Abstract
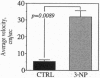
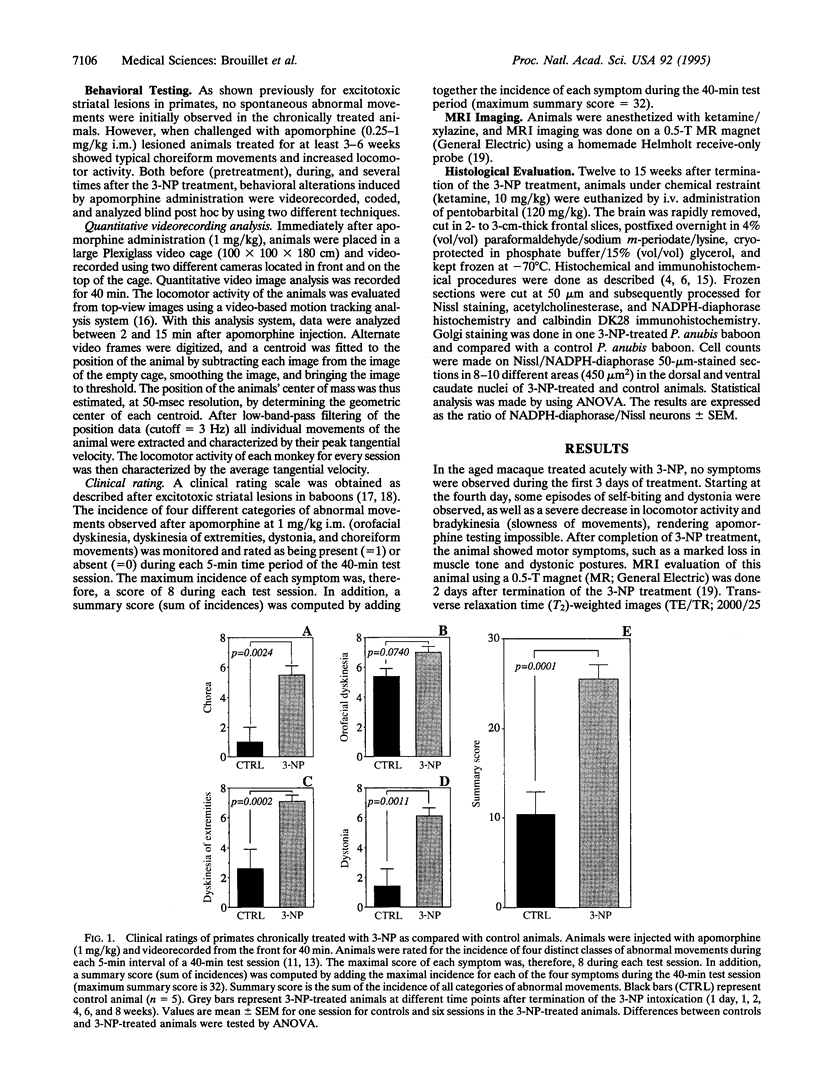
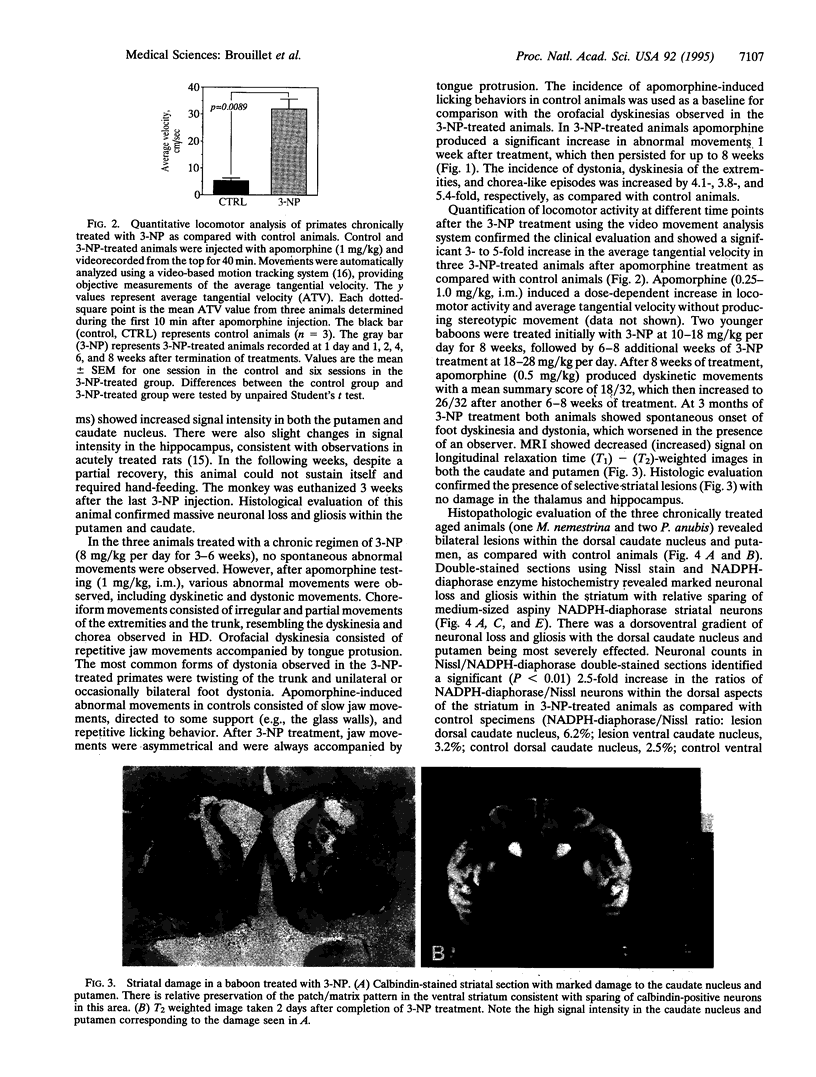
Although the gene defect responsible for Huntington disease (HD) has recently been identified, the pathogenesis of the disease remains obscure. One potential mechanism is that the gene defect may lead to an impairment of energy metabolism followed by slow excitotoxic neuronal injury. In the present study we examined whether chronic administration of 3-nitropropionic acid (3-NP), an irreversible inhibitor of succinate dehydrogenase, can replicate the neuropathologic and clinical features of HD in nonhuman primates. After 3-6 weeks of 3-NP administration, apomorphine treatment induced a significant increase in motor activity as compared with saline-treated controls. Animals showed both choreiform movements, as well as foot and limb dystonia, which are characteristic of HD. More prolonged 3-NP treatment in two additional primates resulted in spontaneous dystonia and dyskinesia accompanied by lesions in the caudate and putamen seen by magnetic resonance imaging. Histologic evaluation showed that there was a depletion of calbindin neurons, astrogliosis, sparing of NADPH-diaphorase neurons, and growth-related proliferative changes in dendrites of spiny neurons similar to changes in HD. The striosomal organization of the striatum and the nucleus accumbens were spared. These findings show that chronic administration of 3-NP to nonhuman primates can replicate many of the characteristic motor and histologic features of HD, further strengthening the possibility that a subtle impairment of energy metabolism may play a role in its pathogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin R. L., Greenamyre J. T. Alternative excitotoxic hypotheses. Neurology. 1992 Apr;42(4):733–738. doi: 10.1212/wnl.42.4.733. [DOI] [PubMed] [Google Scholar]
- Albin R. L., Young A. B., Penney J. B., Handelin B., Balfour R., Anderson K. D., Markel D. S., Tourtellotte W. W., Reiner A. Abnormalities of striatal projection neurons and N-methyl-D-aspartate receptors in presymptomatic Huntington's disease. N Engl J Med. 1990 May 3;322(18):1293–1298. doi: 10.1056/NEJM199005033221807. [DOI] [PubMed] [Google Scholar]
- Alston T. A., Mela L., Bright H. J. 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3767–3771. doi: 10.1073/pnas.74.9.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal M. F. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol. 1992 Feb;31(2):119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Ferrante R. J., Swartz K. J., Kowall N. W. Chronic quinolinic acid lesions in rats closely resemble Huntington's disease. J Neurosci. 1991 Jun;11(6):1649–1659. doi: 10.1523/JNEUROSCI.11-06-01649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal M. F., Kowall N. W., Ellison D. W., Mazurek M. F., Swartz K. J., Martin J. B. