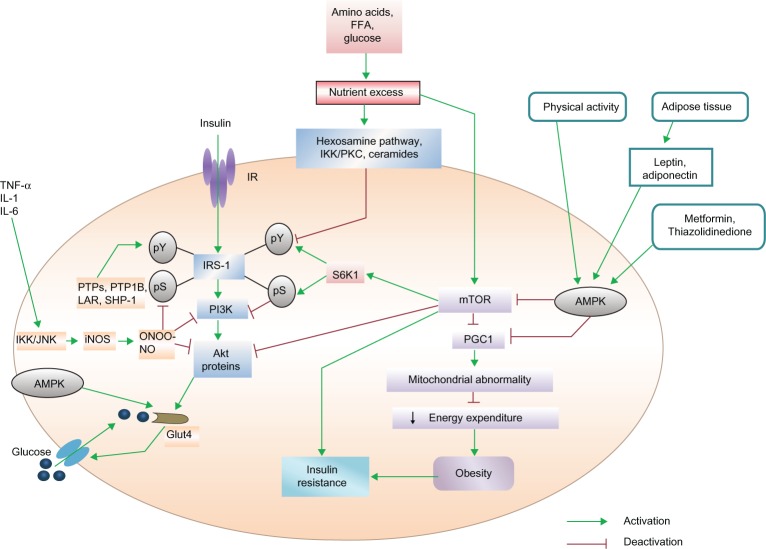
Excess nutrients have been reported to promote insulin resistance by activating the protein kinase mammalian target of the rapamycin (mTOR) pathway.38 mTOR causes serine phosphorylation (pS) of IRS-I, by activating S6 Kinase1 (S6K1) and uncoupling IRS-I from the activation of phosphatidylinositol 3-kinase (PI3K) and Akt proteins, the target proteins of the metabolic pathway of insulin39 (Fig. 2). This type of feedback has been found in myocytes, adipocytes and hepatocytes,34 indicating that in the regulation of glucose homeostasis, the mTOR pathway plays a very important role. Over-expression of mTOR and its effector S6K1 in skeletal muscle, liver and adipose tissue has been reported in genetic and dietary animal models.40 Serine 1101 in the IRS-I protein has been suggested to be a molecular target of S6K1 in the liver of obese animals during the administration of amino acids by infusion in skeletal muscles.4 Adipocytes or macrophages in adipose tissue or skeletal muscle release various types of proinflammatory cytokines (TNF-α, IL-1 and IL-6). These mediators activate c-jun N-terminal kinase (JNK) and inhibitor kappa B kinase (IKK). IKK and JNK inhibit pS of IRS-I, a key element of the insulin-signaling cascade, through the transcriptional activation of inflammatory genes such as iNOS and the promotion of insulin resistance. iNOS activation leads to high levels of nitric oxide (NO) production, which in turn produces the free radical peroxynitrite (ONOO-). This free radical causes s-nitrosylation or nitration of IRS-I, PI3K and/or Akt, to provide inhibitory action on them. Activation of PI3K and Akt is indispensable for glucose transporter 4 (GLUT4) translocation to the cell surface. As stated earlier, when IRS-I is phosphorylated at the serine residue, due to uncoupling of IRS-I, the activation of PI3K and Akt proteins do not occur. Instead, this inhibits the membrane translocation of GLUT4 protein to transport glucose from outside of the cell to the inside. On the other hand, dephosphorylation of tyrosine residue deactivates the IRS-I protein. Excess nutrients cause insulin resistance by activating the mTOR /S6K1 pathway, which in turn inhibits proliferator–activated receptor–gamma coactivator-1 (PGC1) expression. Inhibition of PGC1 gene expression reduces mitochondrial energy expenditure, leading to obesity. On the other hand, excess nutrients reduce IRS tyrosine phosphorylation (pY) via hexosamine, the IKK/protein kinase C (PKC) pathway and ceramides signaling. Activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK) by physical exercise, release of leptin, adinopectin from adipose tissue or pharmacological means (thiazolidinedione and metformin) improve insulin action through the inhibition of iNOS as well as mTOR/S6K1 signaling. Simultaneously, AMPK can also increase glucose transport by triggering GLUT4 translocation. The protein tyrosine phosphatases (PTPs), protein tyrosine phosphatase 1 B (PTP1B), leukocyte antigen-related (LAR) and Src homology 2 (SH2) domain-containing protein-tyrosine-phosphatase-1 (SHP-1) promote and develop insulin resistance by dephosphorylation of tyrosine residues within the insulin receptor. Thus, activation of the mTOR pathway promotes insulin resistance, whereas inhibitory action on these pathways can improve insulin resistance.
