Abstract
To date, the behavior of hyperthermophilic microorganisms in their biotope has been studied only to a limited degree; this is especially true for motility. One reason for this lack of knowledge is the requirement for high-temperature microscopy—combined, in most cases, with the need for observations under strictly anaerobic conditions—for such studies. We have developed a custom-made, low-budget device that, for the first time, allows analyses in temperature gradients up to 40°C over a distance of just 2 cm (a biotope-relevant distance) with heating rates up to ∼5°C/s. Our temperature gradient-forming device can convert any upright light microscope into one that works at temperatures as high as 110°C. Data obtained by use of this apparatus show how very well hyperthermophiles are adapted to their biotope: they can react within seconds to elevated temperatures by starting motility—even after 9 months of storage in the cold. Using the temperature gradient-forming device, we determined the temperature ranges for swimming, and the swimming speeds, of 15 selected species of the genus Thermococcus within a few months, related these findings to the presence of cell surface appendages, and obtained the first evidence for thermotaxis in Archaea.
INTRODUCTION
The discovery of microorganisms by Antonie van Leeuwenhoek in 1676 (published in 1684 by the Royal Society in London, England) was made possible only by the development of his handmade single-lens microscope. Although this discovery has often been called into question, it has been shown unambiguously, by taking pictures through an original Leeuwenhoek microscope, that his device could resolve structures of 700 nm (just a factor of 4 below the resolution of light microscopy) using the biconvex or planoconvex lenses that he fabricated himself (1). Over the centuries, light microscopy has been optimized, e.g., by the use of two-lens microscopes and the introduction of phase-contrast microscopy, and has developed into new, very special fields (for reviews, see references 2 to 4). Special superresolution fluorescence microscopy techniques today overcome the resolution limit of light microscopy (∼200 nm) by a factor of 3 to 20.
Hyperthermophilic microorganisms have attracted much attention because they live in very unusual habitats, often display exotic physiology, and may represent a lifestyle of ancient organisms (5). Physiological studies of such hyperthermophiles are complicated by their often strictly anaerobic nature and the fact that very often, high temperatures (100°C or higher) are needed for experimentation.
Hyperthermophiles occur in naturally heated habitats, such as solfataric fields, self-heating coal refuse piles, or hydrothermal vents. In particular, marine biotopes result in potentially deadly conditions: the vent fluids within black smokers, with temperatures as high as 400°C, would kill any form of life immediately; the surrounding seawater, at ca. 3°C, would not support the physiological needs of hyperthermophiles. The presence of hyperthermophiles in the walls of black (and white) smokers has been proven by 16S rRNA analyses (terminal restriction fragment length polymorphism [T-RFLP] and rRNA gene sequencing) (6). Proof that the organisms found there actually are alive comes from the fact that they can be isolated from wall samples taken from such chimneys (see, e.g., reference 7; for a review, see reference 8). Such pure cultures, then, can be used to study their physiology. However, important questions as to their behavior in the biotope, e.g., their motility, can be answered only to some extent. To overcome these limitations at least partially, a thermomicroscope was developed at our institute (9), which enabled our groups to obtain substantial results, e.g., on the mechanism of formation of the cannulae of Pyrodictium occultum (hollow tubes connecting the periplasm of cells [9]), and to study the motility of hyperthermophiles. The latter studies indicated that some hyperthermophiles can swim very fast and identified Methanocaldococcus villosus as the fastest organism on earth (10), if speed is measured in the relative unit bodies per second (bps). We characterized the swimming behavior of hyperthermophiles as a “relocate-and-seek” strategy: they swim very fast and almost linearly over (relatively) long distances, but close to surfaces, they can change to a much slower, zigzag movement. The “seek” movement was discussed as a prerequisite for attachment to surfaces (10), a hypothesis substantiated by the fact that hyperthermophiles, such as the archaeon Pyrococcus furiosus (11) or M. villosus (12), use their flagella not only for motility but also for adhesion to various surfaces, including their natural substrates.
Nevertheless, many basic questions on the biology of hyperthermophiles, especially with respect to their motility, remain unanswered. Such questions include the following. How fast do hyperthermophiles react to changes in temperature? How fast can various species swim? Do they stop swimming at low temperatures? Do they swim away from temperatures too hot or too cold for them? (In other words, do they show thermotaxis?) Can cells experiencing low temperatures for a prolonged period react quickly to high temperatures by active motility?
We show here how simple light microscopy using our newly developed, low-budget device—the temperature gradient-forming device (TGFD)—can help to answer such very basic questions on the biology of anaerobic, hyperthermophilic microorganisms. For a systematic study of these questions, we have chosen 15 species of the archaeal genus Thermococcus that differ in growth requirements and in the number of cell appendages and for which, to some extent, data on motility were available. Additionally, we analyzed M. villosus for its reaction to a rapid increase in temperature, a typical situation that hyperthermophiles experience in their biotope.
MATERIALS AND METHODS
Microorganisms and growth conditions.