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986 May 8;321(6066):168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- Brouillet E. P., Shinobu L., McGarvey U., Hochberg F., Beal M. F. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol. 1993 Mar;120(1):89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Brouillet E., Jenkins B. G., Hyman B. T., Ferrante R. J., Kowall N. W., Srivastava R., Roy D. S., Rosen B. R., Beal M. F. Age-dependent vulnerability of the striatum to the mitochondrial toxin 3-nitropropionic acid. J Neurochem. 1993 Jan;60(1):356–359. doi: 10.1111/j.1471-4159.1993.tb05859.x. [DOI] [PubMed] [Google Scholar]
- Ferrante R. J., Beal M. F., Kowall N. W., Richardson E. P., Jr, Martin J. B. Sparing of acetylcholinesterase-containing striatal neurons in Huntington's disease. Brain Res. 1987 May 12;411(1):162–166. doi: 10.1016/0006-8993(87)90694-9. [DOI] [PubMed] [Google Scholar]
- Ferrante R. J., Kowall N. W., Beal M. F., Martin J. B., Bird E. D., Richardson E. P., Jr Morphologic and histochemical characteristics of a spared subset of striatal neurons in Huntington's disease. J Neuropathol Exp Neurol. 1987 Jan;46(1):12–27. doi: 10.1097/00005072-198701000-00002. [DOI] [PubMed] [Google Scholar]
- Ferrante R. J., Kowall N. W., Richardson E. P., Jr Proliferative and degenerative changes in striatal spiny neurons in Huntington's disease: a combined study using the section-Golgi method and calbindin D28k immunocytochemistry. J Neurosci. 1991 Dec;11(12):3877–3887. doi: 10.1523/JNEUROSCI.11-12-03877.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould D. H., Gustine D. L. Basal ganglia degeneration, myelin alterations, and enzyme inhibition induced in mice by the plant toxin 3-nitropropanoic acid. Neuropathol Appl Neurobiol. 1982 Sep-Oct;8(5):377–393. doi: 10.1111/j.1365-2990.1982.tb00306.x. [DOI] [PubMed] [Google Scholar]
- Hamilton B. F., Gould D. H. Nature and distribution of brain lesions in rats intoxicated with 3-nitropropionic acid: a type of hypoxic (energy deficient) brain damage. Acta Neuropathol. 1987;72(3):286–297. doi: 10.1007/BF00691103. [DOI] [PubMed] [Google Scholar]
- Hantraye P., Leroy-Willig A., Denys A., Riche D., Isacson O., Maziere M., Syrota A. Magnetic resonance imaging to monitor pathology of caudate-putamen after excitotoxin-induced neuronal loss in the nonhuman primate brain. Exp Neurol. 1992 Oct;118(1):18–23. doi: 10.1016/0014-4886(92)90018-l. [DOI] [PubMed] [Google Scholar]
- Hantraye P., Riche D., Maziere M., Isacson O. A primate model of Huntington's disease: behavioral and anatomical studies of unilateral excitotoxic lesions of the caudate-putamen in the baboon. Exp Neurol. 1990 May;108(2):91–104. doi: 10.1016/0014-4886(90)90014-j. [DOI] [PubMed] [Google Scholar]