Figure 2.
mTOR/S6K1, AMPK and SHP-1 pathways in the development of insulin resistance.
AMPK pathway in insulin resistance
AMPK is a member of a metabolite-sensing protein kinase family, which monitors the energy level of different cells.42 When energy stored in the cells is decreased by AMPK, the functioning of adenosine triphosphate (ATP)-consuming pathway is stopped and that of alternative pathways for ATP regeneration is started.43 By activating AMPK, peroxisome proliferator-activated receptor gamma (PPARγ) agonists inhibit iNOS induction in macrophages, myocytes and adipocytes.44 AMPK switches on the metabolic pathways while turning off inflammation in insulin target tissues and macrophages. The antidiabetic drug thiazolidinedione activates AMPK, which inhibits PPARγ, leading to reduction of PGC1 expression. AMPK is also activated by muscle contraction, exercise and the release of leptin and adinopectin from adipocytes. On the other hand, insulin sensitivity is improved by AMPK because it inhibits the activation of the mTOR/S6K1 pathway (Fig. 2). It is also reported that AMPK is activated by the antidiabetic drug metformin by the inhibition of mTOR/S6K1 in different types of cells.45 AMPK may be a vital therapeutic target as its activation can reduce both inflammatory processes and nutrient-sensing signals, which may play important role against insulin resistance and for type 2 diabetes.
SHP-1 pathway
It has also been reported that the PTPs, PTP1B and LAR are negative regulators of the insulin receptor kinase in liver and peripheral insulin target tissues. In the signaling pathway, pY is a key to insulin signal transduction. PTPs are prominent candidates to negatively regulate insulin action.46 The protein tyrosine phosphatase SHP-1 is an inhibitor of signal transduction in the insulin-signaling pathway in the liver and skeletal muscles.47 Animal models with a deficient SHP-1 protein are glucose tolerant and insulin sensitive for glucose metabolism and these animals show increased insulin-signaling through IRS/PI3K/Akt pathway in the liver and in muscle tissues.48 Therefore, SHP-1 plays a predominant role in regulation of insulin resistance and type 2 diabetes.
Pathway by which free fatty acids promote insulin resistance
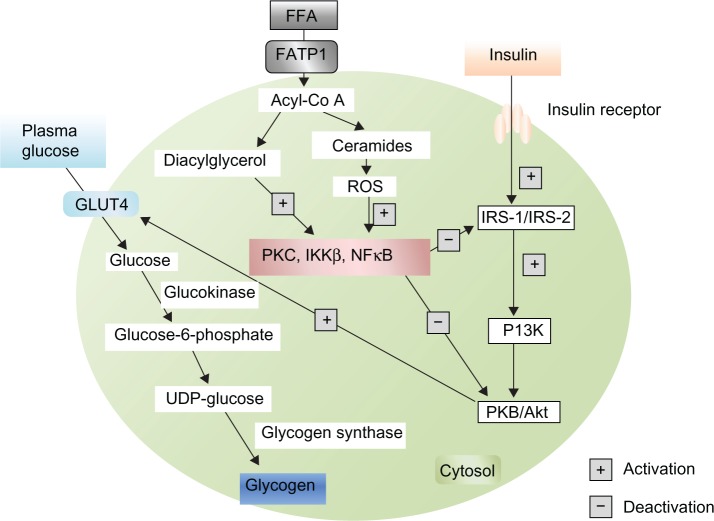
Primarily insulin binds to its receptor, which promotes phosphorylation of IRS, then activates PI3K, which in turn activates protein kinase B (PKB)/Akt, and enhances GLUT4 translocation to the cell surface. FFA metabolites [ceramides, acyl-CoA, diacylglycerol (DAG)] activate serine/threonine kinases [PKC, nuclear factor kB (NFkB), inhibitory kB kinase β (IKKβ)], which inhibit IRS and PKB/Akt, thereby inhibiting insulin signaling and preventing GLUT4 translocation. Elevated levels of FFA thereby inhibit glucose utilization by inhibiting the insulin-signaling pathway and reducing insulin-stimulated muscle glycogen synthesis that also occurs in type 2 diabetes mellitus.
