Abstract
We have assessed whether viscoelastic gels known to inhibit mucociliary clearance can increase lipid-mediated gene transfer. Methylcellulose or carboxymethylcellulose (0.25 to 1.5%) were mixed with complexes of the cationic lipid GL67A and plasmids encoding luciferase and perfused onto the nasal epithelium of mice. Survival after perfusion with 1% CMC or1% MC was 90 and 100%, respectively. In contrast 1.5% CMC was uniformly lethal likely due to the viscous solution blocking the airways. Perfusion with 0.5% CMC containing lipid/DNA complexes reproducibly increased gene expression by approximately 3-fold (n= 16, p<0.05). Given this benefit, likely related to increased duration of contact, we also assessed the effect of prolonging contact time of the liposome/DNA complexes by delivering our standard 80 μg DNA dose over either approximately 22 or 60 min of perfusion. This independently increased gene transfer by 6-fold (n=8, p<0.05) and could be further enhanced by the addition of 0.5% CMC, leading to an overall 25-fold enhancement (n=8, p<0.001) in gene expression. As a result of these interventions CFTR transgene mRNA transgene levels were increased several logs above background. Interestingly, this did not lead to correction of the ion transport defects in the nasal epithelium of cystic fibrosis mice nor for immunohistochemical quantification of CFTR expression. To assess if 0.5% CMC also increased gene transfer in the mouse lung, we used whole body nebulisation chambers. CMC was nebulised for 1 hr immediately before, or simultaneously with GL67A/pCIKLux. The former did not increase gene transfer, whereas co-administration significantly increased gene transfer by 4-fold (p<0.0001, n=18). This study suggests that contact time of non-viral gene transfer agents is a key factor for gene delivery, and suggests two methods which may be translatable for use in man.
Introduction
Although non-viral vectors are less efficient than viral vectors in transfecting airway epithelial cells, the former are currently the most relevant gene transfer agents for the treatment of chronic diseases such as cystic fibrosis (CF). In contrast to most viral vectors, non-viral gene transfer agents are less likely to induce immune responses, thereby allowing for repeated administration, a crucial aspect for the treatment of lifelong diseases. Importantly, proof-of-concept for repeat administration of cationic lipids to airway epithelium has been established in the clinic [1].
The cationic lipid GL67A (Genzyme Corporation) has been used in two CF gene therapy clinical trials [2,3] and has been shown to produce partial correction (approximately 25% towards normal values) of the chloride transport defect [2]. We recently re-assessed the efficacy of GL67A compared two other non-viral vectors suitable for clinical use (manuscript in preparation) and, based on our studies, GL67A remains the most efficient non-viral vector for airway gene transfer. The level of correction of the CF defect that will produce clinical benefit is unknown. The UK Cystic Fibrosis Gene Therapy Consortium has, therefore, initiated a clinical trial programme to assess whether repeat administration of GL67A complexed to plasmid DNA carrying the cystic fibrosis transmembrane conductance regulator (CFTR) cDNA can improve clinically relevant endpoints in the CF lung.
The target for CF gene therapy is the ciliated epithelium of the airways. Mucocilary clearance is a key defence mechanism in the conducting airways, removing inhaled bacteria and pollutants by sweeping them up to the pharynx where they are either coughed out or swallowed. However, this system may also reduce the efficacy of gene transfer by similarly removing gene transfer agents from the lungs. In this context, Sinn et al have interestingly shown that viscoelastic gel formulations, which inhibit mucocilary clearance increase viral gene transfer to the mouse airways [4]. Similarly Seiler et al have shown that thixotropic solutions enhance adenovirus mediated gene tranasfer [5]. No data are currently available for non-viral gene transfer.
Here we, therefore, assessed the effect of two viscoelastic gels, methylcellulose (MC) and carboxymethylcellulose (CMC) on GL67A-mediated gene transfer to the airways of wild-type and CF-knockout mice. These polymers have been shown to be safe for use in man [6–8] and are present in numerous commercial pharmaceuticals including nasal sprays. Preliminary pre-clinical studies indicate that respiratory tissues treated with MC or CMC show no evidence of toxicity [9]. We assessed both the murine nasal epithelium, to allow assessment of correction of the ion transport defect in CF knockout mice, and the lung, given the downstream clinical application.
Material and Methods
Preparation of non-viral gene transfer agents
Eukaryotic expression plasmids carrying no cDNA (pCIK-empty), the luciferase reporter gene cDNA (pCIKLux), the cystic fibrosis transmembrance conductance regulator cDNA (pCIKCFTR) or a GFP-CFTR N-terminal fusion construct (pCIKGFP-CFTR) originally described by Moyer et al [10] (cDNA was a gift from Dr B. Stanton) all under the control of the human cytomegalovirus immediate early promoter/enhancer (CMV) were used. Cationic lipid GL67A was supplied by Genzyme Corporation (Framingham, MA, USA) and complexed with DNA as previously described for nasal perfusion [11] and nebulisation [12]. Briefly, for nasal perfusion 80 μg of plasmid DNA was complexed with GL67A in a 100 μl total volume per mouse. For nebulisation 25 mg plasmid DNA were complexed with GL67A in a total volume of 10 ml. This volume was sufficient to simultaneously nebulise approximately 30 mice. Methylcellulose (MC, Methocel A4C; Dow Chemical Co., Midland, MI, USA) and Carboxymethlycellulose sodium (CMC, Aqualon 7MF; Hercules, Inc., Wilmington, DE, USA) were prepared as 2% (w/v) stock solutions in sterile water as previously described [4]. Lipid/DNA complexes were mixed with MC and CMC to final concentrations ranging from 0.5% to 1.5% in a total volume of 16 ml for nebulisation and 150 to 400 μl for nasal perfusion (see Figures for details). For control groups appropriate amounts of sterile water instead of viscoelastic gels were added to the lipid/DNA complexes.
Radiotracer efflux assay
For the radiotracer efflux assay, HEK293T cells (1×105 cells/well in pre-coated poly- L-lysine 6-well plates (Becton–Dickinson, Oxford, UK)) were seeded 24 h before transfection. Plasmids (1 μg/well) were complexed to Lipofectamine™ 2000 (2.5 μg, Invitrogen Ltd, Paisley, UK) according to manufacturer’s recommendation. CFTR chloride channel activity was assayed by measuring the rate of 125iodide efflux essentially as previously described [13]. The 125iodide efflux rates were normalised to the time of forskolin/IBMX addition (time t0). Curves were constructed by plotting rates of 125iodide efflux against time. To reflect the cumulative levels of 125iodide efflux following agonist-stimulation, all comparisons were based on areas under the time-125iodide efflux curves, as calculated by the trapezium rule. Experiments were carried out in duplicate (n=6 wells/group/experiment).
