Abstract
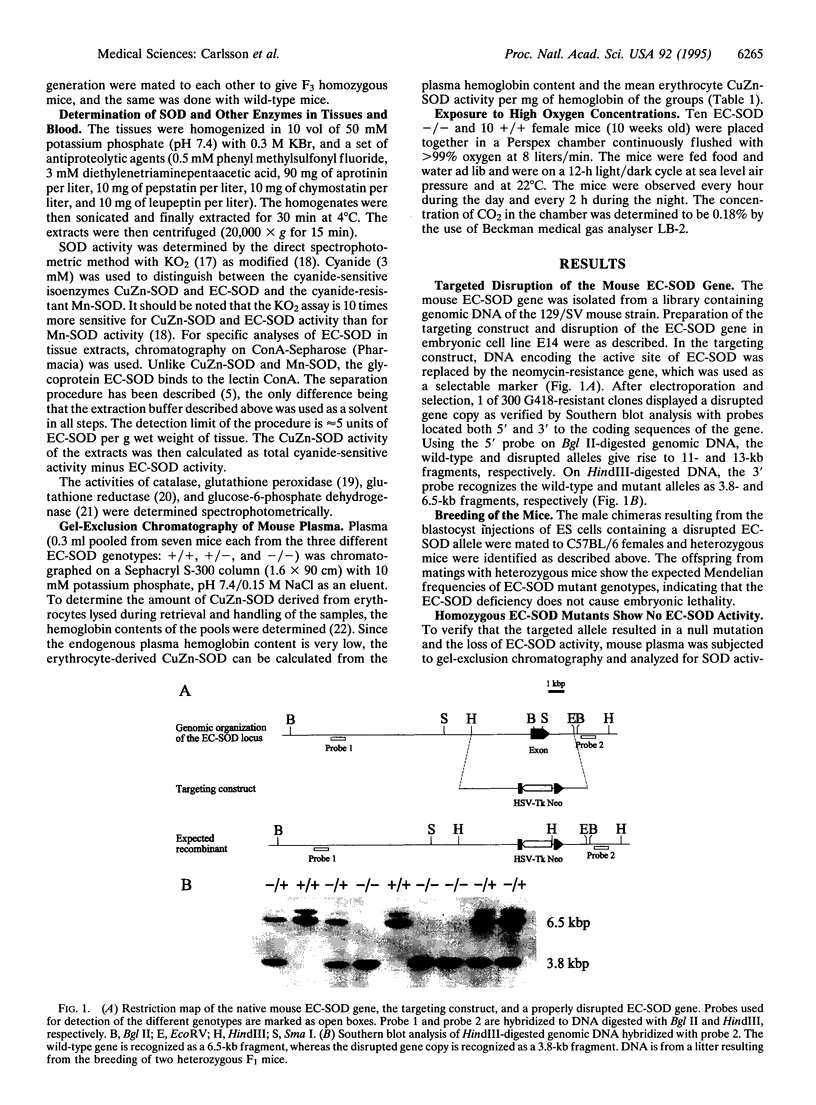
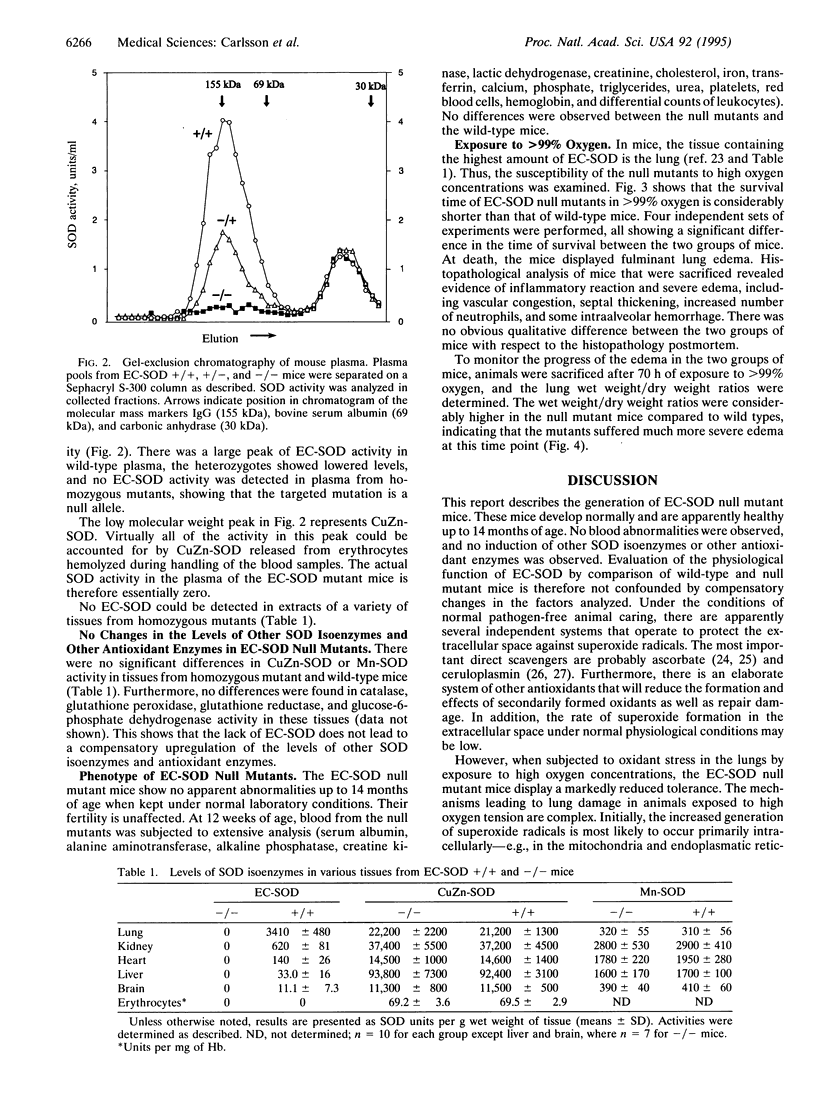
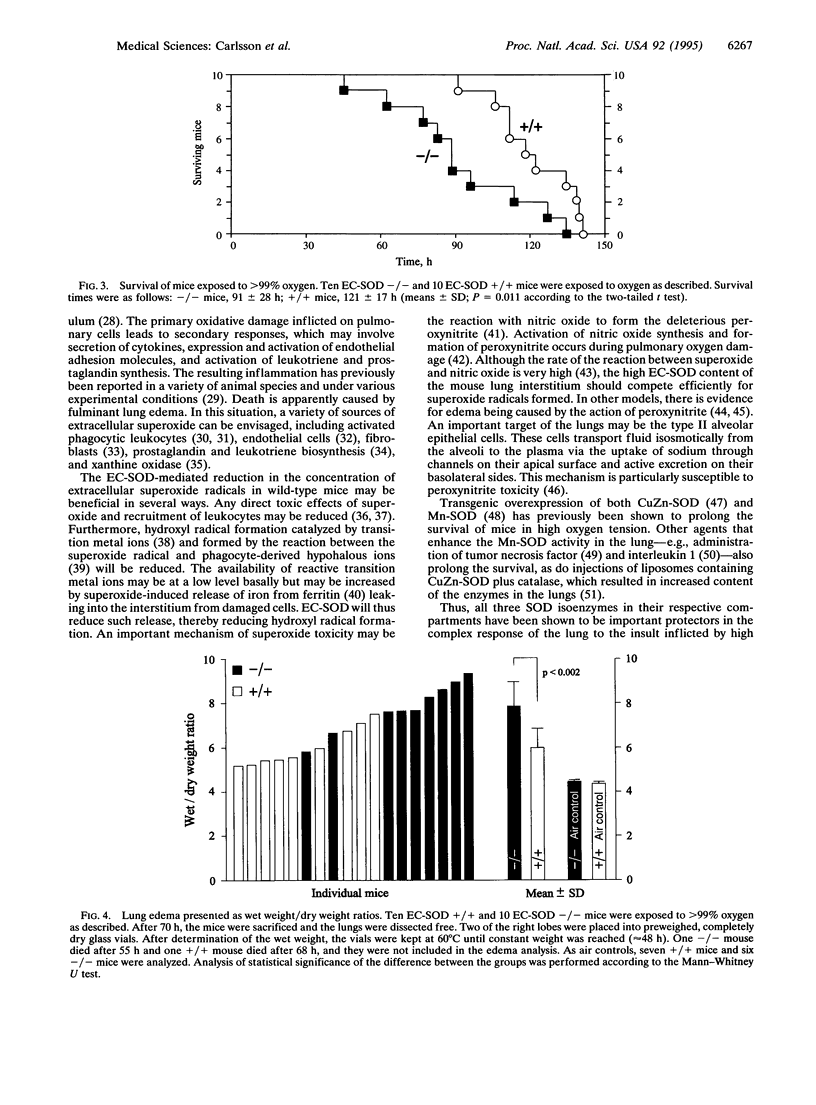
Extracellular superoxide dismutase (EC-SOD; superoxide:superoxide oxidoreductase, EC 1.15.1.1) is a secreted Cu- and Zn-containing tetrameric glycoprotein, the bulk of which is bound to heparan sulfate proteoglycans in the interstitium of tissues. To test the function of EC-SOD in vivo, mice carrying a targeted disruption of the EC-SOD gene were generated. The EC-SOD null mutant mice develop normally and remain healthy until at least 14 months of age. No compensatory induction of other SOD isoenzymes or other antioxidant enzymes was observed. When stressed by exposure to > 99% oxygen, the EC-SOD null mutant mice display a considerable reduction in survival time compared to wild-type mice and an earlier onset of severe lung edema. These findings suggest that while under normal physiological conditions other antioxidant systems may substitute for the loss of EC-SOD; when the animal is stressed these systems are unable to provide adequate protection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkstén B., Marklund S., Hägglöf B. Enzymes of leukocyte oxidative metabolism in Down's syndrome. Acta Paediatr Scand. 1984 Jan;73(1):97–101. doi: 10.1111/j.1651-2227.1984.tb09905.x. [DOI] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folz R. J., Crapo J. D. Extracellular superoxide dismutase (SOD3): tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics. 1994 Jul 1;22(1):162–171. doi: 10.1006/geno.1994.1357. [DOI] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl H. P., Till G. O., Ryan U. S., Ward P. A. Mediator-induced activation of xanthine oxidase in endothelial cells. FASEB J. 1989 Nov;3(13):2512–2518. doi: 10.1096/fasebj.3.13.2806779. [DOI] [PubMed] [Google Scholar]