Normally, plasma glucose enters the cell by GLUT4. Then glucose is converted to glucose-6-phosphate in presence of Glucokinase. Glucose-6-phosphate is converted to uridine diphosphate (UDP)-glucose and ultimately glycogen in the presence of glycogen synthase, to remain stored as glycogen. Excess FFA in plasma inhibits the IRS signaling pathway and leads to reduced GLUT4 translocation and reduced glucose uptake. As a result, the plasma glucose level is elevated and type 2 diabetes develops. Increased deposition of intramyocellular lipid metabolites (eg, fatty acyl-CoAs, DAG) produce increased cytoplasmic levels of acyl-CoA. These molecules stimulate many serine/threonine (Ser/Thr) kinases,22 such as PKC, IKK and cytokines, such as NFkB, TNF-α and IL-6. Proteins of the insulin-signaling pathway are phosphorylated by Ser/Thr kinases. IRS-I is a substrate for PKCs such as protein kinase C μ (PKCμ) or protein kinase C α (PKCα). When IRS-I is phosphorylated on Ser-307, it leads to a decreased IRS-I-associated PI3K activity. As a result, IRS-I inhibition decreases GLUT4 activity and glucose uptake (Fig. 3). Glycogen synthase kinase and PKB/Akt are two important proteins of the insulin-signaling pathway that are inactivated through PKC-dependent mechanisms.49 In mice, inactivation of protein kinase C ζ (PKCζ) was shown to prevent insulin resistance induced by a high-fat diet.50 As discussed above, IKK and NFkB are two other main Ser/Thr kinases activated by lipid metabolites and inflammatory molecules.51 Many studies reported a strong association between oxidative stress and insulin resistance.52 It is also known that the accumulation of intramyocellular fatty acid metabolites produces free radical reactive oxygen species (ROS).53 Recent evidence indicates that insulin resistance also occurs in pancreatic β-cells, leading to type 2 diabetes. According to the ‘lipotoxicity’ hypothesis, chronically elevated FFA may directly damage the cells of the pancreas by increase NO production. Evidence from transgenic mice expressing human islet amyloid polypeptide (IAPP) indicates that increased dietary fat consumption has a direct link with the pancreatic β-cell dysfunction by inducing islet amyloid deposition which promotes insulin resistance and ultimately type 2 diabetes is developed.54,55 High plasma FFA and increased intracellular lipid concentration inhibit insulin signaling in muscle. An increased level of FFA has a toxic effect on pancreatic β-cells and develops insulin resistance, by which a secondary pathogenetic mechanism of type 2 diabetes can be elucidated.
Figure 3.
Inhibitory pathway of GLUT4 translocation by free fatty acids.
Abbreviations: FATP1, fatty acid-transport protein 1; ROS, reactive oxygen species.
Differentiation between Insulin Resistance and Type 2 Diabetes
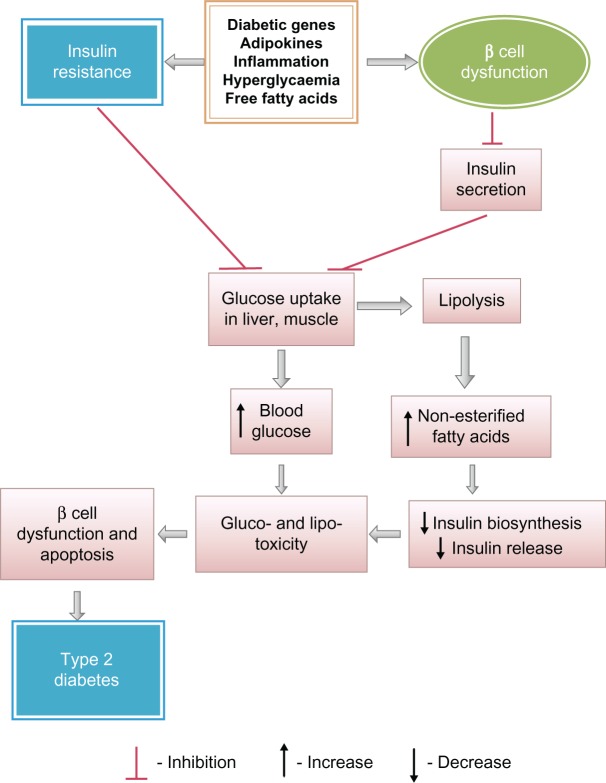
An association between insulin resistance and type 2 diabetes has already been established.56 Insulin resistance is not only one of the most powerful predictors of the future development of type 2 diabetes, but is also a target of therapeutic approaches once hyperglycemia is developed.57 Insulin primarily increases the cellular uptake of glucose. During the development of insulin resistance, hepatocytes, adipocytes and myocytes do not respond well to insulin. The β-cells of pancreatic islets increase insulin release sufficiently to overcome the reduced efficiency of insulin action to maintain normal glucose tolerance. Thus, people with insulin resistance also have hyperinsulinemia.58 When cells are unable to take up glucose well, glucose accumulates in the blood. Insulin resistance thereby causes elevated fasting blood glucose as an indication of type 2 diabetes. Insulin resistance increases the risk of developing type 2 diabetes. Type 2 diabetes mellitus (NIDDM) is a complex metabolic disorder that is characterized by high blood glucose in the context of insulin resistance as well as relative insulin deficiency. Fluctuations in insulin sensitivity occur throughout the normal life cycle including puberty, pregnancy