The various Thermococcus species that we studied are listed in Table 1; they were obtained from the DSMZ and JCM and represent the corresponding type strains. For growth, two different media were used: the “poor medium” (called SMES below) was a modified SME medium (13) containing 0.1% yeast extract and 0.1% peptone with the addition of elemental sulfur. The “rich medium” (called MAYTP below) was a modified MA-YT medium (14) supplemented with 0.5% (each) yeast extract, tryptone, and pyruvate. Cells were grown at 85°C; the temperature ranges for the growth of the various thermococci are given in Table 1. The particular species of the genus Thermococcus selected for this study were chosen for the following reasons. Thermococcus acidaminovorans can use amino acids as the sole carbon and energy source and has been described as motile (15). Thermococcus aegaeicus has been reported to be immotile at room temperature but to possess a single flagellum (16) (this species has been renamed Thermococcus aegaeus [http://www.bacterio.net/]). Thermococcus alcaliphilus was chosen for its growth at high pHs and its description as possessing a single flagellum (17), while Thermococcus atlanticus has been reported to be immotile and to possess no cell appendages (18). Thermococcus celer is the type species for the genus Thermococcus (19). Thermococcus celericrescens has been described as a fast-growing species that forms cell fusions and is motile, with a polar tuft of flagella (20). Thermococcus chitonophagus has an unusual physiology, in that it can use chitin as the sole energy and carbon source (21), and has been described as motile. Thermococcus coalescens has been reported to have two different cell types and to be motile, with a polar tuft of flagella (22). Thermococcus gorgonarius has been described as motile and as possessing “very thin, 5 nm flagella” (23). Thermococcus guaymasensis was described as motile in 1994 and as immotile in 1998 (24, 25). Thermococcus kodakaraensis was chosen because it has been described as highly motile, with a polar tuft of flagella (14) (this species has been renamed Thermococcus kodakarensis [http://www.bacterio.net/]), and because of its genetics (26). Thermococcus pacificus was of interest for its flagella, which differ in diameter from those of T. gorgonarius (23). Thermococcus radiotolerans, with its gamma-irradiation tolerance, has an unusual physiology (27), while Thermococcus stetteri shows an unusually high maximum growth temperature and has been described as “non-motile or motile with a polar tuft of flagella” (28). Finally, Thermococcus thioreducens was chosen due to its description as an obligate S0 reducer (29). All these strains were analyzed for motility after growth at 85°C in the rich MAYTP medium or in the poor SMES medium (T. aegaeus did not grow in MAYTP medium).
TABLE 1.
Temperature ranges of growth and swimming for 15 selected species of the genus Thermococcus
| Thermococcus species | Culture collection no. | Temp range (°C) for: |
Avg/maximal swimming speed [μm/s] at 85°Ca |
TEM analysis of cell appendagesb | ||
|---|---|---|---|---|---|---|
| Growthc | Swimming | In SMES | In MAYTP | |||
| T. acidaminovorans | DSM 11906T | 55–90 | No cell appendages | |||
| T. aegaeus | DSM 12767T | 70–90 | 40–95 | 16/35 | No growth in MAYTP | 1–5 flagella (width, 10–11 nm) |
| T. alcaliphilus | DSM 10322T | 56–90 | 40–95 | 16/27 | 13/24 | 1–10 flagella (width, 9–11 nm) per cell |
| T. atlanticus | DSM 15226T | 70–90 | 25–95 | 21/36 | 1 to 5 flagella (width, 11–13 nm) per cell plus rarely thinner filaments (8 nm) in SMES; no cell appendages in MAYTP | |
| T. celer | DSM 2476T | 50–90 | 25–95 | 46/83 | 110/175 | Polar tuft of flagella (width, 11–12 nm) in MAYTP; 1–5 flagella in SMES |
| T. celericrescens | DSM 17994T | 50–85 | 25–95 | 83/177 | 52/182 | Polar tuft of flagella (width, 9–12 nm) |
| T. chitonophagus | DSM 10152T | 60–95 | 55–95 | 213/343 | 113/182 | Polar tuft of flagella (width, 11–12 nm) |
| T. coalescens | DSM 16538T | 57–90 | 40–95 | 19/32 | 1–5 flagella (width, 12 nm) in SMES; no cell appendages in MAYTP | |
| T. gorgonarius | DSM 10395T | 68–95 | Many polytrichous cell appendages (width, 5–8 nm) | |||
| T. guaymasensis | DSM 11113T | 56–90 | 25–95 | 67/165 | Polar tuft of flagella (width, 10–11 nm) plus rarely thinner filaments (6–7 nm) in SMES; in MAYTP only the thin filaments | |
| T. kodakarensis | JCM 12380T | 60–100 | 25–95 | 72/166 | 68/153 | Polar tuft of flagella (width, 10–12 nm) in MAYTP; in SMES 1–5 flagella |
| T. pacificus | DSM 10394T | 70–95 | 1–5 polytrichous appendages (width, 6–7 nm) plus additional thinner cell surface structures | |||
| T. radiotolerans | DSM 15228T | 55–95 | 25–95 | 20/30 | 19/34 | 1–3 curled flagella (width, 11–12 nm), plus thinner filaments (5–6 nm) in MAYTP |
| T. stetteri | DSM 5262T | 55–98 | 25–95 | 65/108 | 155/322 | Polar tuft of flagella (width, 10–12 nm) plus additional thinner filaments |
| T. thioreducens | DSM 14981T | 55–94 | 50–95 | 21/43 | 15/27 | Polar tuft of flagella (width, 10–12 nm) plus thinner filaments (6–7 nm) |
Determined at least in triplicate for cells grown in poor SMES medium and rich MAYTP medium. Swimming speeds at temperatures other than 85°C may be found in Fig. S1 in the supplemental material.
