Abstract
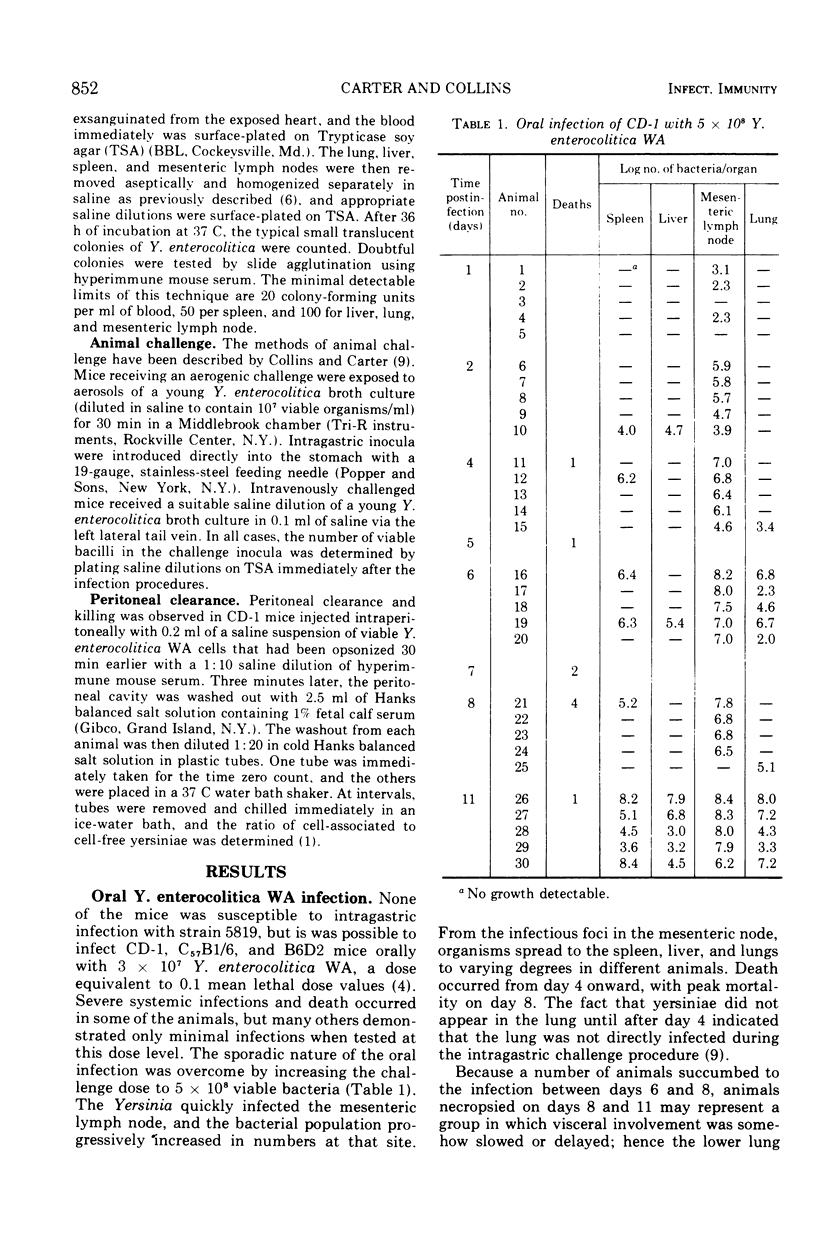
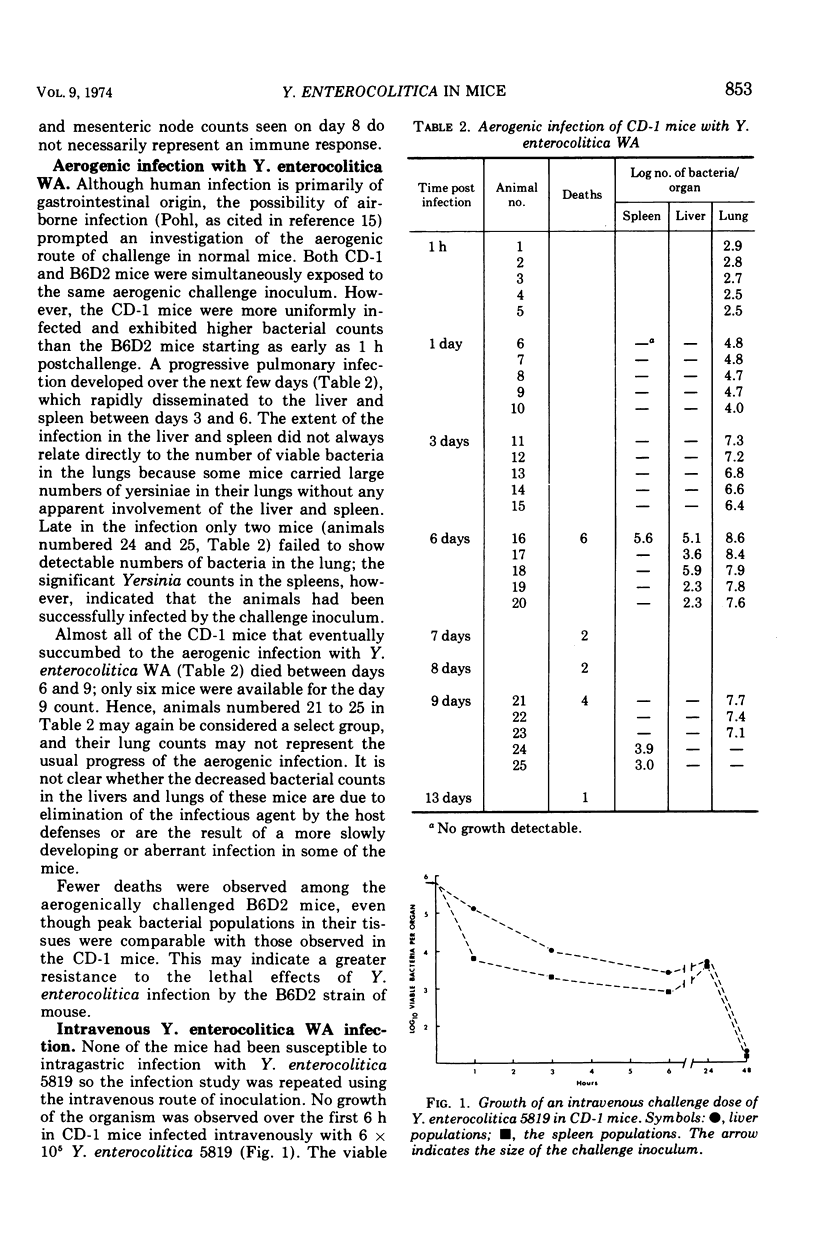
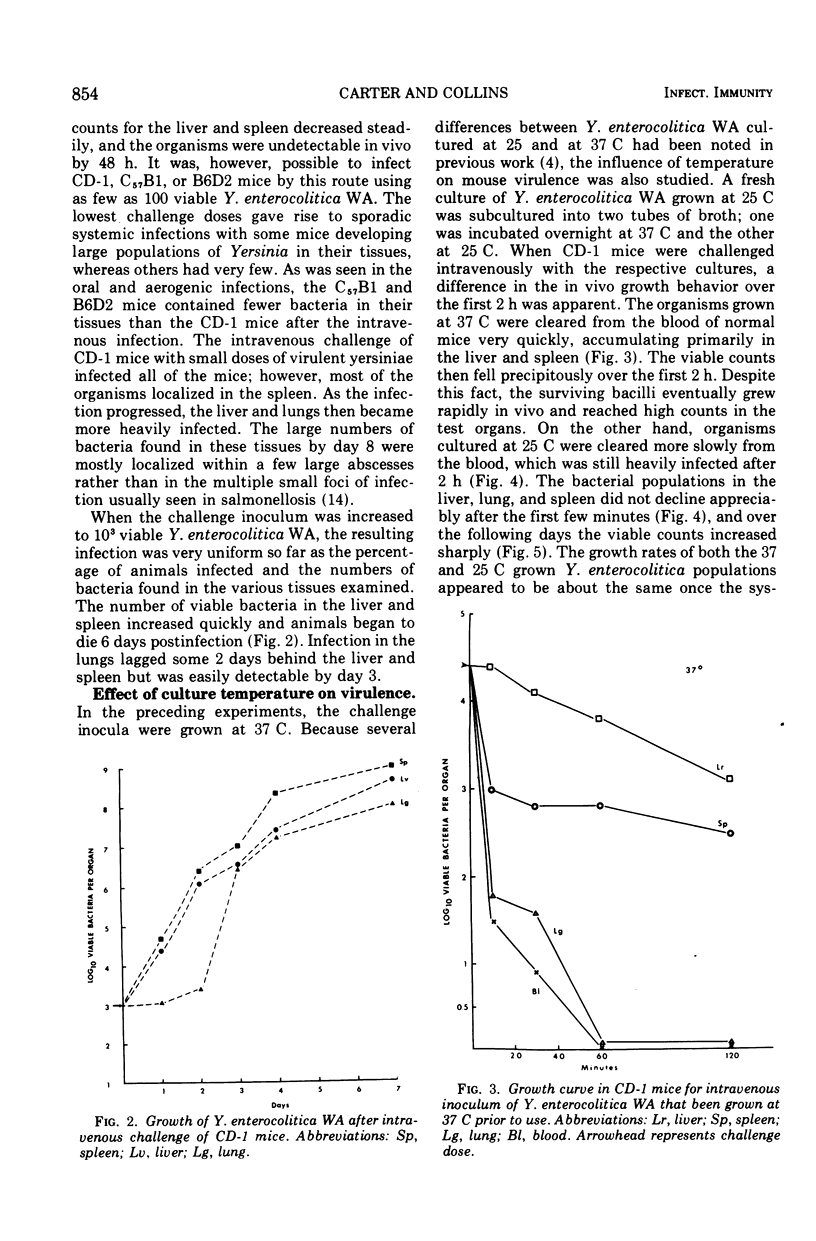
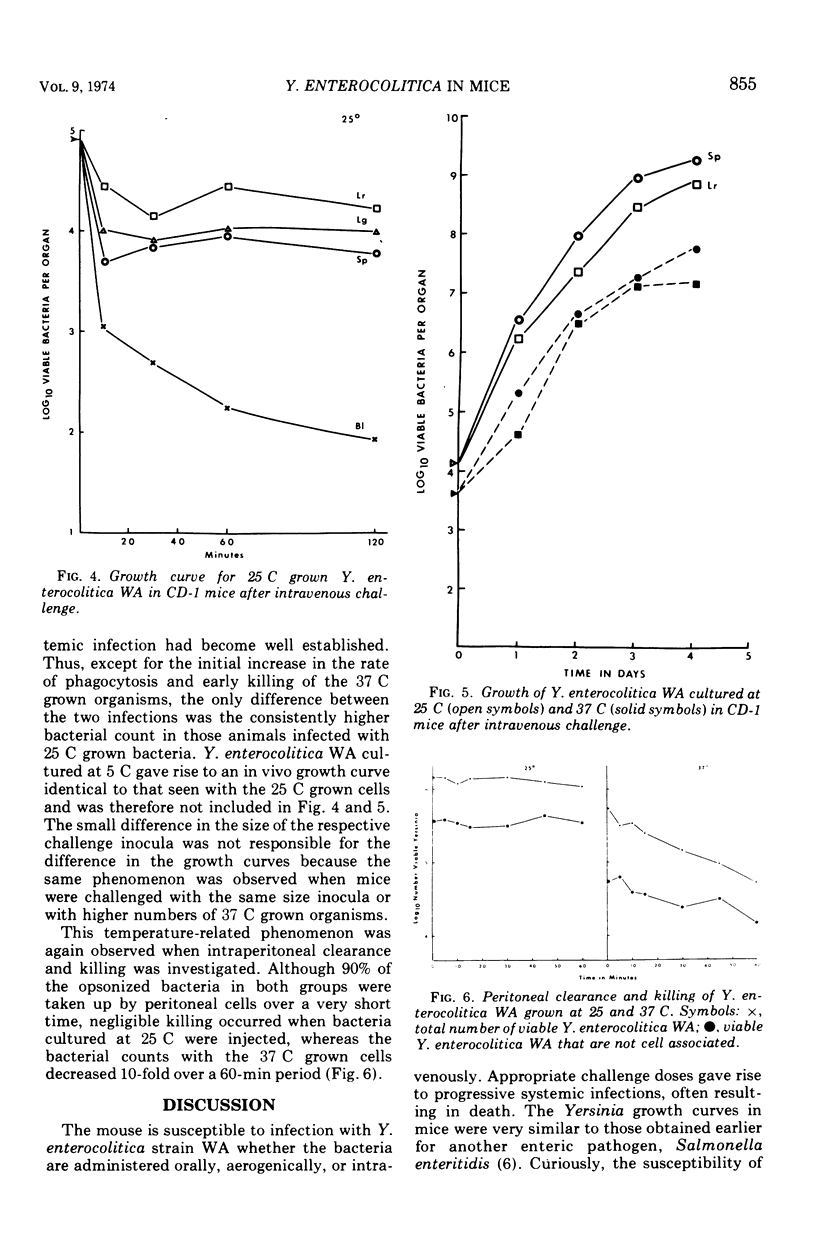
Infection of several strains of laboratory mice with a virulent strain of Yersinia enterocolitica was followed by performing viable bacterial counts on homogenates of selected tissues at intervals after intragastric, aerogenic, or intravenous infection. It is observed that CD-1 mice are more susceptible to Y. enterocolitica infection than either the C57B1/6 or B6D2 strains. Development of an enteric infection is dose dependent; less than 5 × 107 organisms by mouth yields sporadic, low levels of systemic infection, with many of the animals showing no apparent infection. Increasing the challenge inoculum by a factor of 10 eliminates the variability among the animals, giving rise to an enteric infection in all of the mice that moves quickly to the mesenteric lymph node. The bacterial population in the lymph node multiplies rapidly, and the infection is disseminated to the spleen, liver, and lungs, ultimately killing most of the animals. Exposure to an aerogenic challenge of less than 1,000 organisms resulted in a fulminating pneumonitis with an