and aging.59–61 Prediabetes usually occurs in people who already have insulin resistance. Normal β-cells produce an adaptive response to insulin resistance, which results in increasing numbers of β-cells in the islets and enhancement of their function to release more insulin, and normal glucose level is maintained. Although insulin resistance alone does not cause type 2 diabetes, it often sets a stage for the disease by placing high demand on the insulin-producing β-cells, which results in a gradual loss of functions of β-cells and the development of type 2 diabetes. Both obesity and type 2 diabetes are associated with insulin resistance. Some obese and insulin-resistant persons do not develop hyperglycaemia,62 whereas in similar persons with type 2 diabetes, β-cells are unable to release insulin more in response to decreased insulin sensitivity of the cells.63–65 Eventually, β-cell dysfunction occurs, resulting in an impaired glucose tolerance and an enhancement of fasting blood glucose, eventually leading to type 2 diabetes.66 NEFA are potential molecules to link insulin resistance and β-cell dysfunction and apoptosis (Fig. 4) in individuals with type 2 diabetes.67 In order to provide energy to cells during insulin resistance, lipolysis occurs, which provides a high plasma level of NEFA. A high plasma level of NEFA causes lipotoxicity and progressive loss of β-cell function. NEFA also decrease normal insulin release from β-cell with a marked impairment in glucose-stimulated insulin secretion in them and reducing insulin biosynthesis. A progressive loss of β-cell function causes impaired glucose tolerance in normal cells, eventually leading to insulin resistance in those cells and then to type 2 diabetes.69 Thus, development of insulin resistance may be a prediabetic condition playing a pivotal role for the process of development of type 2 diabetes.
Figure 4.
Predominant biochemical alterations for progression of type 2 diabetes from insulin resistance and due to beta cells dysfunction.
Conclusion
An association among elevated FFA, obesity, peripheral insulin resistance and development of type 2 diabetes has been elucidated. In obese individuals, plasma FFA not only produces insulin resistance in skeletal muscles, but also may have additional actions in the liver and the pancreas, which lead to the development of type 2 diabetes. Obesity results in a pro-inflammatory state starting from the metabolic cells and also recruiting immune cells with the release of inflammatory cytokines (TNF-α, IL-6, adiponectin, etc). These inflammatory mediators cause obesity-induced inflammation, the chronic form of which generally leads to complications such as insulin resistance and diabetes mellitus. In type 2 diabetes, excess synthesis of lipid droplets occurs and a formation of lipid peroxides becomes hazardous to health, since it may aggravate the prevalence of a diseased condition or generate new diseases (cardiovascular diseases, cancer, etc) in the body. Thus, prevention of lipid peroxidation is a weapon to slow down the process of development of diabetic complications and other diseases. Ectopic lipids (DAG and possibly ceramides) may be the root cause of liver and muscle insulin resistance and new therapies aimed at decreasing the lipid contents in these organs may represent efficacious therapeutic targets for the treatment of insulin resistance and its associated comorbidities. At a societal level, concerted efforts to restore balance in our diets and behaviors (sedentary life-style, smoking, drinking alcohol, psychological stress, etc) can prevent obesity and ectopic lipid deposition. The involvement of lipid in the development of diabetic complications can no longer be deniable. In the end, lifestyle modifications such as physical activity, diets, change of sedentary to active life format may not be enough to control diabetes, the metabolic disease, in obese patients. Instead, more research is required to find the molecular targets to achieve proper therapeutic strategies to combat type 2 diabetes, the notoriously slow killer.