- Hantraye P., Riche D., Maziere M., Isacson O. Intrastriatal transplantation of cross-species fetal striatal cells reduces abnormal movements in a primate model of Huntington disease. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4187–4191. doi: 10.1073/pnas.89.9.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins B. G., Koroshetz W. J., Beal M. F., Rosen B. R. Evidence for impairment of energy metabolism in vivo in Huntington's disease using localized 1H NMR spectroscopy. Neurology. 1993 Dec;43(12):2689–2695. doi: 10.1212/wnl.43.12.2689. [DOI] [PubMed] [Google Scholar]
- Kanazawa I., Kimura M., Murata M., Tanaka Y., Cho F. Choreic movements in the macaque monkey induced by kainic acid lesions of the striatum combined with L-dopa. Pharmacological, biochemical and physiological studies on neural mechanisms. Brain. 1990 Apr;113(Pt 2):509–535. doi: 10.1093/brain/113.2.509. [DOI] [PubMed] [Google Scholar]
- Ludolph A. C., He F., Spencer P. S., Hammerstad J., Sabri M. 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Can J Neurol Sci. 1991 Nov;18(4):492–498. doi: 10.1017/s0317167100032212. [DOI] [PubMed] [Google Scholar]
- Novelli A., Reilly J. A., Lysko P. G., Henneberry R. C. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988 Jun 7;451(1-2):205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- Ryan A. S., Treuth M. S., Rubin M. A., Miller J. P., Nicklas B. J., Landis D. M., Pratley R. E., Libanati C. R., Gundberg C. M., Hurley B. F. Effects of strength training on bone mineral density: hormonal and bone turnover relationships. J Appl Physiol (1985) 1994 Oct;77(4):1678–1684. doi: 10.1152/jappl.1994.77.4.1678. [DOI] [PubMed] [Google Scholar]
- Vonsattel J. P., Myers R. H., Stevens T. J., Ferrante R. J., Bird E. D., Richardson E. P., Jr Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985 Nov;44(6):559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Weller M., Paul S. M. 3-Nitropropionic acid is an indirect excitotoxin to cultured cerebellar granule neurons. Eur J Pharmacol. 1993 Oct 1;248(3):223–228. doi: 10.1016/0926-6917(93)90048-u. [DOI] [PubMed] [Google Scholar]
- Young A. B., Greenamyre J. T., Hollingsworth Z., Albin R., D'Amato C., Shoulson I., Penney J. B. NMDA receptor losses in putamen from patients with Huntington's disease. Science. 1988 Aug 19;241(4868):981–983. doi: 10.1126/science.2841762. [DOI] [PubMed] [Google Scholar]
- Zeevalk G. D., Nicklas W. J. Chemically induced hypoglycemia and anoxia: relationship to glutamate receptor-mediated toxicity in retina. J Pharmacol Exp Ther. 1990 Jun;253(3):1285–1292. [PubMed] [Google Scholar]
- Zeevalk G. D., Nicklas W. J. Evidence that the loss of the voltage-dependent Mg2+ block at the N-methyl-D-aspartate receptor underlies receptor activation during inhibition of neuronal metabolism. J Neurochem. 1992 Oct;59(4):1211–1220. doi: 10.1111/j.1471-4159.1992.tb08430.x. [DOI] [PubMed] [Google Scholar]
- Zeevalk G. D., Nicklas W. J. Mechanisms underlying initiation of excitotoxicity associated with metabolic inhibition. J Pharmacol Exp Ther. 1991 May;257(2):870–878. [PubMed] [Google Scholar]