In vivo transfections
Male and female C57Bl/6 mice (Charles River), or “gut-corrected” CF-knockout mice, [14] were used at approximately 6–12 weeks of age. All experiments were carried out with approval of appropriate local Ethics Committees and according to Home Office regulations.
a.) Nasal perfusion
Mice were anaesthetised with Ketaset/Domitor (76 mg/kg and 1 mg/kg, respectively, National Veterinary Service, Stoke-on-Trent, UK). Catheters (<0.5 mm outer diameter) were inserted into the nostrils (approximately 2.5 mm) and using a syringe pump (Cole-Palmer, Illinois, USA) gene transfer agents (150 to 400 μl) were then perfused onto the nasal epithelium (7 μl/min) for approximately 22 to 60 min. At the end of the procedure mice, were injected i.p. with Antisedan (1 mg/kg, National Veterinary Services) to reverse the anaesthesia and placed in a recovery box at 30°C for 2 hours.
b.) Nebulisation
Mice were placed into an exposure chamber and exposed to an aerosol generated by a PARI LC+ nebuliser (PARI GmbH, Starnberg, Germany) at a pressure of 22 psi for 1 hr; 24 hours after transfection gene expression was quantified.
Murine endpoint assays
Preparation of tissue homogenate from mouse nose
Mice were culled by cervical dislocation and the snout, skin and nasal plate were removed. The septum was dissected and placed in 200 μl 1xRLB buffer (Promega, Southampton, UK). Nasal tissue was homogenised for 15 seconds and incubated at room temperature for 15 minutes, followed by three freeze-thaw cycles (−80°C for 30 min, thawed at room temperature) and centrifugation (5 min at 16,000gav). The supernatant was frozen at −80°C for luciferase quantification.
Preparation of tissue homogenate from mouse lung
The lungs were placed in 300 μl 1xRLB buffer (Promega, Southampton, UK) and homogenized using a Fast-Prep homogenizer (Thermo Fisher Scientific, Waltham, MA, USA) set to 4 m/sec for 45 seconds followed by 15 min incubation at room temperature. The supernatant was removed and transferred to a QiaShredder column (Qiagen, Crawley, Sussex) and centrifuged (1 min at 16,000gav) followed by an additional centrifugation (5 min at 16,000gav). The supernatant was frozen at −80°C for luciferase quantification.
Luciferase assay
Luciferase activity was measured in the supernatant using a standard luciferase assay kit (Promega) and a TD-20e luminometer (Turner BioSystems, Sunnyvale, CA). Total protein per sample was determined using the BioRad protein assay kit (BioRad laboratories, Hercules, CA) and luciferase activity was expressed as arbitrary relative light units (RLU)/mg total protein.
Real-time RT-PCR
An interdental brush (Curaprox CPS 07, Dent-o-Care Ltd, London, UK) was inserted 2 mm into the right nasal cavity and then rolled along the septum to isolate respiratory epithelial cells. This harvesting method allowed collection of samples enriched to >80% for murine airway epithelial cells (E Holder, manuscript submitted). Cells were resuspended in RLT buffer (Qiagen Ltd, West Sussex, England) containing 1% β-mercaptoethanol and spun through a QIAshredder (Qiagen Ltd) to lyse the cells. Total RNA was extracted using the Qiagen RNeasy Mini kit (Qiagen Ltd). Contaminating DNA was removed using Ambion DNA-free™ kit (Ambion Ltd, Huntingdon, UK) and RNA purified using the Qiagen RNA clean-up protocol. Absolute levels of mRNA were quantified by two-step, real-time quantitative TaqMan RT-PCR using the ABI PRISM 7700 Sequence Detection System and Sequence Detector v1.6.3 software (Applied Biosystems, Warrington, Cheshire, UK), using plasmid or endogenous mRNA-specific primers and fluorogenic probes. Sequences of the forward (F), reverse (R), and probe (P) oligonucleotides used for each mRNA target are listed below, with the corresponding concentrations for TaqMan PCR. The R primer or a separate R2 primer were utilised in reverse transcriptase reactions. For pCIK-CFTR and pCIKGFP-CFTR, R2: 5′-GTC GTA TTA AGT ACT CTA GCC TT-3′, F: 5′-GCT TCT GAC ACA ACA GTC TCG AA-3′, R: 5′-GGA GTG GAC ACC TGC CCC A-3′, P: 5′-FAM-TGC CTC ACG ACC AAC TTC TGC AGC-3′; for murine Cftr, F: F: 5′-AGC CAG CTT TAT CTC CAA ACT CTT C-3′, R: 5′-GCT GTC TGT ACC CTT TCC TCA AA-3′, P: 5′-VIC-TCA GCT GGA GCA CAG C-3′, respectively. Controls included no template control and no-reverse transcriptase (RT-) control where total RNA or MultiScribe reverse transcriptase and RNase inhibitor were omitted from the reverse transcriptase reaction, respectively. The number of copies of plasmid-derived mRNA was calculated from standard curves of in vitro transcribed RNA fragments that surrounded the amplified region [15].
Immunohistochemistry
In our experience anti-CFTR antibodies are not particularly sensitive and we therefore decided to use a N-terminal GFP-CFTR fusion cDNA to monitor CFTR expression. CF knockout mice were transfected with pCIKGFP-CFTR or a control plasmid (pCIK empty) (n=10/group). In addition some mice were transfected with SeV-GFP-CFTR [16] at a dose of 108 TU/mouse (n=6) as a positive control for the assay. Mice were anaesthetized 48 hr after transfection and perfused with phosphate-buffered saline (PBS, Sigma-Aldrich Steinheim, Germany) through the right ventricle to remove excess blood. The nasal septum lined with the epithelium was removed and fixed overnight in 4% paraformaldehyde (Sigma) in PBS at 4°C. Tissues were incubated in 30% sucrose in PBS for approximately 6 hr and embedded into optimum cutting temperature compound (OCT, Sacura Finetek Europe, B.V) using standard histological procedures. Serial 6 μm sections (100 μm apart) were cut (Bright Co, Ltd cryostat) approximately 2–3 mm from the tip of the nose, a region known to consist predominantly of respiratory epithelium, and placed on poly-L-lysine-coated slides (Thermo Shandon Ltd, Runcorn, Cheshire, UK). Slides were washed twice in PBS and incubated for 10 min at 100°C in EDTA buffer (1mM EDTA in H20, pH 8, Sigma). Slides were cooled down for 20 mins and the EDTA buffer was replaced with 0.1% Triton X100 (Sigma) in PBS (= wash buffer). Slides were washed in wash buffer 4 times and incubated with polyclonal primary anti-GFP antibody (1:200 dilution, Molecular Probes, Leiden, Holland) for 1 hr. Slides were washed and then incubated with secondary goat anti-rabbit antibody (1:200 dilution) conjugated to AlexaFluor 594 (Molecular Probes) for 1 hr. Slides were then washed 4 times in wash buffer, DAPI stained and mounted in Vectashield (Vector Laboratories, Burlingame, USA). Tissues were analysed using a Zeiss microscope (Zeiss Axioscop2 plus, Carl Zeiss, Gottingen, Germany, objective 63×/1.4, filter emission BP 515–565 ). All microscopy was performed blinded. 3 to 4 sections (300–400 cells/section) were analysed per mouse.