Footnotes
Author Contributions
Contributed to the writing of the manuscript: BM, CMH, LM, PP, MKG. Agree with manuscript results and conclusions: BM, CMH, LM, PP, MKG. Jointly developed the structure and arguments for the paper: BM, CMH, LM, PP, MKG. Made critical revisions and approved final version: BM. All authors reviewed and approved of the final manuscript.
Funding
We are indebted to Department of Science and Technology (Govt. of India) grant no. DST/inspire/fellowship/2010(87) and Indian Council of Medical Research, Grant no. 58/7/2009-BMS, for this work.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
References
- 1.Mukherjee B, Mukherjee JR, Chatterjee M. Lipid peroxidation, glutathione levels and changes in glutathione-related enzyme activities in streptozotocin-induced diabetic rats. Immunology and Cell Biology. 1994;72:109–14. doi: 10.1038/icb.1994.17. [DOI] [PubMed] [Google Scholar]
- 2.Kumawat M, Singh I, Singh N, Singh V, Kharb S. Lipid peroxidation and lipid profile in type II diabetes mellitus. Webmed Central Biochemistry. 2012;3(3):WMC003147. [Google Scholar]
- 3.Kangralkar VA, Patil SD, Bandivadekar MR. Oxidative stress and diabetes: a review. International Journal of Pharmaceutical Applications. 2010;1(1):38–45. [Google Scholar]
- 4.Barathmanikanth S, Kalishwaralal K, Sriram M, et al. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. Journal of Nanobiotechnology. 2010;8:16. doi: 10.1186/1477-3155-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayaraghavan K. Treatment of dyslipidemia in patients with type 2 diabetes. Lipids in Health and Disease. 2010;9:144–56. doi: 10.1186/1476-511X-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotto AM., Jr Jeremiah Metzger Lecture: Cholesterol, inflammation and atherosclerotic cardiovascular disease: is it all LDL? Transactions of the American Clinical and Climatological Association. 2011;122:256–87. [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer EJ, Gleason JA, Dansinger ML. Dietary fructose and glucose differentially affect lipid and glucose homeostasis. Journal of Nutrition. 2009;139(6):1257S–62S. doi: 10.3945/jn.108.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2011;123:2292–333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. American Journal of Physiology—Endocrinology and Metabolism. 2009;297:E271–88. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 10.Targher G, Bellis A, Fornengo P, et al. Prevention and treatment of nonalcoholic fatty liver disease. Digestive and Liver Disease. 2010;42(5):331–40. doi: 10.1016/j.dld.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Tacer KF, Rozman D. Nonalcoholic fatty liver disease: Focus on lipoprotein and lipid deregulation. Journal of Lipids. 2011;2011:783976. doi: 10.1155/2011/783976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. The Journal of Clinical Endocrinology and Metabolism. 2008;93(11):S57–63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 2009;17:1872–7. doi: 10.1038/oby.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosoyamada K, Uto H, Imamura Y, et al. Fatty liver in men is associated with high serum levels of small, dense low-density lipoprotein cholesterol. Diabetology and Metabolic Syndrome. 2012;4:34. doi: 10.1186/1758-5996-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proceedings of the National Academy of Sciences (USA) 2009;106(36):15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–89. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsuyoshi H, Yasui K, Harano Y, et al. Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatology Research. 2009;39(4):366–73. doi: 10.1111/j.1872-034X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 18.Schreuder TC, Verwer BJ, van Nieuwkerk CM, Mulder CJ. Nonalcoholic fatty liver disease: An overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. 2008;14(16):2474–86. doi: 10.3748/wjg.14.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghassab RK, Gohari LH, Firoozray M, Yegane MN. Determination of low density lipoprotein particle size by polyacrylamide gradient gel electrophoresis in patients with coronary artery stenosis. Lab Medicine. 2010;4(3):164–6. [Google Scholar]
- 20.Fujita K, Nozaki Y, Wada K, et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50(3):772–80. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 21.Koletzko B, Lien E, Agostoni C, et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. Journal of Perinatal Medicine. 2008;36(1):5–14. doi: 10.1515/JPM.2008.001. [DOI] [PubMed] [Google Scholar]
- 22.Corpeleijn E, Saris WH, Blaak EE. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obesity Reviews. 2009;10:178–93. doi: 10.1111/j.1467-789X.2008.00544.x. [DOI] [PubMed] [Google Scholar]