invariably fatal outcome. Intravenous challenge with 500 organisms caused a rapidly fatal, systemic infection. The growth of the bacteria in the intravenously infected mouse depends upon the temperature at which the challenge inoculum had been grown in vitro. At temperatures below 26 C, the bacteria are cleared from the blood at a slower rate and are more resistant to intracellular killing, as compared to organisms grown at 37 C. This effect results in the inoculum increasing to greater numbers in the tissues in a shorter period of time.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON G. A., BURROWS T. W. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956 Oct;37(5):481–493. [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. The genus Yersinia: biochemistry and genetics of virulence. Curr Top Microbiol Immunol. 1972;57:111–158. doi: 10.1007/978-3-642-65297-4_4. [DOI] [PubMed] [Google Scholar]
- CAVANAUGH D. C., RANDALL R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959 Oct;83:348–363. [PubMed] [Google Scholar]
- Carter P. B., Varga C. F., Keet E. E. New strain of Yersinia enterocolitica pathogenic for rodents. Appl Microbiol. 1973 Dec;26(6):1016–1018. doi: 10.1128/am.26.6.1016-1018.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Carter P. B. Comparative immunogenicity of heat-killed and living oral Salmonella vaccines. Infect Immun. 1972 Oct;6(4):451–458. doi: 10.1128/iai.6.4.451-458.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Immunity to enteric infection in mice. Infect Immun. 1970 Mar;1(3):243–250. doi: 10.1128/iai.1.3.243-250.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Mechanisms in antimicrobial immunity. J Reticuloendothel Soc. 1971 Jul;10(1):58–99. [PubMed] [Google Scholar]
- Collins F. M. Salmonellosis in orally infected specific pathogen-free C57B1 mice. Infect Immun. 1972 Feb;5(2):191–198. doi: 10.1128/iai.5.2.191-198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN W. A., LAWTON W. D., FUKUI G. M., SURGALLA M. J. THE PATHOGENESIS OF PLAGUE. I. A STUDY OF THE CORRELATION BETWEEN VIRULENCE AND RELATIVE PHAGOCYTOSIS RESISTANCE OF SOME STRAINS OF PASTEURELLA PESTIS. J Infect Dis. 1963 Sep-Oct;113:139–143. doi: 10.1093/infdis/113.2.139. [DOI] [PubMed] [Google Scholar]
- Janssen W. A., Surgalla M. J. Plague bacillus: survival within host phagocytes. Science. 1969 Feb 28;163(3870):950–952. doi: 10.1126/science.163.3870.950. [DOI] [PubMed] [Google Scholar]