- Gaboury J., Woodman R. C., Granger D. N., Reinhardt P., Kubes P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol. 1993 Sep;265(3 Pt 2):H862–H867. doi: 10.1152/ajpheart.1993.265.3.H862. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin. A scavenger of superoxide anion radicals. J Biol Chem. 1979 May 25;254(10):4040–4045. [PubMed] [Google Scholar]
- Haddad I. Y., Pataki G., Hu P., Galliani C., Beckman J. S., Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest. 1994 Dec;94(6):2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Ischiropoulos H., Beckman J. S., Matalon S. Peroxynitrite inhibition of oxygen consumption and sodium transport in alveolar type II cells. Am J Physiol. 1994 Jun;266(6 Pt 1):L628–L634. doi: 10.1152/ajplung.1994.266.6.L628. [DOI] [PubMed] [Google Scholar]
- Huie R. E., Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18(4):195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Jamieson D. Oxygen toxicity and reactive oxygen metabolites in mammals. Free Radic Biol Med. 1989;7(1):87–108. doi: 10.1016/0891-5849(89)90103-2. [DOI] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Binding of human extracellular-superoxide dismutase C to cultured cell lines and to blood cells. Lab Invest. 1989 May;60(5):659–666. [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J. 1988 Oct 1;255(1):223–228. [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Sandström J., Edlund A., Marklund S. L. Turnover of extracellular-superoxide dismutase in tissues. Lab Invest. 1994 May;70(5):705–710. [PubMed] [Google Scholar]
- Koppenol W. H., Moreno J. J., Pryor W. A., Ischiropoulos H., Beckman J. S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992 Nov-Dec;5(6):834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- Kukreja R. C., Kontos H. A., Hess M. L., Ellis E. F. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res. 1986 Dec;59(6):612–619. doi: 10.1161/01.res.59.6.612. [DOI] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Marklund S. L., Bjelle A., Elmqvist L. G. Superoxide dismutase isoenzymes of the synovial fluid in rheumatoid arthritis and in reactive arthritides. Ann Rheum Dis. 1986 Oct;45(10):847–851. doi: 10.1136/ard.45.