TEM analyses used at least 15 cells for each species; examples are given in Fig. S3 in the supplemental material.
It should be noted that the classification of three species is ambiguous. First, 16S rRNA gene analyses placed T. chitonophagus as a close relative of Pyrococcus furiosus. Second, T. guaymasensis possesses exactly the same 16S rRNA gene sequence as T. kodakarensis (our own data, not shown), but the two species differ with respect to motility (Table 1). Third, during the course of this study, T. radiotolerans was removed from the official DSMZ list of thermococci.
M. villosus was grown as described recently in MGG medium at 80°C under an H2–CO2 (80:20) atmosphere (12).
High-temperature light microscopy.
The original thermomicroscope (9) was used as described previously (10), including data acquisition and handling. Briefly, cells in the logarithmic growth phase were transferred to rectangular glass capillaries (order no. 5010-100; VitroCom, Mountain Lakes, NJ, USA), which were closed by cyanoacrylate glue (Pattex Uni-Rapide [Henkel AG, Düsseldorf, Germany] or UHU Super Power [UHU Gmbh, Bühl, Germany]), and motility was analyzed using an Olympus BX50 microscope. Movies were recorded with a pco.1600 camera (2-GB internal memory) and were analyzed using the open-source software ImageJ (version 1.410) with the add-on modules ParticleTracker and Manual Tracking. Tracks of at least 20 consecutive frames were used for calculations. The speeds of at least 10 different cells from one sample were averaged, and experiments were run in at least 3-fold repetitions in order to reduce experimental variation.
TGFD.
The temperature gradient-forming device (TGFD) is described in detail in Results. It can be placed on the XY stage of any (upright, phase-contrast) light microscope and therefore is generally applicable. The TGDF, including the controlling unit, will be made available at a price of ca. €3,000; details are available upon request. The system was calibrated by filling glass capillaries with solid chemicals, such as azobenzene and naphthalene, and observing their melting behavior in temperature gradients. For swimming analyses, a rectangular glass capillary was filled aerobically with cells grown to logarithmic phase and was placed in the milled groove of the TGFD. Movies were recorded and analyzed as described previously (10).
A minor disadvantage of the TGFD in its present form is its limitation to the use of air objectives. Experiments are under way to overcome this limitation so as to allow the use of electrically heated oil immersion objectives also, as in the original thermomicroscope (9). Such a high magnification will be needed, however, only for very special experimental setups. All the data given here were obtained with an unheated 40-fold magnifying objective.
Electron microscopy.
We used established procedures for electron microscopic investigations (30). Cells in the logarithmic growth phase were fixed with glutardialdehyde, carefully concentrated (at a maximum of 3,000 × g), and dropped onto copper grids (400 mesh). After washing and negative staining with uranyl acetate, specimens were analyzed for the presence of cell appendages by transmission electron microscopy (TEM).
RESULTS
The TGFD.
The original thermomicroscope (9) has worked reliably for more than 15 years, but experiments are quite time-consuming (ca. 30 min of heating time to reach the maximum temperature of 95°C) and are restricted to one preset temperature. In cooperation with the electronic workshop of the Faculty for Biology of the University of Regensburg, we developed a new apparatus, replacing the thermomicroscope. We call this low-budget device a “temperature gradient-forming device,” because it allows one to establish a temperature gradient of ca. 40°C over a distance of just 2 cm within a few minutes. The maximum temperature is 110°C; therefore, in the TGFD, we can mimic temperature gradients found in the natural habitat.
The TGFD itself (Fig. 1) is made from a stainless steel plate into which a rectangular groove is milled, to hold a rectangular glass capillary with a length of 3 cm and an inner width of 100 μm. After the capillary is filled with cells in growth medium by capillary action, it is closed at both ends by super glue. Anaerobiosis during filling was not necessary, since the redox indicator resazurin, present in the media, never changed its color to pink, which would have indicated aerobiosis. The rectangular groove of the TGFD has 5 observation holes over a distance of 2 cm. Temperature sensors are placed at the back side of the steel plate close to the holes to allow determination of the actual temperature at each observation hole. An electronic controller allows the setting of the two independent working heating elements, located to the left and right of the groove holding the capillary, to various temperatures. The actual temperature at the observation holes is measured by the heat sensors. Two larger holes allow the placement of the glass capillary (with a drop of super glue at both ends) directly into the groove to achieve optimal heat transfer. The whole setup is contained in a circuit board, which can be placed on the XY stage of a normal light microscope to convert it to a thermomicroscope.
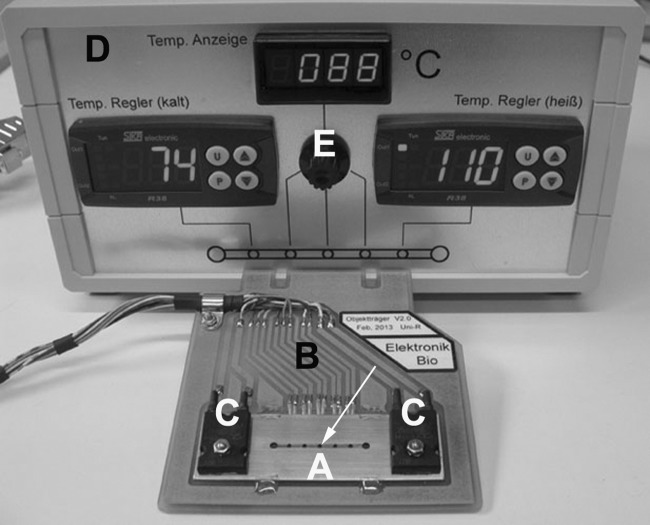
FIG 1.