Nasal potential difference
PD measurements in the mouse nose were performed as previously described [17], with minor modifications. In brief, mice were anaesthetised with Ketaset/Domitor, which minimised movement of mice during the procedure. The perfusion catheter was inserted 2.5 mm into the mouse nose. The baseline (BL) was measured and buffer containing amiloride was perfused for 5 min using a syringe pump. The amiloride response was read at the lowest point, when a plateau was reached. Low chloride solution was perfused for 8–10 min and the response was measured 7 min after starting perfusion.
Statistical analysis
Statistical analyses were performed by ANOVA and Kruskal-Wallis followed by post-hoc analysis appropriate for parametric and non-parametric data. The null hypothesis was rejected at p<0.05.
Results
Mouse nasal perfusion
The formation of lipid/DNA complexes is based on electrostatic interactions between the cationic lipid and the anionic DNA, and is particularly sensitive to the addition of charged molecules. To minimise disturbance of complex formation, MC and CMC (0.1 to 1.5% final concentration) were added approximately 30 min after mixing the lipid and DNA. Using visual observation we did not observe any precipitation over a 2 hr period, despite the fact that viscosity, based on visual observation, increased with increasing concentration of the compounds.
We next determined if mice tolerated nasal perfusion of GL67A/DNA complexes (80 μg DNA in 400 μl total volume) containing CMC or MC. Survival after 60 min perfusion with a final concentration of 1% CMC or MC was 90 and 100%, respectively (n=8), but mortality increased to 100% (n=3) when mice were perfused with 1.5% CMC, likely due to the viscous solution blocking the airways. For ethical reasons we did not expose mice to 1.5% MC because this solution was more viscous than 1.5% CMC. Subsequent nasal perfusion experiments were performed with a maximum of 1% MC and CMC final concentration.
Dose-related increase in gene transfer
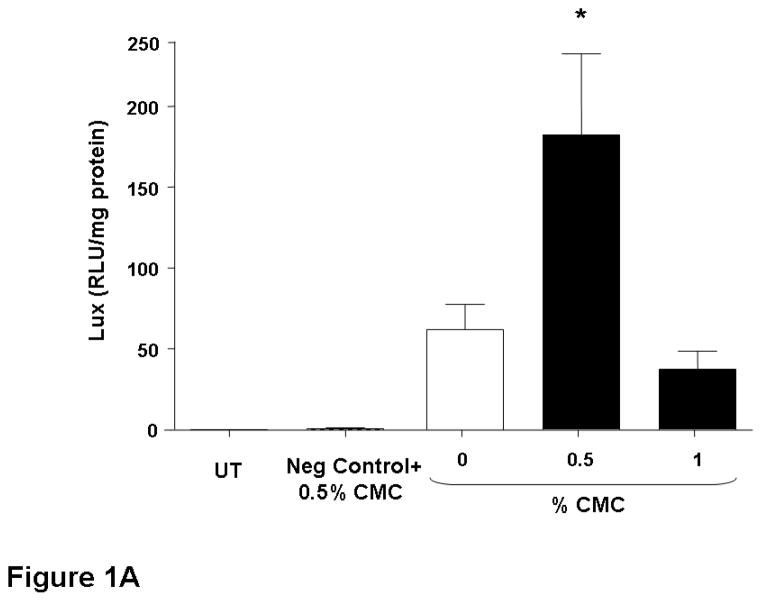
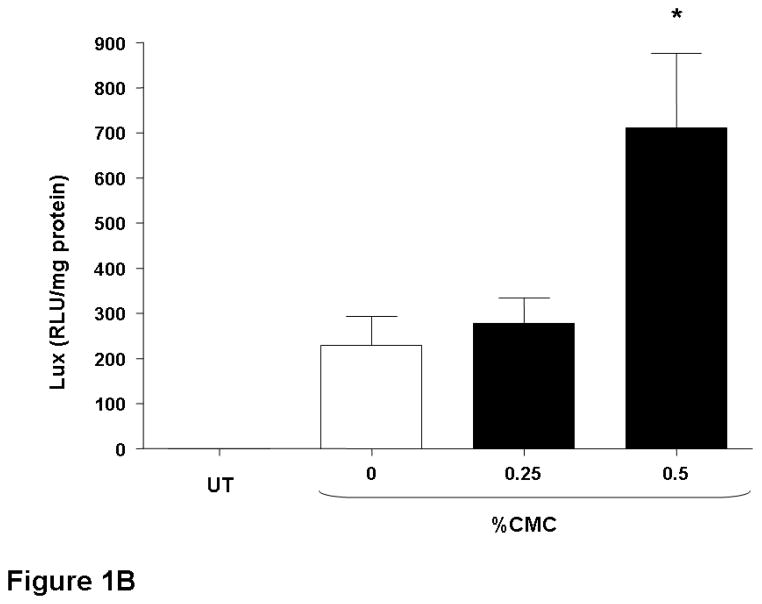
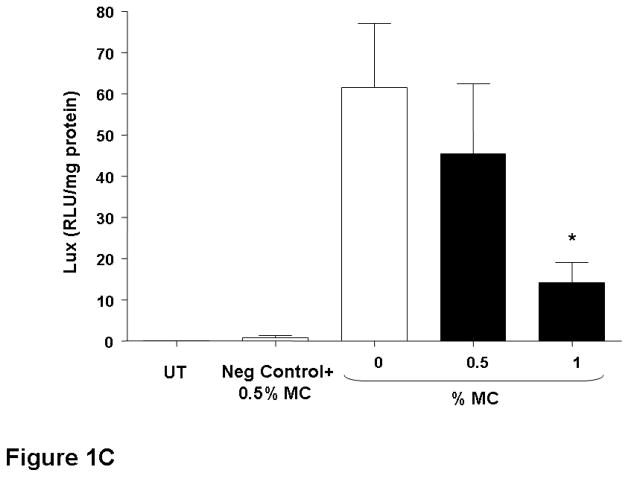
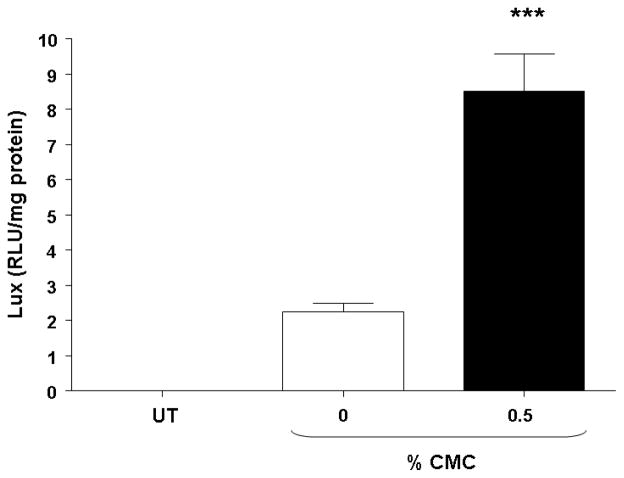
The nasal epithelium of mice was initially perfused with GL67A/pCIKLux complexes (80 μg in 400 μl total volume) containing 0.5% and 1% CMC. Appropriate control groups receiving GL67A/pCIKLux without CMC (0%) and animals transfected with an irrelevant control plasmid, as well as untransfected mice, were also included (n=8/group). Nasal perfusion with 0.5% CMC containing GL67A/pCIKLux complexes significantly (p<0.05) increased gene expression approximately 3-fold compared to 0% CMC control group, whereas the addition of 1% CMC did not alter gene expression (Figure 1A). To confirm the results and to assess if lower concentrations of CMC might be beneficial, mice were perfused with 0.25% and 0.5% CMC containing GL67A/pCIKLux complexes (n=8/group). The significant increase with 0.5% CMC previously obtained was reproducible (p<0.05), but 0.25% CMC had no effect (Figure 1B). In contrast, 0.5% MC did not alter gene expression and 1%MC reduce gene expression (p<0.05, n=8/group) (Figure 1C). Thus, the effects of viscoelastic gels on non-viral gene transfer are formulation and concentration dependent, and subsequent nasal perfusion experiments were performed with a final concentration of 0.5% CMC.