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Ceruloplasmin, extracellular-superoxide dismutase, and scavenging of superoxide anion radicals. J Free Radic Biol Med. 1986;2(4):255–260. doi: 10.1016/s0748-5514(86)80007-1. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Expression of extracellular superoxide dismutase by human cell lines. Biochem J. 1990 Feb 15;266(1):213–219. doi: 10.1042/bj2660213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984 Sep 15;222(3):649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Invest. 1984 Oct;74(4):1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L., Holme E., Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982 Nov 24;126(1):41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Regulation by cytokines of extracellular superoxide dismutase and other superoxide dismutase isoenzymes in fibroblasts. J Biol Chem. 1992 Apr 5;267(10):6696–6701. [PubMed] [Google Scholar]
- Marklund S. Determination of plasma or serum haemoglobin by peroxidase activity employing 2,2'-azino-di-(3-ethyl-benzthiazolinsulphonate-6) as chromogen. Scand J Clin Lab Invest. 1978 Oct;38(6):543–547. doi: 10.1080/00365517809108817. [DOI] [PubMed] [Google Scholar]
- Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem. 1976 Dec 10;251(23):7504–7507. [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965 Nov;240(11):4470–4480. [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986 Nov 15;137(10):3295–3298. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J. 1989 Oct 15;263(2):539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun. 1975 Mar 17;63(2):463–468. doi: 10.1016/0006-291x(75)90710-x. [DOI] [PubMed] [Google Scholar]