The temperature gradient-forming device, including the control unit. This low-budget custom-made device consists of a stainless steel plate (A) located on a circuit board (B). Two independently working heating elements (C) located at the left and right ends of the steel plate allow heating up to 110°C. A rectangular glass capillary containing the cells to be analyzed is placed into the rectangular groove milled into the steel plate; the five small holes (the middle one is indicated by the arrow) for observation of cells are 5 mm apart each. On the back side of the circuit board, five temperature sensors are located close to the observation holes. The control unit (D) shows the actual temperatures at the left- and rightmost observation holes (here 74 and 110°C). The temperatures of the three middle observation holes (here 88°C for the middle hole indicated by the arrow) can be accessed using the black switch (E). The placement of the circuit board onto the xy-stage of any conventional upright light microscope converts it into a thermomicroscope.
Swimming speed and temperature range for swimming observed for various thermococci.
The data obtained for the 15 selected species are given in Table 1. We want to stress especially the following points.
(i) The temperature ranges for swimming differ greatly among the various species. Most species exhibited swimming within an extremely broad temperature range, with 25°C as the lowest and 95°C as the highest temperature. The swimming speed at the lowest temperature, however, was severely reduced from that at higher temperatures in all cases (see Fig. S1 in the supplemental material).
(ii) For the four species T. aegaeus, T. alcaliphilus, T. coalescens, and T. thioreducens, the temperature range of swimming resembled the temperature range of growth more closely than for the other motile species (Table 1).
(iii) Our data clearly prove that no universal statement can be made regarding a temperature dependence of the swimming speed. Cells of T. stetteri exhibited such dependence in a very pronounced way, as did T. celer in MAYTP medium. Very interestingly, however, T. celer did not show such dependence in SMES medium (see Fig. S1 in the supplemental material).
(iv) Swimming speeds differ greatly among the various species. T. aegaeus is one of the slowest swimmers, and T. stetteri is a fast swimmer (at 85°C), with average velocities of 16 and 155 μm/s, respectively (Table 1). As mentioned above, the very fast swimming species T. chitonophagus (213 μm/s) might be reclassified in future work as a species of the genus Pyrococcus.
(v) The growth medium had a strong influence on swimming. Very interestingly, the motility of the three species T. atlanticus, T. coalescens, and T. guaymasensis was completely repressed in MAYTP medium. On the other hand, MAYTP medium led to >2-fold increases in the swimming speeds of T. celer and T. stetteri.
(vi) We noted, furthermore, that species with a lower motility limit of ca. 50°C showed a preference for “zigzag movement” (which we have argued earlier is a seeking mode of cells [10] enabling them finally to adhere to surfaces) at this low temperature; these species were swimming in straight lines only to a limited degree. The zigzag swimming at low temperatures was observed not only for cells located close to the walls of the glass capillaries but also for cells in the middle focus plane of the capillary, i.e., at a distance of ca. 50 μm from the glass wall.
(vii) Since a temperature gradient can be established in the TGFD, we were able to ask if cells would actively swim to certain temperature regions, i.e., if they would show thermotaxis. The first data obtained for T. stetteri indicated such behavior (see Fig. S2 and accompanying text in the supplemental material).
Numbers and kinds of cell appendages in the selected Thermococcus species.
In addition to the swimming studies, cells of all selected species of the genus Thermococcus were grown to logarithmic phase in SMES and MAYTP media and were analyzed by transmission electron microscopy. We found that all of them possess cell appendages (except for T. acidaminovorans), which differed in number from one single filament (e.g., T. radiotolerans) to multiple filaments, as in T. stetteri (Table 1; see also Fig. S3 in the supplemental material). The diameters of the cell appendages were either in the range of 9 to 12 nm, corresponding to the width of archaeal flagella, or in the range of 5 to 8 nm. We continue here to call these motility structures archaeal flagella. It has been suggested recently to rename them archaella (31), but this has been called into question (32). The thinner filaments were the only kind of cell appendage in T. gorgonarius (as many as 30 polytrichous appendages) and T. pacificus (1 to 5 appendages) or were, rarely, discovered together with flagella (representing at most 10% of total cell appendages) in T. atlanticus, T. guaymasensis, T. radiotolerans, T. stetteri, and T. thioreducens. Interestingly, the occurrence/number of cell appendages differed for some species depending on the growth medium; T. atlanticus, for example, possessed cell appendages in SMES medium but not in MAYTP medium.
Reactions of hyperthermophilic Archaea, especially Methanocaldococcus villosus, to changes in temperature.
