Abstract
The isozyme form of eukaryotic initiation factor 4F [eIF-(iso)4F] from wheat germ is composed of a p28 subunit that binds the 7-methylguanine cap of mRNA and a p86 subunit having unknown function. The p86 subunit was found to have limited sequence similarity to a kinesin-like protein encoded by the katA gene of Arabidopsis thaliana. Native wheat germ eIF-(iso)4F and bacterially expressed p86 subunit and p86-p28 complex bound to taxol-stabilized maize microtubules (MTs) in vitro. Binding saturation occurred at 1 mol of p86 per 5-6 mol of polymerized tubulin dimer, demonstrating a substoichiometric interaction of p86 with MTs. No evidence was found for a direct interaction of the p28 subunit with MTs. Unlike kinesin, cosedimentation of eIF-(iso)4F with MTs was neither reduced by MgATP nor enhanced by adenosine 5'-[gamma-imido]triphosphate. Both p86 subunit and p86-p28 complex induced the bundling of MTs in vitro. The p86 subunit was immunolocalized to the cytosol in root maize cells and existed in three forms: fine particles, coarse particles, and linear patches. Many coarse particles and linear patches were colocalized or closely associated with cortical MT bundles in interphase cells. The results indicate that the p86 subunit of eIF-(iso)4F is a MT-associated protein that may simultaneously link the translational machinery to the cytoskeleton and regulate MT disposition in plant cells.
Full text
PDF


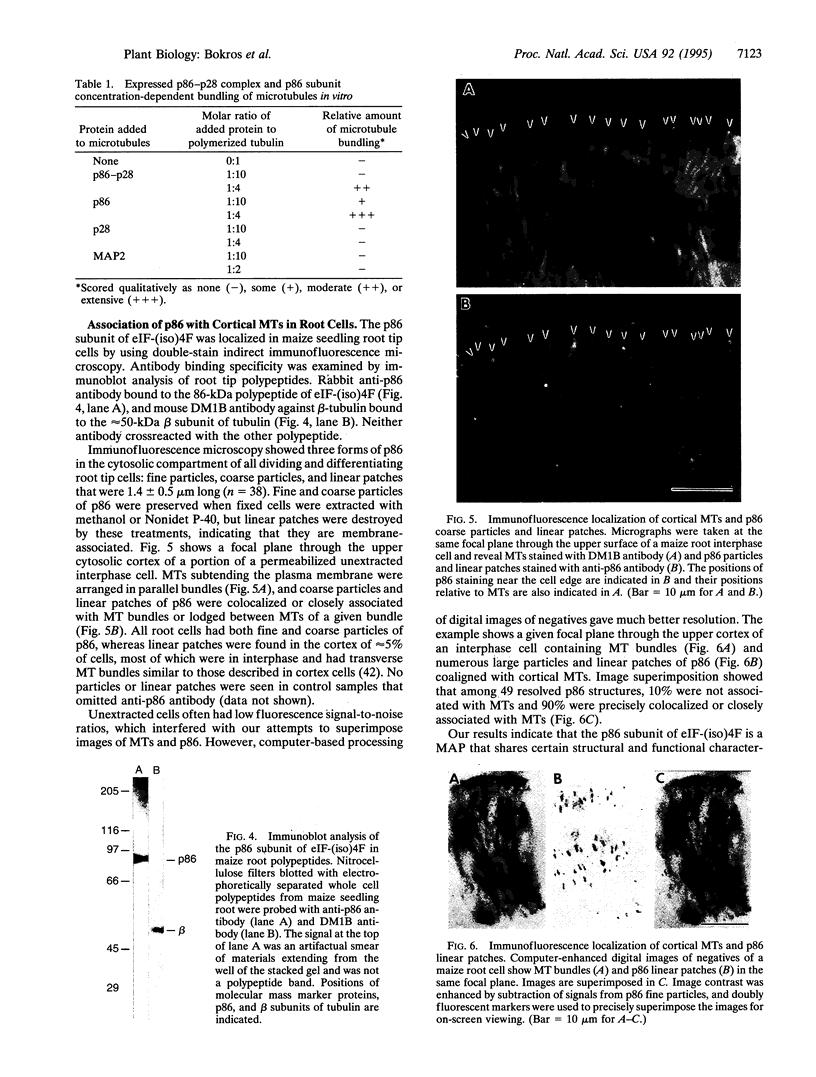

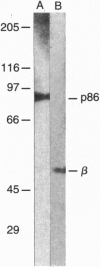
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. L., Metz A. M., Timmer R. T., Rhoads R. E., Browning K. S. Isolation and sequence of the cDNAs encoding the subunits of the isozyme form of wheat protein synthesis initiation factor 4F. J Biol Chem. 1992 Nov 15;267(32):23232–23236. [PubMed] [Google Scholar]
- Bloom G. S., Luca F. C., Vallee R. B. Microtubule-associated protein 1B: identification of a major component of the neuronal cytoskeleton. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5404–5408. doi: 10.1073/pnas.82.16.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blose S. H., Meltzer D. I., Feramisco J. R. 10-nm filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J Cell Biol. 1984 Mar;98(3):847–858. doi: 10.1083/jcb.98.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokros C. L., Hugdahl J. D., Hanesworth V. R., Murthy J. V., Morejohn L. C. Characterization of the reversible taxol-induced polymerization of plant tubulin into microtubules. Biochemistry. 1993 Apr 6;32(13):3437–3447. doi: 10.1021/bi00064a030. [DOI] [PubMed] [Google Scholar]
- Bokros C. L., Hugdahl J. D., Hanesworth V. R., Murthy J. V., Morejohn L. C. Characterization of the reversible taxol-induced polymerization of plant tubulin into microtubules. Biochemistry. 1993 Apr 6;32(13):3437–3447. doi: 10.1021/bi00064a030. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Browning K. S., Humphreys J., Hobbs W., Smith G. B., Ravel J. M. Determination of the amounts of the protein synthesis initiation and elongation factors in wheat germ. J Biol Chem. 1990 Oct 15;265(29):17967–17973. [PubMed] [Google Scholar]
- Browning K. S., Webster C., Roberts J. K., Ravel J. M. Identification of an isozyme form of protein synthesis initiation factor 4F in plants. J Biol Chem. 1992 May 15;267(14):10096–10100. [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Fulton A. B., Wan K. M. Many cytoskeletal proteins associate with the hela cytoskeleton during translation in vitro. Cell. 1983 Feb;32(2):619–625. doi: 10.1016/0092-8674(83)90481-6. [DOI] [PubMed] [Google Scholar]