Figure 1. Addition of CMC increases gene transfer to mouse nasal epithelium.
Mouse nasal epithelium was perfused with pCIKlux (80 μg/mouse) or pCIKempty (negative control) complexed to GL67A or remained untransfected (UT) (perfusion rate: 7 μl/min). Viscoelastic gels to final concentrations ranging from 0.25% to 1% were added to the lipid/DNA complexes (final volume 150 μl/mouse). 24 hr after transfection luciferase expression was quantified in tissue homogenate. (A and B) carboxymethylcellulose (CMC), (C) methylcellulose (MC). Bars represent group mean ±SEM (n=8/group). *=p<0.05 compared to 0% CMC.
Prolonged contact time
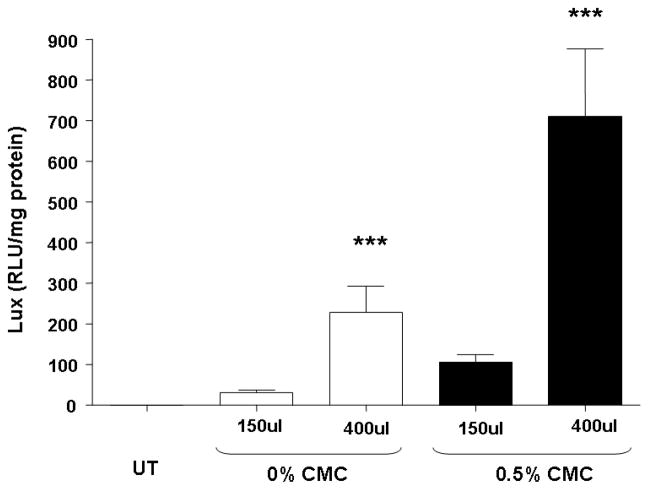
Our standard conditions for GL67A murine nasal perfusion studies include 80 μg of plasmid DNA in a total volume of 100 μl, perfused at a rate of approximately 7 μl/min over approximately 15 min [18]. We next assessed, if an extension of the perfusion time, and thereby prolongation of contact time between the gene transfer agent and the airway epithelium, could increase gene transfer efficiency. All mice were exposed to 80 μg pCIKLux complexed to GL67 in either 150 or 400 μl using a constant perfusion speed of 7μl/min. The prolonged perfusion (60 min vs 22 min) of 400 μl lipid/DNA complexes increased gene transfer by 6-fold (n=8, p<0.05) (Figure 2). Prolonging contact time further to a total of 5 hours, however, did not further improve gene transfer (60 min: 193.4±56.9, 300 min: 231.5±73.7 RLU/mg total protein, n=8).
Figure 2. Prolonged perfusion increases gene expression in mouse nasal epithelium.
Mouse nasal epithelium was perfused with pCIKlux (80 μg/mouse) complexed to GL67A or remained untransfected (UT). Carboxymethylcellulose (CMC) to a final concentration of 0.5% was added to the lipid/DNA complexes. To increase contact time between epithelium and gene transfer agents the lipid/DNA complexes were either administered in 150 μl or 400 μl total volume and were then perfused onto the nasal epithelium (7 μl/min) for approximately 23 and 60 min, respectively. 24 hr after transfection luciferase expression was quantified in tissue homogenate. Bars represent group mean ±SEM (n=8/group). ***=p<0.005 compared to 150 μl group.
Prolonged contact time and viscoelastic gels
We speculated that a combination of prolonged perfusion and the addition of 0.5% CMC may have an additive effect on non-viral gene transfer in the mouse nose. All mice were exposed to 80 μg pCIKLux complexed to GL67 containing either 0% or 0.5% CMC in either 150 or 400 μl using a constant perfusion speed of approximately 7 μl/min. Importantly, a combination of prolonged perfusion and CMC further increased transfection efficiency to an overall 25-fold enhancement (n=8, p<0.001) in gene expression compared to our standard formulation (Figure 2).
CMC for nebulisation
We also assessed if CMC increased gene transfer in the mouse lung using whole body nebulisation chambers. 0.5% CMC was either nebulised for 1 hr immediately before, or simultaneously with, GL67A/pCIKLux. The former did not increase gene transfer (data not shown), whereas co-administration significantly increased gene transfer approximately 4-fold (p<0.0001, n=18) (Figure 3). Attempts to increase the concentration of CMC from 0.5% to 1% were unsuccessful as the high viscosity of the solution prevented nebulisation.