To investigate the direct reactions of thermococci to high temperatures, we preheated the TGFD to 85°C, loaded a glass capillary into it, and immediately conducted light microscopic analysis. From those experiments, it became clear that cells reacted within less than 5 s to a shift from room temperature to 85°C by starting to swim (data not shown). Considering that the heating of the liquid in the glass capillary takes a short time, we feel confident in stating that the exposure of cells to their optimal growth temperature results in their immediate ability to move.
In addition to the various species of the genus Thermococcus, we used M. villosus to study the reaction to changes in temperature in more detail. An experiment in which the glass capillary was heated from ca. 20°C (a temperature supporting neither motility [10] nor the growth of this hyperthermophile [12]) to 90°C (a temperature supporting motility and growth) is presented in Movie S1 in the supplemental material. At a heating rate of ca. 5°C/s, the first motile cells are observed after ca. 10 s, i.e., at ca. 70°C. We conclude from these data that cells can react immediately to temperatures supporting their life, by starting motility. We extended these studies, not only using freshly grown cells but also “ageing” a culture of M. villosus for various times via storage at 6°C. This treatment was taken as a proxy for the conditions that cells experience in the ocean: after being swept away from their biotope, e.g., hydrothermal vents or black smokers (at high temperatures), cells are thought to stay in normal (low-temperature) seawater in a kind of hibernating stage. How fast then, would they react to high temperatures, indicating their presence in new, life-supporting surroundings?
It turned out that cells can react immediately to such high temperatures, even after 9 months of storage in the cold. Movie S2 in the supplemental material shows the reaction of M. villosus cells that had been stored in the cold for 6 months to heating from ca. 20°C to 90°C. For a culture in which ca. 50% of all cells showed good motility at 80°C, “cold ageing” resulted in ca. 30% motile cells after 1 day, ca. 20% motile cells after 7 days, ca. 10% motile cells after 1 and 3 months, and 5% motile cells after 6 and 9 months. Movie S3 in the supplemental material shows the motility of cells in a temperature gradient from 90 to 140°C (the highest temperature we could reach using a “zero model” of the TGFD), in the same capillary as that analyzed in Movie S2 in the supplemental material, after an additional incubation for 5 min at 90°C. Interestingly, not only the percentage of motile M. villosus cells but also their swimming speeds increased (from ∼100 μm/s at 90°C to ∼375 μm/s at 140°C) when they were subjected to such nonphysiologically high temperatures.
DISCUSSION
Microorganisms living in hydrothermal vent systems encounter special problems: the vent fluids, with temperatures as high as 400°C, are far too hot to allow growth, but the surrounding seawater, at 2 to 3°C, is far too cold to support the physiological needs of these microorganisms. Furthermore, the vent fluids emanate from the sea ground at a velocity of ca. 1 m/s; for a microorganism with a diameter of 1 μm, this translates into ca. 106 body lengths/s. If a hyperthermophile were translocated for just 1 s in such a fluid stream and could swim directionally back to its original location at a speed of 100 μm/s, it would need 10,000 s (nearly 3 h) to reach its original location. Such movement, however, would take place in aerobic, cold seawater, which is detrimental to its physiology. How then can hyperthermophiles cope with these conditions?
Reactions to changes in temperature.
We have shown here that hyperthermophiles can react very rapidly to changes in temperature. In particular, their ability to start active movement the moment they encounter temperatures close to their growth temperature is advantageous. These temperatures can indicate to the microorganisms their presence in a habitat potentially supporting life. But as outlined above, such surroundings also pose two potential risks for the life of hyperthermophiles: temperatures might rise so high as to be lethal, or cells might be swept away into cold, aerobic seawater. We have shown here that at least M. villosus can react to extremely high temperatures, such as 140°C, by very fast swimming for a short time. We cannot determine whether this “hypermotility” represents a thermodynamic effect (the cellular machinery works faster at a higher temperature) or a true emergency escape reaction. In either case, however, the result would be the same, namely, the ability to swim away from deadly high temperatures.
The ability to move actively via flagella in hot surroundings is obviously advantageous for hyperthermophiles. Their motility organelles can be used for two different swimming modes (10): either rapid, more or less straight movements to cover long distances or a slow “zigzag seeking mode.” In addition, flagella can also be used for adhesion to surfaces (11, 12). Taking the data together, we argue that hyperthermophiles can react rapidly to their (novel) presence in hot surroundings by actively swimming to a region with a temperature that supports their life and staying there via adhesion.
Swimming speeds and temperature ranges of swimming observed for various species of the genus Thermococcus.
The genus Thermococcus, consisting exclusively of hyperthermophilic species, is very abundant in marine habitats. Its members are characterized as strictly anaerobic obligate and facultative sulfur respirers occurring in marine and, less frequently, terrestrial thermal environments. The cocci are described as nonmotile or motile with polar tufts of flagella and as exhibiting optimal growth at temperatures between 75 and 88°C (33). Indeed, a total of 25 validly described Thermococcus species were available in 2012 from the DSMZ alone, while ca. 260 isolates and 782 ribotypes have been described (http://www.bacterio.net/). Hence, the 15 species selected for this study represent the physiological spectrum of this genus over its complete range. Their average swimming speeds, 16 to 155 μm/s, are in a medium range for Archaea. The slowest Archaea (ca. 5 μm/s) have been reported to be Halobacterium salinarum (10) and Haloferax volcanii (34), while the fastest Archaea (500 to 600 μm/s) belong to the genus Methanocaldococcus (10).
