Abstract
The prevalence of eating disorders and obesity in Western societies is epidemic and increasing in severity. Preclinical research focused on the development of animal models which can mimic the maladaptive patterns of food intake observed in certain forms of eating disorders and obesity. This study was aimed at characterizing a recently established model of palatable diet alternation in female rats. For this purpose, females rats were fed either continuously with a regular chow diet (Chow/Chow) or intermittently with a regular chow diet for 2 days and a palatable, high-sucrose diet for 1 day (Chow/Palatable). Following diet cycling, rats were administered rimonabant (0, 0.3, 1, 3 mg/kg i.p.) during access to either palatable diet or chow diet and were assessed for food intake and body weight. Finally, rats were pretreated with rimonabant (0 – 3 mg/kg, i.p.) and tested in the elevated plus maze during withdrawal from the palatable diet. Female rats with alternating access to palatable food cycled their intake, overeating during access to the palatable diet, and under-eating upon returning to the regular chow diet. Rimonabant treatment resulted in increased chow hypophagia and anxiety-like behavior in Chow/Palatable rats. No effect of drug treatment was observed on the compulsive eating of palatable food in the diet cycled rats. The results of this study suggest that withdrawal from alternating access to the palatable diet makes individuals vulnerable to the anxiogenic effects of rimonabant and provide etiological factors potentially responsible for the emergence of severe psychiatric side-effects following rimonabant treatment in obese patients.
Keywords: Eating disorders, diet cycling, palatable food, withdrawal, rimonabant, female, rat
Introduction
The prevalence of eating disorders and obesity in Western societies is epidemic and increasing in severity. One of the recognized causes of pathological overeating is the increased availability of palatable, calorie-dense foods, high in fats and refined sugars, which are more rewarding than low calorie foods. Individuals who repeatedly try to abstain from obesogenic foods by switching to safer low energy alternatives ultimately crave highly palatable foods, relapse and binge eat (Polivy and Herman, 1985). Vicious circles of abstinence and uncontrollable eating of palatable food have been proposed to share similarities with the intoxication/withdrawal cycles observed in drug addiction (Corwin and Grigson, 2009; Cottone et al, 2012).
In recent years, preclinical research has focused on the development of animal models that can mimic the maladaptive patterns of food intake observed in certain forms of disordered eating. A widely accepted approach to induce overeating is to provide intermittent access to highly palatable diets in free-fed experimental animals (Hagan and Moss, 1997; Avena et al, 2006; Cifani et al, 2009; Cottone et al, 2009b; Corwin et al, 2011; Parylak et al, 2012; Velazquez-Sanchez et al, 2014). This strategy allows the development of excessive intake of highly palatable food as well as undereating of safer alternatives. It is based on the relative palatability of the different diets provided to animals rather than on energy alternations induced by food restriction.
In an effort to better understand the etiological factors of maladaptive feeding behavior, we have recently developed a model of diet alternation in male Wistar rats which is characterized by short 3-day cycles of access to differentially rewarding diets (Dore et al, 2013b). In this procedure, subjects quickly escalated food intake and increased body weight gain when intermittently fed the palatable diet for 1 day. The palatable diet was cyclically withdrawn for 2 days and replaced with a regular chow diet, during which period male rats developed spontaneous and progressively increasing hypophagia and body weight loss (Dore et al, 2013b).
Whether female rats under a shortened palatable diet cycling procedure would show a maladaptive feeding behavior is currently unknown. The female sex is generally underrepresented in preclinical research, limiting the study of eating disorders, which have been extensively documented to occur to a greater extent in women compared to men (Oakley Browne et al, 2006; Hudson et al, 2007; Hay et al, 2008; Preti et al, 2009). Indeed, women show earlier onsets of eating disorders (Kessler et al, 2013) and worse outcomes compared to men (Stoving et al, 2011).
Therefore, the first aim of this study was to characterize a shortened model of diet alternation in female rats. For this purpose we monitored food intake, body weight and anxiety-like behavior in female rats which received cycles of 2 days of access to a regular chow diet (C phase) followed by 1 day of access to a highly palatable, sugary diet (P phase).
The endocannabinoid system represents a promising target for the development of novel pharmacological agents that reduce food intake (Di Marzo, 2008; Kirkham, 2009; Berner et al, 2011; DiPatrizio and Piomelli, 2012; Mendez-Diaz et al, 2012). Antagonism of the type-1 cannabinoid (CB1) receptor has been demonstrated to be a successful pharmacological approach for overeating and obesity. However, the CB1 receptor antagonist rimonabant, initially approved for the treatment of obesity, was withdrawn from the market shortly after due to serious psychiatric side effects, including the emergence of a negative emotional affect (Akbas et al, 2009; Moreira et al, 2009).