Figure 3. Addition of CMC increased gene transfer after nebulisation in mouse lung.
Mouse lungs were transfected with pCIKlux complexed to GL67A for one hour using a nebulisation chamber or remained untransfected (UT). Carboxymethylcellulose (CMC) to a final concentration of 0.5% was added to the lipid/DNA complexes (25 mg DNA in 16 ml final volume). 24 hr after transfection luciferase expression was quantified in the lung homogenate. Bars represent group mean ±SEM (n=8/group). *=p<0.05 compared to 0% CMC
Expression of vector-specific CFTR mRNA
To date the levels of gene transfer achieved by non-viral vectors has been insufficient to detect recombinant CFTR protein or correction of the ion transport defect in the nasal epithelium of CF mice [18]. However, since a combination of prolonged contact time, and the addition of CMC, improved gene transfer in the mouse nasal epithelium by 25-fold compared to our previous formulation, we felt, that this increase in gene expression warranted a re-evaluation of whether these biomarkers could be altered by non-viral gene transfer.
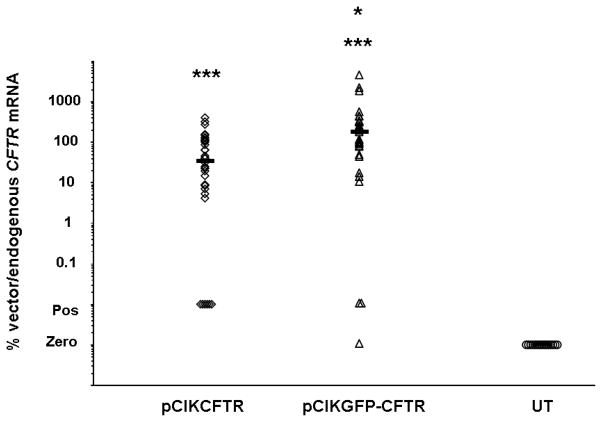
CF knockout mice were first transfected with pCIK-CFTR or pCIKGFP-CFTR complexed to GL67A containing 0.5% CMC, or remained untransfected (n=35/group) and mRNA was quantified 24 hr after transfection using quantitative RT-PCR. Gene expression was detectable in 69 out of 70 transfected mice. Median levels of gene expression were equivalent to 36% and 170% of endogenous CFTR in mice transfected with pCIK-CFTR and pCIKGFP-CFTR, respectively (Figure 4). mRNA levels were significantly (p<0.01) higher in pCIKGFP-CFTR transfected mice when compareed to pCIKCFTR.
Figure 4. Expression of vector-specific CFTR mRNA in mouse nasal epithelium.
The nasal epithelium of CF knockout mice was perfused with pCIKCFTR or pCIKGFPCFTR (80 μg DNA/mouse) complexed to GL67A including 0.5% CMC (final volume 150 μl/mouse) or remained untransfected (UT). 24 hr after transfection vector-specific and endogenous CFTR mRNA were quantified in nasal epithelium using real-time quantitative RT-PCR. Data are expressed as the ratio of vector-specific over endogenous CFTR mRNA. Each symbol represents one mouse (n=35/group). Horizontal bars indicates group median. In some samples mRNA was detectable, but not accurately quantifiable. These samples are labelled positive (pos). *=p<0.05 compared to pCIKCFTR, ***=p<0.001 compared to untransfected.
Expression of GFP-CFTR fusion protein
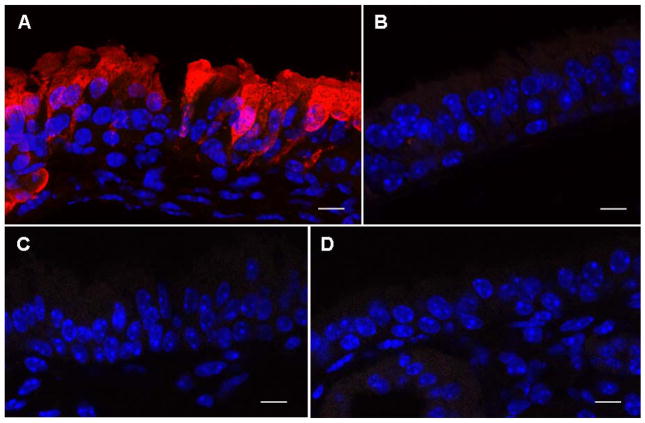
CF-knockout mice were transfected with pCIKGFP-CFTR complexed to GL67A or a control plasmid without cDNA (pCIK-empty) (n=10/group). As a positive control for the immunohistochemistry (IHC), mice (n=6) were transfected with recombinant Sendai-virus (SeV) vector carrying the GFP-CFTR fusion contruct (SeV-GFP-CFTR, 108 pfu/mouse). We have previously shown that this virus transduces the nasal epithelium highly efficiently [16]. Despite extensive analysis of all tissues (300–400 respiratory epithelial cells per level, ~1000 cells per mouse) we did not detect recombinant protein in pCIKGFP-CFTR transfected mice. In contrast, in mice transduced SeV-GFP-CFTR protein was detectable (Figure 5).
Figure 5. Detection of GFP-CFTR fusion protein in mouse nasal epithelium.
The nasal epithelium of CF knockout mice was perfused with pCIKGFPCFTR complexed to GL67A plus 0.5% CMC. A recombinant Sendai virus (SeV) carrying GFP-CFTR was used as positive control and untransfected animals were negative controls. 24 hr after transfection nasal tissue was processed for immunohistochemistry and expression of GFP-CFTR fusion protein was visualized using an anti-GFP antibody and a secondary antibody conjugated to AlexaFluor 594. (A) SeVGFP-CFTR with anti-GFP primary antibody, (B) SeV-GFP-CFTR without anti-GFP primary antibody, (C) pCIKGFP-CFTR with anti-GFP primary antibody, (D) untransfected mouse with anti-GFP primary antibody. GFP-CFTR protein appears in red. DAPI stained nuclei appear in blue. Representative images are shown. n=10 mice/group. Scale bar=10 μm.