- 23.Gabrielsen JS, Gao Y, Simcox JA, et al. Adipocyte iron regulates adiponectin and insulin sensitivity. The Journal of Clinical Investigation. 2012;122(10):3529–40. doi: 10.1172/JCI44421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60(10):2441–9. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Layden BT, Durai V, Lowe WL., Jr G-Protein-coupled receptors, pancreatic islets, and diabetes. Nature Education. 2010;3(9):13. [Google Scholar]
- 26.Imbeault P, Haman F, Blais JM, et al. Obesity and type 2 diabetes prevalence in adults from two remote first nations communities in northwestern Ontario, Canada. Journal of Obesity. 2011;2011:267509–14. doi: 10.1155/2011/267509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant RW, Moore AF, Florez JC. Genetic architecture of type 2 diabetes: recent progress and clinical implications. Diabetes Care. 2009;32(6):1107–14. doi: 10.2337/dc08-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez de Morentin PB, Varela L, Fernø J, Nogueiras R, Diéguez C, López M. Hypothalamic lipotoxicity and the metabolic syndrome. Biochimica et Biophysica Acta. 2010;1801(3):350–61. doi: 10.1016/j.bbalip.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocrine-Related Cancer. 2009;16:1103–23. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 30.Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13(1):9–19. [PMC free article] [PubMed] [Google Scholar]
- 31.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocrine Reviews. 2008;29(3):351–66. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Reviews In Endocrine and Metabolic Disorders. 2011;12(3):163–72. doi: 10.1007/s11154-011-9168-2. [DOI] [PubMed] [Google Scholar]
- 33.Esteve E, Ricart W, Real JMF. Adipocytokines and insulin resistance: The possible role of lipocalin-2, retinol binding protein-4, and adiponectin. Diabetes Care. 2009;32(Suppl 2):S362–7. doi: 10.2337/dc09-S340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emanuela F, Grazia M, Marco DR, Paola LM, Giorgio F, Marco B. Inflammation as a link between obesity and metabolic syndrome. Journal of Nutrition and Metabolism. 2012;2012:476380. doi: 10.1155/2012/476380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Molecular Medicine. 2008;14:11–12. 741–51. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinozaki S, Choi CS, Shimizu N, et al. Liver-specific inducible nitric-oxide synthase expression is sufficient to cause hepatic insulin resistance and mild hyperglycemia in mice. Journal of Biological Chemistry. 2011;286(40):34959–75. doi: 10.1074/jbc.M110.187666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. The Journal of the Federation of American Societies for Experimental Biology (FASEB) 2011;25(7):2399–407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weickert MO. Nutritional modulation of insulin resistance. Scientifica. 2012;2012:1–15. doi: 10.6064/2012/424780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. American Journal of Physiology—Endocrinology and Metabolism. 2009;296(4):E581–91. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 40.Melnik BC, John SM, Carrera-Bastos P, Cordain L. The impact of cow’s milk-mediated mTORC1-signaling in the initiation and progression of prostate cancer. Nutrition and Metabolism. 2012;9:74. doi: 10.1186/1743-7075-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tremblay F, Brûlé S, Hee Um S, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(35):14056–61. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. The American Journal of Clinical Nutrition. 2011;93(4):891S–6S. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- 43.Lefort N, St. Amand E, Morasse S, Côte′ CH, Marette A. The alpha-subunit of AMPK is essential for submaximal contraction mediated glucose transport in skeletal muscle in vitro. American Journal of Physiology—Endocrinology and Metabolism. 2008;295(6):E1447–54. doi: 10.1152/ajpendo.90362.2008. [DOI] [PubMed] [Google Scholar]
- 44.Dallaire P, Bellmann K, Laplante M, et al. Obese mice lacking inducible nitric oxide synthase are sensitized to the metabolic actions of peroxisome proliferator–activated receptor-γ agonism. Diabetes. 2008;57(8):1999–2011. doi: 10.2337/db08-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song CW, Lee H, Dings RP, et al. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Scientific Reports. 2012;2:362–71. doi: 10.1038/srep00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajala RVS, Wiskur B, Tanito M, Callegan M, Rajala A. Diabetes reduces autophosphorylation of retinal insulin receptor and increases protein-tyrosine phosphatase-1B activity. Investigative Ophthalmology and Visual Science. 2009;50(3):1033–40. doi: 10.1167/iovs.08-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubois MJ, Bergeron S, Kim HJ, et al. The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nature Medicine. 2006;12(5):549–56. doi: 10.1038/nm1397. [DOI] [PubMed] [Google Scholar]