- Knapp W., Fahrländer H., Hartweg H. Zur Atiologie der akuten regionären Enteritis (Ileitis. Schweiz Med Wochenschr. 1970 Feb 21;100(8):364–368. [PubMed] [Google Scholar]
- Knapp W., Lysy J., Knapp C., Stille W., Goll U. Enterale Infektionen beim Menschen durch Yersinia enterocolitica und ihre Diagnose. Infection. 1973;1(2):113–125. doi: 10.1007/BF01638486. [DOI] [PubMed] [Google Scholar]
- MOLLARET H. H., CHEVALIER A. CONTRIBUTION 'A L''ETUDE D'UN NOUVEAU GROUPE DE GERMES PROCHES DU BACILLE DE MALASSEZ ET VIGNAL. I. CARACT'ERES CULTURAUX ET BIOCHIMIQUES. Ann Inst Pasteur (Paris) 1964 Jul;107:121–127. [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V., Collins F. M. Host-parasite relations in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollaret H. H., Guillon J. C. Contribution à l'étude d'un nouveau groupe de germes (Yersinia enterocolitica) proches du bacille de Malassez et Vignal. II. Pouvoir pathogène expérimental. Ann Inst Pasteur (Paris) 1965 Oct;109(4):608–613. [PubMed] [Google Scholar]
- Mollaret H. H. L'infection humaine a "Yersinia enterocolitica" en 1970, a la lumière de 642 cas récents. Aspects cliniques et perspectives épidémiologiques. Pathol Biol (Paris) 1971 Feb;19(3):189–205. [PubMed] [Google Scholar]