Nasal potential difference measurements
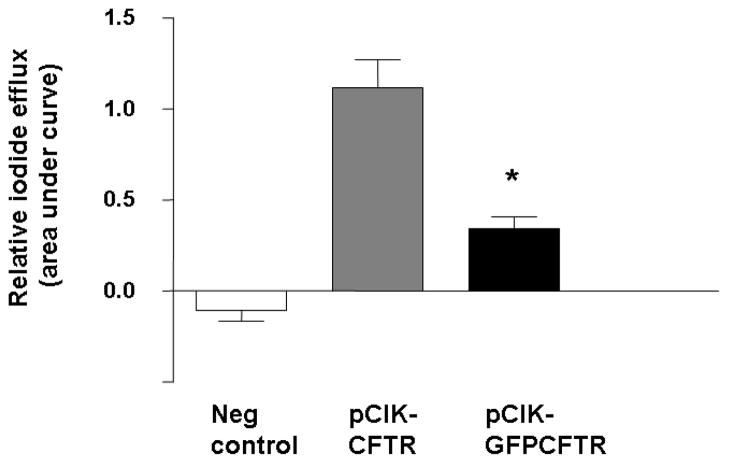
To ensure plasmids to be used in the study in vivo were able to generate functional CFTR channels, an in vitro radiotracer efflux assay was first performed. pCIKCFTR and pCIKGFP-CFTR both generated significant CFTR-mediated ion efflux, although the fusion cDNA lead to significantly (p<0.05) lower efflux activity compared to pCIKCFTR (Figure 6).
Figure 6. Assessment of CFTR function in a radio-tracer efflux assay.
293T cells were transfected with either pCIKCFTR, pCIKGFP-CFTR or a negative control plasmid (pCIK-βgal) complexed to Lipofectamine 2000. 48 hr after transfection iodide efflux was quantified. Bars represent group mean ± SEM (n=6/group). *=p<0.05
Next, CF-knockout mice were transfected with pCIKCFTR, pCIKGFP-CFTR or pCI-empty complexed to GL67A and 0.5% CMC as described above (n=30/group) and nasal Potential Difference (NPD) measurements were performed 24 hr after transfection. The baseline NPD and amiloride response are measures of sodium absorption, which are elevated in CF. The response to perfusion with a low chloride solution is a measure of CFTR-mediated chloride transport, which is absent or reduced in CF. There were no significant changes in baseline PD, amiloride response, or low chloride responses (Table 1) between mice treated with CFTR expressing plasmids and controls.
Table 1.
Nasal potential difference measurements in CF knockout mice transfected with CFTR-expression plasmids complexed to the cationic lipid GL67
| Plasmid | Baseline (absolute PD) | Amiloride (ΔPD) | Low Chloride (ΔPD) | n |
|---|---|---|---|---|
| pCIKCFTR | −14.65 (1.33) | 10.80 (1.08) | −0.48 (0.38) | 31 |
| pCIKGFP-CFTR | −15.11 (1.31) | 8.84 (1.33) | −0.49 (0.37) | 30 |
| pCI-empty | −15.99 (1.41) | 11.61 (1.35) | −0.47 (0.52) | 29 |
Numbers indicate group mean (SEM). For reference non-CF low chloride responses are 9.5±3.5 mV.
Discussion
Here, we have shown that carboxymethylcellulose, a viscoelastic gel, significantly increases cationic lipid (GL67A)-mediated gene transfer to the airway epithelium. Gene transfer was further increased by prolonging contact time between the vector and the target cells; these two interventions were additive providing an approximately 25-fold increase in reporter gene expression and up to 170% of endogenous CFTR mRNA levels in knockout-mice. Interestingly, despite these changes neither CFTR protein nor changes in bioelectric properties could be detected.
Sinn et al have previously shown that the viscoelastic gels MC and CMC significantly reduce mucociliary transport rates [4], which is likely to prolong contact time between the gene transfer agent and the lung epithelium and may explain the increased transfection efficiency of non-viral gene transfer agents observed in this study. MC and CMC did not appear to open tight junctions in a polarised human airway epithelial cell model [4]. However, we observed, that the baseline nasal potential difference was reduced in the CF knockout mice treated with CMC (mean baseline PD approximately −15 mV) compared to our previously published CF mouse baseline values of approximately −24 mV [19]. This drop in baseline PD may be caused by the opening of tight junctions, or epithelial cell damage, which may also affect gene transfer efficiency. Interestingly, we have previously shown that tight junction openers increase non-viral gene transfer in murine airway epithelium [20]. It is, therefore, unclear if the effect of CMC is solely due to increasing contact time between vector and target cell.
We observed a number of similarities and differences compared to the study by Sinn et al. which assessed the effect of CMC on virus-mediated transduction to airway epithelium [4]. (a) The maximum feasible concentration for nasal administration was similar. At concentrations above 1% CMC and MC survival during nasal perfusion was low, likely due to mice being obligate nose breathers with the highly viscous solutions leading to blockage of the nasal passages. The maximum feasible concentration for nebulisation was determined by the ability to nebulise with the PARI LC+ nebuliser. It may be feasible to increase further the concentration by switching to other jet or ultrasonic nebulisers, or the most recently introduced mesh-based nebulisers, (b) 1% CMC increased viral, but not non-viral gene transfer which may reflect the different properties or cellular uptake of viral and non-viral gene transfer agents, (c) MC improved viral, but not non-viral gene transfer. However, MC was less effective in reducing mucociliary clearance (MCC) than CMC [4], which may in part explain the results observed here.
We also increased gene expression by prolonging perfusion of the lipid/DNA complexes onto the nasal epithelium. Lipid/DNA complexes enter cells through endocytosis and the amount of vector taken into the cell will, in part, depend on the endocytosis rate of the cells. It is, therefore, readily conceivable that prolonged contact time will allow more vector to be endocytosed, which in turn is likely to increase gene expression. Interestingly, we show here, that the effects of CMC and prolonged perfusion on gene transfer were additive. As mentioned above, it is unclear, if the cilia-static action of CMC is the main factor involved in increasing transfection or if CMC-induced opening of tight junctions also plays an important role. Independent of the mode of action, it is likely that prolonged contact time through longer perfusion would further enhance the effect.
Using these optimised transfection conditions we next attempted to identify transfected cells using immunohistochemistry (IHC). In a previous study [18], we tried to quantify lipid-mediated CFTR expression by IHC in transfected CF knockout mice using a variety of anti-CFTR antibodies [18]. Interestingly, no CFTR expression could be detected even in control experiments following transduction with a Sendai virus (SeV) vector carrying a CFTR cDNA, which has been shown to transfect close to 100% of airway epithelial cells and partially corrects the chloride transport defect in the CF mouse nose [21,22]. Here we, therefore, used a GFP-CFTR fusion construct [10] and anti-GFP antibodies to visualise transfected cells. We first showed that GFP-CFTR mRNA is detectable after GL67A/pCIKGFP-CFTR mediated gene transfer. Importantly, we used a novel cell harvesting technique that allowed detection of vector-specific mRNA in samples enriched to >80% for murine airway epithelial cells, rather than tissue homogenates. Interestingly, pCIKGFP-CFTR-derived mRNA levels were significantly higher than pCIKCFTR-derived levels. The reason for this is unknown, but an mRNA stabilising effect of the N-terminal GFP fusion may be responsible. However, although the anti-GFP antibody detected the GFP-CFTR fusion protein in SeV-GFP-CFTR transduced mice (positive control), we were unable to visualise protein expression in GL67A/pCIKGFP-CFTR transfected animals. After transduction the SeV vector replicates in the cytoplasm of transduced cells which likely leads to higher levels of recombinant protein expression per cell than GL67/pCIKGFP-CFTR expression, which may explain the results.