The temperature ranges of swimming for the various species of the genus Thermococcus differ considerably, with some species showing slow, but clearly measurable, motility even at 25°C. The cells swim fastest at very high temperatures, and the upper limit for motility, 95°C, is above the maximum growth temperature. Even faster, but only short-term (1 to 3 min), motility was observed for M. villosus at the extraordinarily high temperature of 140°C; thereafter, cells died off.
In our view. both the swimming speed and the temperature range of swimming that we observed are advantageous for hyperthermophilic microorganisms. The ability to swim fastest at very high temperatures would allow them to escape from deadly high-temperature surroundings. Swimming at temperatures lower than those required for cell division can also “make sense,” since it enables cells to return to temperatures supporting life. Very fast swimming at low temperatures, on the other hand, would be very costly energetically and would deprive cells of ATP, which—in contrast to the swimming of Bacteria—is used to rotate archaeal flagella (35). It seems to us that energy (ATP) limits the swimming speed in some, but not all, thermococci. For example, we argue that temperature limits ATP availability in T. stetteri but not in T. celer, resulting in the temperature-independent speed of T. celer in SMES medium (see Fig. S1 in the supplemental material). In the case of T. stetteri, an increase in temperature leads to an increase in ATP production and therefore in swimming speed. Indeed, an Arrhenius plot (data not shown) clearly shows a linear relationship between ln[speed] and 1/T for T. stetteri, indicative of the dependence of its swimming speed on temperature.
In addition, we note that the number of “true flagella” per cell correlates with the ability of the species tested to move and, to a certain extent, with the swimming speed. For cells of T. gorgonarius and T. pacificus, which possessed only appendages with diameters of 5 to 8 nm, no swimming motility was detected in any experiment. T. atlanticus, T. coalescens, and T. guaymasensis expressed flagella in SMES medium but not in MAYTP medium, in agreement with the observation that swimming was detected only in SMES medium. Species for which we observed the presence of a polar tuft of flagella (more than ca. 20 flagella/cell) were found to be faster than species possessing 1 to 5 flagella per cell.
Conclusions.
The TGFD presented here has the following advantages for the study of the behavior of hyperthermophiles: (i) it can be added to any upright light microscope; (ii) the device can be heated up to 110°C; (iii) cells can be analyzed in parallel at 5 different temperatures differing by as much as 40°C; (iv) heating rates allow analyses at rapidly changing temperatures; (v) the time required for motility analyses is more than 5-fold shorter than that with the original thermomicroscope.
Our data obtained with the TGFD show clearly that statements on motility should not and cannot be made if cells are analyzed under nonphysiological conditions. Light microscopy at 20°C under aerobic conditions would have indicated that all 15 species of the genus Thermococcus analyzed here were immotile. Our swimming studies, performed anaerobically at the appropriate temperatures, identified only 3 of them as truly immotile. For other reasons also, caution should be exercised in making absolute statements regarding motility. Very clearly, for example, T. guaymasensis was motile only in SMES medium (SME medium with added S0), not in MAYTP medium or in MAYTP medium with added S0.
Furthermore, we argue that to describe cell appendages as flagella simply because long, thin filaments are present on cells is inaccurate. Without functional studies—i.e., light microscopic observation at high temperatures under anaerobic conditions—the presence of such cell appendages can be misleading. We have shown here that the thin filaments of T. gorgonarius described previously (24) are not used for swimming. According to our measurements, they have a diameter of ca. 5 to 8 nm, much too small for true archaeal flagella. Even a diameter of 10 to 14 nm, considered characteristic of archaeal flagella (in contrast to archaeal fimbriae, with a diameter of ca. 5 nm [36]), is not sufficient to define cell appendages as motility organelles. The fibers of Ignicoccus hospitalis, e.g., have a diameter of 14 nm, but this archaeon was demonstrated to be immotile (37).
Finally, and most importantly, our data prove that anaerobic (hyper)thermophiles are extremely well adapted to their special biotopes because they can react immediately to a rise in temperature by starting motility; they are able to swim rapidly to a region of adequate temperature; and they show two different swimming modes (10), enabling them to use their motility organelles for adhesion as well. Differences in temperature ranges of motility and swimming speeds might very well reflect the various strategies used by different species to meet the requirements for survival in their biotopes. In this respect, it might be interesting to conduct further experiments to analyze the colonization of an artificial biotope under various conditions, such as a temperature gradient, by a mixture of Thermococcus species.
Supplementary Material
ACKNOWLEDGMENTS
The expert technical help of E. Papst is gratefully acknowledged. We especially thank A. Probst for help with phylogenetic analyses and R. Rachel for providing equipment for electron microscopy.
Footnotes
Published ahead of print 23 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00984-14.