- Goodson H. V., Kang S. J., Endow S. A. Molecular phylogeny of the kinesin family of microtubule motor proteins. J Cell Sci. 1994 Jul;107(Pt 7):1875–1884. doi: 10.1242/jcs.107.7.1875. [DOI] [PubMed] [Google Scholar]
- Hamill D., Davis J., Drawbridge J., Suprenant K. A. Polyribosome targeting to microtubules: enrichment of specific mRNAs in a reconstituted microtubule preparation from sea urchin embryos. J Cell Biol. 1994 Nov;127(4):973–984. doi: 10.1083/jcb.127.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. Translational initiation factor and ribosome association with the cytoskeletal framework fraction from HeLa cells. Cell. 1984 May;37(1):85–93. doi: 10.1016/0092-8674(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Hugdahl J. D., Bokros C. L., Hanesworth V. R., Aalund G. R., Morejohn L. C. Unique functional characteristics of the polymerization and MAP binding regulatory domains of plant tubulin. Plant Cell. 1993 Sep;5(9):1063–1080. doi: 10.1105/tpc.5.9.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl J. D., Morejohn L. C. Deficient nucleation during co-polymerization of mammalian MAP2 and tobacco tubulin. Biochem Mol Biol Int. 1994 Sep;34(2):375–384. [PubMed] [Google Scholar]
- Kuriyama R., Savereide P., Lefebvre P., Dasgupta S. The predicted amino acid sequence of a centrosphere protein in dividing sea urchin eggs is similar to elongation factor (EF-1 alpha). J Cell Sci. 1990 Feb;95(Pt 2):231–236. doi: 10.1242/jcs.95.2.231. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lax S. R., Browning K. S., Maia D. M., Ravel J. M. ATPase activities of wheat germ initiation factors 4A, 4B, and 4F. J Biol Chem. 1986 Nov 25;261(33):15632–15636. [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Ngo N. In vitro assembly of multiprotein complexes containing alpha, beta, and gamma tubulin, heat shock protein HSP70, and elongation factor 1 alpha. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3028–3032. doi: 10.1073/pnas.90.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui H., Yamaguchi-Shinozaki K., Shinozaki K., Nishikawa K., Takahashi H. Identification of a gene family (kat) encoding kinesin-like proteins in Arabidopsis thaliana and the characterization of secondary structure of KatA. Mol Gen Genet. 1993 Apr;238(3):362–368. doi: 10.1007/BF00291995. [DOI] [PubMed] [Google Scholar]
- Mitsui H., Yamaguchi-Shinozaki K., Shinozaki K., Nishikawa K., Takahashi H. Identification of a gene family (kat) encoding kinesin-like proteins in Arabidopsis thaliana and the characterization of secondary structure of KatA. Mol Gen Genet. 1993 Apr;238(3):362–368. doi: 10.1007/BF00291995. [DOI] [PubMed] [Google Scholar]
- Morejohn L. C., Bureau T. E., Tocchi L. P., Fosket D. E. Tubulins from different higher plant species are immunologically nonidentical and bind colchicine differentially. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1440–1444. doi: 10.1073/pnas.81.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morejohn L. C., Fosket D. E. Higher plant tubulin identified by self-assembly into microtubules in vitro. Nature. 1982 Jun 3;297(5865):426–428. doi: 10.1038/297426a0. [DOI] [PubMed] [Google Scholar]