- 48.Princen F, Bard E, Sheikh F, et al. Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death. Molecular and Cellular Biology. 2009;29(2):378–88. doi: 10.1128/MCB.01661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luna-Ulloa LB, Hernandez-Maqueda JG, Santoyo-Ramos P, Castaneda-Patlan MC, Robles-Flores M. Protein kinase C ζ is a positive modulator of canonical Wnt signaling pathway in tumoral colon cell lines. Carcinogenesis. 2011;32(11):1615–24. doi: 10.1093/carcin/bgr190. [DOI] [PubMed] [Google Scholar]
- 50.Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148(5):852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Austin RL, Rune A, Bouzakri K, Zierath JR, Krook A. siRNA-mediated reduction of inhibitor of nuclear factor-kappaB kinase prevents tumor necrosis factor-alpha–induced insulin resistance in human skeletal muscle. Diabetes. 2008;57(8):2066–73. doi: 10.2337/db07-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alsultan AI, Seif MA, Amin TT, Naboli M, Alsuliman AM. Relationship between oxidative stress, ferritin and insulin resistance in sickle cell disease. European Review for Medical and Pharmacological Sciences. 2010;14(6):527–38. [PubMed] [Google Scholar]
- 53.Martins AR, Nachbar RT, Gorjao R, et al. Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: importance of the mitochondrial function. Lipids in Health and Disease. 2012;11:30. doi: 10.1186/1476-511X-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic β cells. Trends in Endocrinology and Metabolism. 2012;23(9):477–87. doi: 10.1016/j.tem.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. European Journal of Clinical Investigation. 2002;32(3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 56.Morino K, Petersen KF, Sono S, et al. Regulation of mitochondrial biogenesis by lipoprotein lipase in muscle of insulin-resistant offspring of parents with type 2 diabetes. Diabetes. 2012;61(4):877–87. doi: 10.2337/db11-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor R. Insulin resistance and type 2 diabetes. Diabetes. 2012;61:778–9. doi: 10.2337/db12-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and Hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Suppl 2):S262–8. doi: 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- 59.Moran A, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–44. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 60.Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B- cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. American Journal of Obstetrics and Gynecology. 1990;162:1008–14. doi: 10.1016/0002-9378(90)91306-w. [DOI] [PubMed] [Google Scholar]
- 61.DeFronzo RA. Glucose intolerance of aging. Evidence for tissue insensitivity to insulin. Diabetes. 1979;28:1095–101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- 62.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: An overview. Indian Journal of Medical Research. 2007;125:451–72. [PubMed] [Google Scholar]
- 63.Perley M, Kipnis DM. Plasma insulin responses to glucose and tolbutamide of normal weight and obese diabetic and nondiabetic subjects. Diabetes. 1966;15:867–74. doi: 10.2337/diab.15.12.867. [DOI] [PubMed] [Google Scholar]
- 64.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and B-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–72. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 65.Kahn SE. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. Journal of Clinical Endocrinology and Metabolism. 2001;86:4047–58. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- 66.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(14):840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 67.Cnop M. Fatty acids and glucolipotoxicity in the pathogenesis of Type 2 diabetes. Biochemical Society Transactions. 2008;36(Pt 3):348–52. doi: 10.1042/BST0360348. [DOI] [PubMed] [Google Scholar]
- 68.DeFronzo RA. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–95. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Journal of Clinical Investigation. 1999;104:787–94. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sako Y, Grill VE. A 48-hour lipid infusion in the rat time-dependently inhibits glucose induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology. 1990;127:1580–9. doi: 10.1210/endo-127-4-1580. [DOI] [PubMed] [Google Scholar]
- 71.Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. Journal of Clinical Investigation. 1994;93:870–6. doi: 10.1172/JCI117042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. American Journal of Physiology. 1999;276:E1055–66. doi: 10.1152/ajpendo.1999.276.6.E1055. [DOI] [PubMed] [Google Scholar]