REFERENCES
- 1.Ford BJ. 2011. The clarity of images from early single-lens microscopes captured on video. Microsc. Anal. 2011(March):15–17 [Google Scholar]
- 2.Keller P, Schmidt A, Marshall G, Yoshida K, Saar BG, Zeng Y, Willig K, Nägerl V, Urban N, Bonhoeffer T, Hell SW. 2009. Magnifying power. Nature 459:629. 10.1038/459629a [DOI] [PubMed] [Google Scholar]
- 3.Kasper R, Huang B. 2011. Light microscopy. Cell 147:1198. 10.1016/j.cell.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 4.Coltharp C, Xiao J. 2012. Superresolution microscopy for microbiology. Cell. Microbiol. 14:1808–1818. 10.1111/cmi.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stetter KO. 1995. Microbial life in hyperthermal environments. ASM News 61:285–290 [Google Scholar]
- 6.Takai K, Komatsu T, Inagaki F, Horikoshi K. 2001. Distribution of archaea in a black smoker chimney structure. Appl. Environ. Microbiol. 67:3618–3629. 10.1128/AEM.67.8.3618-3629.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blöchl E, Rachel R, Burggraf S, Hafenbradl D, Jannasch HW, Stetter KO. 1997. Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113°C. Extremophiles 1:14–21. 10.1007/s007920050010 [DOI] [PubMed] [Google Scholar]
- 8.Stetter KO. 2006. Hyperthermophiles in the history of life. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361:1837–1843. 10.1098/rstb.2006.1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horn C, Paulmann B, Junker G, Huber H. 1999. In vivo observation of cell division of anaerobic hyperthermophiles by using a high-intensity dark-field microscope. J. Bacteriol. 181:5114–5118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzog B, Wirth R. 2012. Swimming behavior of selected species of Archaea. Appl. Environ. Microbiol. 78:1670–1674. 10.1128/AEM.06723-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Näther DJ, Rachel R, Wanner G, Wirth R. 2006. Flagella of Pyrococcus furiosus: multifunctional organelles, made for swimming, adhesion to various surfaces, and cell-cell contacts. J. Bacteriol. 188:6915–6923. 10.1128/JB.00527-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellack A, Huber H, Rachel R, Wanner G, Wirth R. 2011. Methanocaldococcus villosus sp. nov., a heavily flagellated archaeon that adheres to surfaces and forms cell-cell contacts. Int. J. Syst. Evol. Microbiol. 61:1239–1245. 10.1099/ijs.0.023663-0 [DOI] [PubMed] [Google Scholar]
- 13.Stetter KO, König H, Stackebrandt E. 1983. Pyrodictium gen. nov., a new genus of submarine disc-shaped sulphur reducing archaebacteria growing optimally at 105°C. Syst. Appl. Microbiol. 4:535–551. 10.1016/S0723-2020(83)80011-3 [DOI] [PubMed] [Google Scholar]
- 14.Atomi H, Fukui T, Kanai T, Morikawa M, Imanaka T. 2004. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1:263–267. 10.1155/2004/204953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dirmeier R, Keller M, Hafenbradl D, Braun F-J, Rachel R, Burggraf S, Stetter KO. 1998. Thermococcus acidaminovorans sp. nov., a new hyperthermophilic alkalophilic archaeon growing on amino acids. Extremophiles 2:109–114. 10.1007/s007920050049 [DOI] [PubMed] [Google Scholar]
- 16.Arab H, Völker H, Thomm M. 2000. Thermococcus aegaeicus sp. nov. and Staphylothermus hellenicus sp. nov., two novel hyperthermophilic archaea isolated from geothermally heated vents off Palaeochori Bay, Milos, Greece. Int. J. Syst. Evol. Microbiol. 50:2101–2108. 10.1099/00207713-50-6-2101 [DOI] [PubMed] [Google Scholar]
- 17.Keller M, Braun FJ, Dirmeier R, Hafenbradl D, Burggraf S, Rachel R, Stetter KO. 1995. Thermococcus alcaliphilus sp. nov., a new hyperthermophilic archaeum growing on polysulfide at alkaline pH. Arch. Microbiol. 164:390–395. 10.1007/BF02529736 [DOI] [PubMed] [Google Scholar]
- 18.Cambon-Bonavita MA, Lesongeur F, Pignet P, Wery N, Lambert C, Godfroy A, Querellou J, Barbier G. 2003. Thermococcus atlanticus sp. nov., a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent in the Mid-Atlantic Ridge. Extremophiles 7:101–109 [DOI] [PubMed] [Google Scholar]
- 19.Zillig W, Holz I, Janekovic D, Schäfer W, Reiter WD. 1983. The archaebacterium Thermococcus celer represents a novel genus within the thermophilic branch of the archaebacteria. Syst. Appl. Microbiol. 4:88–94. 10.1016/S0723-2020(83)80036-8 [DOI] [PubMed] [Google Scholar]
- 20.Kuwabara T, Minaba M, Ogi N, Kamakura M. 2007. Thermococcus celericrescens sp. nov., a fast-growing and cell-fusing hyperthermophilic archaeon from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 57:437–443. 10.1099/ijs.0.64597-0 [DOI] [PubMed] [Google Scholar]