Therefore, the second aim of our study was to investigate whether the endocannabinoid system (eCB) plays a role in the consummatory and emotional outcomes of the shortened diet cycling procedure in female rats. For this purpose, we investigated the effects of systemic rimonabant administration on i) escalated excessive intake of palatable diet ii) regular chow hypophagia, and iii) anxiety-like behavior during withdrawal from palatable food.
Methods
Subjects
Female Wistar rats (N=20), 41–47 days old at arrival weighing 126–150 g (Charles River, Wilmington, MA), were single housed in wire-topped, plastic cages (27 × 48 × 20 cm) in a 12-hour reverse light cycle (lights off at 11:00h), in an AAALAC-approved humidity- (60%) and temperature-controlled (22°C) vivarium. Rats had access to corn-based chow (Harlan Teklad LM-485 Diet 7012 [65% (kcal) carbohydrate, 13% fat, 21% protein, metabolizable energy 310 cal/100 g; Harlan, Indianapolis, IN]) and water ad libitum at all times, unless otherwise specified. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85-23, revised 1996) and the Principles of Laboratory Animal Care (http://www.nap.edu/readingroom/bookslabrats), and were approved by the Institutional Animal Care and Use Committees (IACUC) of Boston University.
Development of a shortened, ad libitum palatable diet alternation in female rats
Intermittent, extended access to a palatable diet procedure in female rats was performed using a protocol previously described in male rats only (Dore et al, 2013b) and shortened from previous protocols (Cottone et al, 2008a, 2009a,b; Iemolo et al, 2012), in order to produce a faster experimental cycling procedure. After 1 week of acclimation, rats were divided were divided in two groups of ten subjects, matched for food intake, body weight and feed efficiency from a 3–4 days baseline period. One group was then provided a chow diet (‘Chow’) ad libitum (Chow/Chow), whereas a second group was provided chow ad libitum for 2 days, followed by 1 day of ad libitum access to a highly palatable, chocolate-flavored, high-sucrose diet (‘Palatable’; Chow/Palatable). The chow diet was the above described Harlan Teklad LM-485 Diet 7012. The palatable diet was a nutritionally complete, chocolate-flavored, high-sucrose (50% kcal), AIN-76A-based diet that is comparable in macronutrient proportions and energy density to the chow diet [chocolate-flavored Formula 5TUL: 66.7% (kcal) carbohydrate, 12.7% fat, 20.6% protein, metabolizable energy 344 cal/100 g; TestDiet, Richmond, IN; formulated as 45 mg precision food pellets to increase its preferredness]. For brevity, the first 2 days (chow only) and the following 1 day (chow or palatable according to experimental group) are referred in all experiments as phases C and P. Diets were never concurrently available. Food intake and body weight were measured daily. Average food intake was calculated as the kcal intake in a certain phase divided by the number of days of that phase (2 for phase C, 1 for phase P). Average body weight change was calculated as the difference between the body weight at the end and at the beginning of a phase divided by the number of days of that phase (2 for phase C, 1 for phase P). Average and cumulative feed efficiency was calculated as mg body weight gained in a certain time interval (phase or cycle) divided by the kcal food intake in the same time interval. Cumulative food intake and cumulative body weight gain was calculated as the kcal food intake or body weight gained at the end of each cycle since the beginning of the study. To evaluate the escalation of palatable diet intake, food intake was measured 1 hafter the diet switch at the beginning of each P phase. For the time course studies, food intake was measured at the beginning of either the C or the P phase (at the time of the diet switch), and then 1, 3, 6 and 24 h later. As previously published (Cottone et al, 2008a, 2009a,b) the 5TUL Chocolate Diet (sugary palatable diet) was uniformly preferred compared to the Harlan LM-485 chow diet.
Effects of rimonabant on intake of regular chow and palatable diet
Rimonabant (0, 0.3, 1, 3 mg/kg, i.p., −30 minutes) was administered at the onset of either the P or the C phase, in a within-subject Latin square design. Rats were then provided with a pre-weighed amount of food, and intake was recorded 1, 3, and 24 h later. Body weights were recorded immediately before and 24 h following drug administration.