We were also unable to detect changes in murine nasal bioelectrics. There may be several reasons for this: (1) Although significant levels of vector-specific mRNA are produced, these may only come from a very few highly expressing cells, rather than from many cells expressing CFTR at moderate to low levels. This would not be sufficient to correct the chloride transport defect, as in vitro mixing experiments imply that a minimum of 5% of cells expressing CFTR may be required [23]. Importantly, the quantitative RT-PCR assay does not currently provide information on how many cells express vector-specific CFTR mRNA, and further refinements of RT-PCR down to single-cell quantification are required. However, since SeV vector which transfects close to 100% of murine nasal epithelial cells, but only achieves modest correction (approximately 20% towards wild-type) [21], other factors may play a role. (2) Although the optimised transfection conditions used here led to 170% of vector-specific mRNA compared to endogenous Cftr expression in the CF mice, it is important to note that murine Cftr expression is approximately 15-fold lower in the FABp-CF mice used here compared to wild-type mice[24], possibly due to increased mRNA degradation caused by knock-in of the stop codon and the neomycin resistance gene [25], or strain variation in Cftr expression. Thus, the maximum transgene levels achieved here, represent approximately 12% of endogenous wild-type Cftr levels, which may still be too low to correct mouse nasal PD. Importantly, however, CF subjects with “mild mutations” which maintain low levels of residual CFTR expression and function (≥5%) have generally less severe lung disease [26]. (3) Although, previous studies have shown that GFP fused to the N-terminus of CFTR neither affected chloride channel function nor the correct apical localization of CFTR [10,27], we observed a significant reduction in a radio-tracer efflux assay. Differences may be due to different models and assays being used in these studies. We cannot exclude that the fusion construct may have reduced chloride channel function in the mouse nose in vivo, although we have previously shown that when incorporated into SeV vector, partial correction of chloride transport can be achieved with this construct in CF mice [16]. Importantly, the non-modified CFTR cDNA also did not achieve correction. (4) The suitability of the CF mouse nasal epithelium as a model for respiratory gene transfer has been put into question by two recent publications showing that the nasal bioelectrics are dominated by the olfactory rather than the respiratory epithelium [28,29]. Our experience is in keeping with this observation. Transduction with SeV vector, which targets both the respiratory and olfactory epithelium led to increases in chloride transport [21], whereas non-viral gene transfer agents such as GL67A and DC-Chol/DOPE, which have been optimised to transfect the airway epithelium, were unable to alter ion transport in the mouse nose.
Prolonged contact time between the airway epithelium and gene transfer agents may also increase transfection efficiency in the human lung. Extending the nebulisation time may be one way to achieve this, although patient tolerability may become a rate limiting factor. As mentioned above CMC has been shown to be safe for use in man and may, therefore, be suitable for inclusion in gene therapy clinical trials. However, efficacy and tolerability have to be carefully assessed in a suitable pre-clinical model. We routinely use wild-type sheep because lung structure and cell composition are similar to human. The recently developed CF pig and ferret models [30,31] may allow assessment of these novel strategies in the context of CF lung disease. However, it remains to be seen, if CF pigs and ferrets develop CF-like lung disease and when these models will become available for the wider research community.
Conclusions
We have shown that prolonged contact time, achieved by either exposure to a viscoelastic gel or prolonged perfusion, or a combination of both, increases cationic lipid-mediated gene transfer to the mouse nasal epithelium. However, the achieved levels remain insufficient for detection of transfected cells with immunohistochemistry, or correction of ion transport defects in CF knockout mice.
Acknowledgments
We thank Drs A van Heeckeren and M Drumm (Case Western Reserve, USA) for making FABp-CF breeding pairs available and Lucinda Somerton (Imperial College) for help with preparing the manuscript. This work was supported by the Cystic Fibrosis Trust and a Dr Benjamin Angel Senior Fellowship (UG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hyde SC, Southern KW, Gileadi U, Fitzjohn EM, Mofford KA, Waddell BE, et al. Repeat administration of DNA/liposomes to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 2000;7(13):1156–1165. doi: 10.1038/sj.gt.3301212. [DOI] [PubMed] [Google Scholar]
- 2.Alton EW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J, et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet. 1999;353(9157):947–954. doi: 10.1016/s0140-6736(98)06532-5. [DOI] [PubMed] [Google Scholar]
- 3.Sorscher EJ, Logan JJ, Frizzell RA, Lyrene RK, Bebok Z, Dong JY, et al. Gene therapy for cystic fibrosis using cationic liposome mediated gene transfer: a phase I trial of safety and efficacy in the nasal airway. Hum Gene Ther. 1994;5(10):1259–1277. doi: 10.1089/hum.1994.5.10-1259. [DOI] [PubMed] [Google Scholar]
- 4.Sinn PL, Shah AJ, Donovan MD, McCray PB., Jr Viscoelastic gel formulations enhance airway epithelial gene transfer with viral vectors. Am J Respir Cell Mol Biol. 2005;32(5):404–410. doi: 10.1165/rcmb.2004-0410OC. [DOI] [PubMed] [Google Scholar]
- 5.Seiler MP, Luner P, Moninger TO, Karp PH, Keshavjee S, Zabner J. Thixotropic solutions enhance viral-mediated gene transfer to airway epithelia. Am J Respir Cell Mol Biol. 2002;27(2):133–140. doi: 10.1165/ajrcmb.27.2.4793. [DOI] [PubMed] [Google Scholar]