- Negrutskii B. S., Stapulionis R., Deutscher M. P. Supramolecular organization of the mammalian translation system. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):964–968. doi: 10.1073/pnas.91.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K., Toriyama M., Miyazaki M., Murofushi H., Hosoda S., Endo S., Sakai H. The mitotic apparatus-associated 51-kDa protein from sea urchin eggs is a GTP-binding protein and is immunologically related to yeast polypeptide elongation factor 1 alpha. J Biol Chem. 1990 Feb 25;265(6):3240–3247. [PubMed] [Google Scholar]
- Ornelles D. A., Fey E. G., Penman S. Cytochalasin releases mRNA from the cytoskeletal framework and inhibits protein synthesis. Mol Cell Biol. 1986 May;6(5):1650–1662. doi: 10.1128/mcb.6.5.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos S. C., Binder L. I. Phosphorylation determines two distinct species of Tau in the central nervous system. Cell Motil Cytoskeleton. 1987;8(3):210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Wagner M. C., Stenoien D. L., Brady S. T., Bloom G. S. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989 Apr;108(4):1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root D. D., Wang K. Silver-enhanced copper staining of protein blots. Anal Biochem. 1993 Feb 15;209(1):15–19. doi: 10.1006/abio.1993.1077. [DOI] [PubMed] [Google Scholar]
- Shestakova E. A., Motuz L. P., Gavrilova L. P. Co-localization of components of the protein-synthesizing machinery with the cytoskeleton in G0-arrested cells. Cell Biol Int. 1993 Apr;17(4):417–424. doi: 10.1006/cbir.1993.1080. [DOI] [PubMed] [Google Scholar]
- Shestakova E. A., Motuz L. P., Minin A. A., Gavrilova L. P. Study of localization of the protein-synthesizing machinery along actin filament bundles. Cell Biol Int. 1993 Apr;17(4):409–416. doi: 10.1006/cbir.1993.1079. [DOI] [PubMed] [Google Scholar]
- Shiina N., Gotoh Y., Kubomura N., Iwamatsu A., Nishida E. Microtubule severing by elongation factor 1 alpha. Science. 1994 Oct 14;266(5183):282–285. doi: 10.1126/science.7939665. [DOI] [PubMed] [Google Scholar]
- Suprenant K. A., Tempero L. B., Hammer L. E. Association of ribosomes with in vitro assembled microtubules. Cell Motil Cytoskeleton. 1989;14(3):401–415. doi: 10.1002/cm.970140310. [DOI] [PubMed] [Google Scholar]
- Toriyama M., Ohta K., Endo S., Sakai H. 51-kd protein, a component of microtubule-organizing granules in the mitotic apparatus involved in aster formation in vitro. Cell Motil Cytoskeleton. 1988;9(2):117–128. doi: 10.1002/cm.970090204. [DOI] [PubMed] [Google Scholar]
- Wiche G., Oberkanins C., Himmler A. Molecular structure and function of microtubule-associated proteins. Int Rev Cytol. 1991;124:217–273. doi: 10.1016/s0074-7696(08)61528-4. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Laymon R. A., Goldstein L. S. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989 Mar 10;56(5):879–889. doi: 10.1016/0092-8674(89)90692-2. [DOI] [PubMed] [Google Scholar]
- You W., Abe S., Davies E. Cosedimentation of pea root polysomes with the cytoskeleton. Cell Biol Int Rep. 1992 Jul;16(7):663–673. doi: 10.1016/s0309-1651(06)80008-1. [DOI] [PubMed] [Google Scholar]
- van Heerden A., Browning K. S. Expression in Escherichia coli of the two subunits of the isozyme form of wheat germ protein synthesis initiation factor 4F. Purification of the subunits and formation of an enzymatically active complex. J Biol Chem. 1994 Jul 1;269(26):17454–17457. [PubMed] [Google Scholar]