- 21.Huber R, Stöhr J, Hohenhaus S, Rachel R, Burggraf S, Jannasch HW, Stetter KO. 1995. Thermococcus chitonophagus sp. nov., a novel, chitin-degrading, hyperthermophilic archaeum from a deep-sea hydrothermal vent environment. Arch. Microbiol. 164:255–264. 10.1007/BF02529959 [DOI] [Google Scholar]
- 22.Kuwabara T, Minaba M, Iwayama Y, Inouye I, Nakashima M, Marumo K, Maruyama A, Sugai A, Itoh T, Ishibashi J, Urabe T, Kamekura M. 2005. Thermococcus coalescens sp. nov., a cell-fusing hyperthermophilic archaeon from Suiyo Seamount. Int. J. Syst. Evol. Microbiol. 55:2507–2514. 10.1099/ijs.0.63432-0 [DOI] [PubMed] [Google Scholar]
- 23.Miroshnichenko ML, Gongadze GM, Rainey FA, Kostyukova AS, Lysenko AM, Chernyh NA, Bonch-Osmolovskaya EA. 1998. Thermococcus gorgonarius sp. nov. and Thermococcus pacificus sp. nov.: heterotrophic extremely thermophilic archaea from New Zealand submarine hot vents. Int. J. Syst. Bacteriol. 48:23–29. 10.1099/00207713-48-1-23 [DOI] [PubMed] [Google Scholar]
- 24.Canganella F, Jones WJ. 1994. Microbial characterization of thermophilic Archaea isolated from the Guaymas Basin hydrothermal vent. Curr. Microbiol. 28:299–306. 10.1007/BF01573210 [DOI] [PubMed] [Google Scholar]
- 25.Canganella F, Jones WJ, Gamabacorta A, Antranikian G. 1998. Thermococcus guaymasensis sp. nov. and Thermococcus aggregans sp. nov., two novel thermophilic archaea isolated from the Guaymas Basin hydrothermal vent site. Int. J. Syst. Bacteriol. 48:1181–1185. 10.1099/00207713-48-4-1181 [DOI] [PubMed] [Google Scholar]
- 26.Santangelo TJ, Čuboňová L, Reeve JN. 2010. Thermococcus kodakarensis genetics: TK1827-encoded β-glycosidase, new positive-selection protocol, and targeted and repetitive deletion technology. Appl. Environ. Microbiol. 76:1044–1052. 10.1128/AEM.02497-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jolivet E, Corre E, L'Haridon S, Forterre P, Prieur D. 2004. Thermococcus marinus sp. nov. and Thermococcus radiotolerans sp. nov., two hyperthermophilic archaea from deep-sea hydrothermal vents that resist ionizing radiation. Extremophiles 8:219–227. 10.1007/s00792-004-0380-9 [DOI] [PubMed] [Google Scholar]
- 28.Miroshnichenko ML, Bonch-Osmolovskaya EA, Neuner A, Kostrikina NA, Chernych NA, Alekseev VA. 1989. Thermococcus stetteri sp. nov., a new extremely thermophilic marine sulfur-metabolizing archaebacterium. Syst. Appl. Microbiol. 12:257–262. 10.1016/S0723-2020(89)80071-2 [DOI] [Google Scholar]
- 29.Pikuta EV, Marsic D, Itoh T, Bej AK, Tang J, Whitman WB, Ng JD, Garriott OK, Hoover RB. 2007. Thermococcus thioreducens sp. nov., a novel hyperthermophilic, obligately sulfur-reducing archaeon from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 57:1612–1618. 10.1099/ijs.0.65057-0 [DOI] [PubMed] [Google Scholar]
- 30.Rachel R, Meyer C, Klingl A, Gürster S, Heimerl T, Wasserburger N, Burghardt T, Küper U, Bellack A, Schopf S, Wirth R, Huber H, Wanner G. 2010. Analysis of the ultrastructure of archaea by electron microscopy. Methods Cell Biol. 96:47–69. 10.1016/S0091-679X(10)96003-2 [DOI] [PubMed] [Google Scholar]
- 31.Jarrell KF, Albers S-V. 2012. The archaellum: an old motility structure with a new name. Trends Microbiol. 20:307–312. 10.1016/j.tim.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 32.Wirth R. 2012. Response to Jarrell and Albers: seven letters less does not say more. Trends Microbiol. 20:511–512. 10.1016/j.tim.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 33.Zillig W, Reysenbach A-L. 2001. Class IV. Thermococci class. nov., p 341–348 In Boone DR, Castenholz RW, Garrity GM. (ed), Bergey's manual of systematic bacteriology, 2nd ed. Springer, New York, NY [Google Scholar]
- 34.Tripepi M, Esquivel RN, Wirth R, Pohlschröder M. 2013. Haloferax volcanii cells lacking the flagellin FlgA2 are hypermotile. Microbiology 159:2249–2258. 10.1099/mic.0.069617-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streif S, Staudinger WF, Marwan W, Oesterhelt D. 2008. Flagellar rotation in the archaeon Halobacterium salinarum depends on ATP. J. Mol. Biol. 384:1–8. 10.1016/j.jmb.2008.08.057 [DOI] [PubMed] [Google Scholar]
- 36.Thoma C, Frank M, Rachel R, Schmid S, Näther D, Wanner G, Wirth R. 2008. The Mth60 fimbriae of Methanothermobacter thermoautotrophicus are functional adhesins. Environ. Microbiol. 10:2785–2795. 10.1111/j.1462-2920.2008.01698.x [DOI] [PubMed] [Google Scholar]
- 37.Müller D, Meyer C, Gürster S, Küper U, Huber H, Rachel R, Wanner G, Wirth R, Bellack A. 2009. The Iho670 fibers of Ignicoccus hospitalis: a new type of archaeal cell surface appendage. J. Bacteriol. 191:6465–6468. 10.1128/JB.00858-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.