- 6.Grindel JM, Jaworski T, Piraner O, Emanuele RM, Balasubramanian M. Distribution, metabolism, and excretion of a novel surface-active agent, purified poloxamer 188, in rats, dogs, and humans. J Pharm Sci. 2002;91(9):1936–1947. doi: 10.1002/jps.10190. [DOI] [PubMed] [Google Scholar]
- 7.Ismail FA, Napaporn J, Hughes JA, Brazeau GA. In situ gel formulations for gene delivery: release and myotoxicity studies. Pharm Dev Technol. 2000;5(3):391–397. doi: 10.1081/pdt-100100555. [DOI] [PubMed] [Google Scholar]
- 8.Johnston TP, Miller SC. Toxicological evaluation of poloxamer vehicles for intramuscular use. J Parenter Sci Technol. 1985;39(2):83–89. [PubMed] [Google Scholar]
- 9.Ugwoke MI, Agu RU, Jorissen M, Augustijns P, Sciot R, Verbeke N, et al. Toxicological investigations of the effects carboxymethylcellulose on ciliary beat frequency of human nasal epithelial cells in primary suspension culture and in vivo on rabbit nasal mucosa. Int J Pharm. 2000;205(1–2):43–51. doi: 10.1016/s0378-5173(00)00484-1. [DOI] [PubMed] [Google Scholar]
- 10.Moyer BD, Loffing J, Schwiebert EM, Loffing-Cueni D, Halpin PA, Karlson KH, et al. Membrane trafficking of the cystic fibrosis gene product, cystic fibrosis transmembrane conductance regulator, tagged with green fluorescent protein in madin-darby canine kidney cells. J Biol Chem. 1998;273(34):21759–21768. doi: 10.1074/jbc.273.34.21759. [DOI] [PubMed] [Google Scholar]
- 11.Lee ER, Marshall J, Siegel CS, Jiang C, Yew NS, Nichols MR, et al. Detailed analysis of structures and formulations of cationic lipids for efficient gene transfer to the lung. Hum Gene Ther. 1996;7(14):1701–1717. doi: 10.1089/hum.1996.7.14-1701. [DOI] [PubMed] [Google Scholar]
- 12.Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol. 2008;26(5):549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- 13.Derand R, Bulteau-Pignoux L, Becq F. Comparative pharmacology of the activity of wild-type and G551D mutated CFTR chloride channel: effect of the benzimidazolone derivative NS004. J Membr Biol. 2003;194(2):109–117. doi: 10.1007/s00232-003-2030-z. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994;266(5191):1705–1708. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- 15.Rose AC, Goddard CA, Colledge WH, Cheng SH, Gill DR, Hyde SC. Optimisation of real-time quantitative RT-PCR for the evaluation of non-viral mediated gene transfer to the airways. Gene Ther. 2002;9(19):1312–1320. doi: 10.1038/sj.gt.3301792. [DOI] [PubMed] [Google Scholar]
- 16.Ban H, Inoue M, Griesenbach U, Munkonge F, Chan M, Iida A, et al. Expression and maturation of Sendai virus vector-derived CFTR protein: functional and biochemical evidence using a GFP-CFTR fusion protein. Gene Ther. 2007;14(24):1688–1694. doi: 10.1038/sj.gt.3303032. [DOI] [PubMed] [Google Scholar]
- 17.Smith SN, Middleton PG, Chadwick S, Jaffe A, Bush KA, Rolleston S, et al. The in vivo effects of milrinone on the airways of cystic fibrosis mice and human subjects. Am J Respir Cell Mol Biol. 1999;20(1):129–134. doi: 10.1165/ajrcmb.20.1.3278. [DOI] [PubMed] [Google Scholar]
- 18.Griesenbach U, Sumner-Jones SG, Holder E, Munkonge FM, Wodehouse T, Smith SN, et al. Limitations of the Murine Nose in the Development of Non-viral Airway Gene Transfer. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0075OC. Eprint. [DOI] [PubMed] [Google Scholar]
- 19.Griesenbach U, Smith SN, Farley R, Singh C, Alton EW. Validation of Nasal Potential Difference Measurements in Gut-corrected CF Knockout Mice. Am J Respir Cell Mol Biol. 2008;39 (4):490–496. doi: 10.1165/rcmb.2007-0385OC. [DOI] [PubMed] [Google Scholar]
- 20.Larsen MD, Griesenbach U, Alton EWFW. Tight junction openers increase non-viral gene transfer to the airway epithelium in vivo. Hum Gene Ther. 2008;19(10):1125. [Google Scholar]
- 21.Ferrari S, Griesenbach U, Iida A, Farley R, Wright AM, Zhu J, et al. Sendai virus-mediated CFTR gene transfer to the airway epithelium. Gene Ther. 2007;14(19):1371–1379. doi: 10.1038/sj.gt.3302991. [DOI] [PubMed] [Google Scholar]
- 22.Griesenbach U, Cassady RL, Ferrari S, Fukumura M, Muller C, Schmitt E, et al. The nasal epithelium as a factory for systemic protein delivery. Mol Ther. 2002;5(2):98–103. doi: 10.1006/mthe.2002.0524. [DOI] [PubMed] [Google Scholar]
- 23.Johnson LG, Olsen JC, Sarkadi B, Moore KL, Swanstrom R, Boucher RC. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet. 1992;2(1):21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 24.Holder E, Stevenson B, Farley R, Hilliard TN, Wodehouse T, Somerton L, et al. Detection of CFTR transgene mRNA expression in respiratory epithelium isolated from the murine nasal cavity. J Gene Med. 2009 doi: 10.1002/jgm.1413. In Press. [DOI] [PubMed] [Google Scholar]
- 25.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257(5073):1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 26.Dorfman R, Zielenski J. Genotype-Phenotype Correlations in Cystic Fibrosis. In: Bush A, Alton EWFW, Davies JC, Griesenbach U, Jaffe A, editors. Cystic Fibrosis in the 21st Century. London: Karger; 2009. pp. 61–68. [Google Scholar]
- 27.Oceandy D, McMorran B, Schreiber R, Wainwright BJ, Kunzelmann K. GFP-tagged CFTR transgene is functional in the G551D cystic fibrosis mouse colon. J Membr Biol. 2003;192(3):159–167. doi: 10.1007/s00232-002-1072-y. [DOI] [PubMed] [Google Scholar]
- 28.Ostrowski LE, Yin W, Diggs PS, Rogers TD, O’Neal WK, Grubb BR. Expression of CFTR from a ciliated cell-specific promoter is ineffective at correcting nasal potential difference in CF mice. Gene Ther. 2007;14(20):1492–1501. doi: 10.1038/sj.gt.3302994. [DOI] [PubMed] [Google Scholar]
- 29.Grubb BR, Rogers TD, Boucher RC, Ostrowski LE. Ion transport across CF and normal murine olfactory and ciliated epithelium. Am J Physiol Cell Physiol. 2009;296(6):C1301–C1309. doi: 10.1152/ajpcell.00578.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321(5897):1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun X, Yan Z, Yi Y, Li Z, Lei D, Rogers CS, et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest. 2008;118(4):1578–1583. doi: 10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]