- Oury T. D., Piantadosi C. A., Crapo J. D. Cold-induced brain edema in mice. Involvement of extracellular superoxide dismutase and nitric oxide. J Biol Chem. 1993 Jul 25;268(21):15394–15398. [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos C. L., Pou S., Britigan B. E., Cohen M. S., Rosen G. M. Spin trapping evidence for myeloperoxidase-dependent hydroxyl radical formation by human neutrophils and monocytes. J Biol Chem. 1992 Apr 25;267(12):8307–8312. [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Santana T. A., Lee C. Y. Tracheal insufflation of tumor necrosis factor protects rats against oxygen toxicity. J Appl Physiol (1985) 1990 Mar;68(3):1211–1219. doi: 10.1152/jappl.1990.68.3.1211. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Treanor C., Shaffer J. B. Molecular basis for tumor necrosis factor-induced increase in pulmonary superoxide dismutase activities. Am J Physiol. 1990 Dec;259(6 Pt 1):L506–L512. doi: 10.1152/ajplung.1990.259.6.L506. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Crapo J. D., Freeman B. A. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J Clin Invest. 1984 Jan;73(1):87–95. doi: 10.1172/JCI111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Crapo J. D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch Biochem Biophys. 1982 Sep;217(2):411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973 Jul 10;248(13):4793–4796. [PubMed] [Google Scholar]
- White C. W., Avraham K. B., Shanley P. F., Groner Y. Transgenic mice with expression of elevated levels of copper-zinc superoxide dismutase in the lungs are resistant to pulmonary oxygen toxicity. J Clin Invest. 1991 Jun;87(6):2162–2168. doi: 10.1172/JCI115249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wispé J. R., Warner B. B., Clark J. C., Dey C. R., Neuman J., Glasser S. W., Crapo J. D., Chang L. Y., Whitsett J. A. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992 Nov 25;267(33):23937–23941. [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]