Effects of rimonabant on anxiety-like behavior
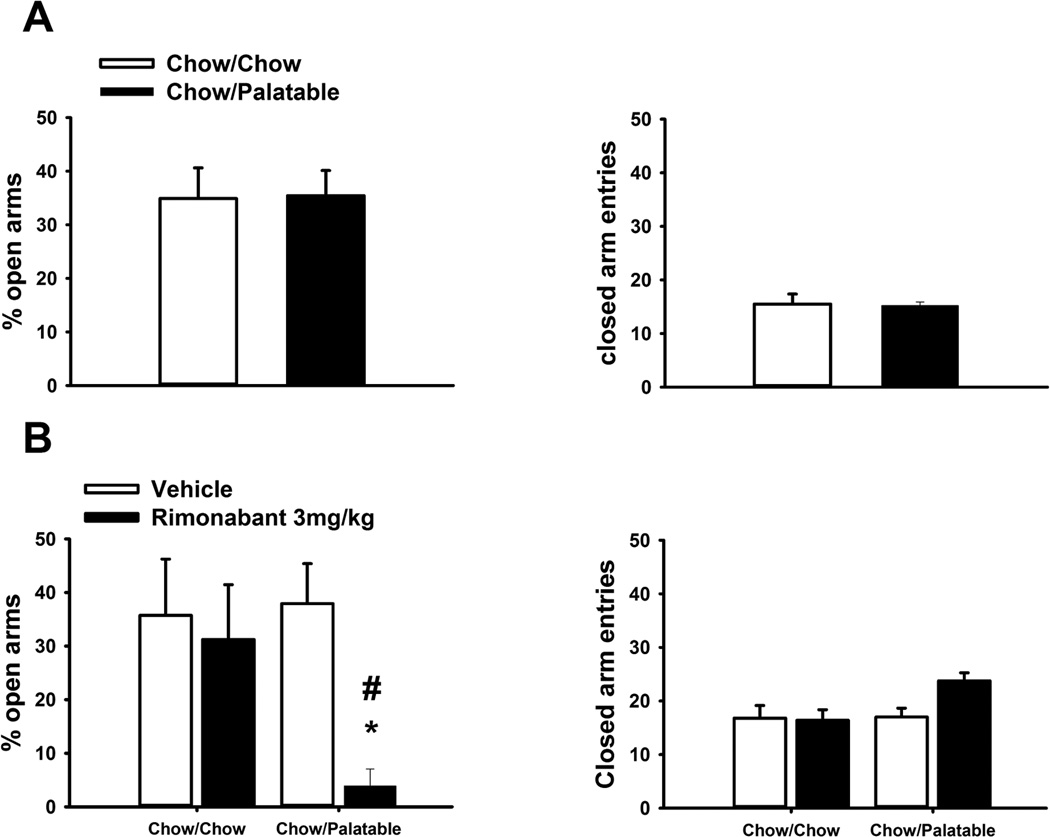
The elevated plus-maze apparatus (Cottone et al, 2007, 2008b; Dore et al, 2013a) was made of black Plexiglas and consisted of four arms (50 cm long 10 cm wide). Two arms had 40-cm-high dark walls (enclosed arms), and two arms had 0.5-cm-high ledges (open arms). The maze was elevated to a height of 50 cm. Open arms received 1.5–2.0 lux of illumination. Animals were habituated to the anteroom the day before testing. On the day of testing, rats were kept in the quiet, dark anteroom for at least 2 h before testing. White noise (70 dB) was present throughout habituation and testing. For testing anxiety-like behavior induced by withdrawal from spontaneous palatable food, rats were placed individually onto the center of the maze facing a closed arm and were then removed after a 5-min period. The primary measures were the percentage of total arm time directed towards the open arms [i.e., 100*open arm/(open arm+closed arm)], a validated index of anxiety-related behavior (Fernandes and File, 1996) and the number of closed arm entries, a specific index of locomotor activity (Cruz et al, 1994). During the 12th cycle, Chow/Palatable rats were tested in a between-subjects design 5h after the switch from the palatable diet to chow. The elevated plus-maze test was repeated after 26 cycles of diet alternation, 5h after the switch from the palatable diet to chow. Allowing 3 weeks later or more before re-testing prevents an increase in the time spent in the open arms (Adamec and Shallow, 2000; Adamec et al, 2005; Walf and Frye, 2007). During this second assessment, subjects were pretreated with rimonabant (0, 3 mg/kg, i.p., −30 minutes). Chow/Chow control rats were tested concurrently in a between-subjects design. Chow diet was available ad libitum until the time of testing.
Drug
Rimonabant [SR141716A or 5-(4-Chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide × HCl] was synthesized as reported previously (Aggarwal et al, 2008); it was solubilized in an 18:1:1 mixture of saline : ethanol : cremophor and administered i.p. (0, 0.3, 1, 3 mg/kg, 1 ml/kg, 30 minutes pre-treatment).
Statistical analysis
Average daily food intake, average daily body weight change and average daily feed efficiency were analyzed using three-way mixed analyses of variance (ANOVAs), with Diet Schedule (the schedule of food access, either continuous access to chow (Chow/Chow) or alternating access to chow and palatable diet (Chow/Palatable)) as a between-subjects factor, Phase (each period of 2 days (Phase C, chow only) or 1 day (chow or palatable according to experimental group)) and Cycle (a 3-day period consisting of a C and a P Phase) as within-subject factors. Intake in the first hour of palatable food consumption, cumulative food intake, cumulative body weight change and cumulative feed efficiency were analyzed using two-way ANOVAs with Diet Schedule as a between-subjects factor and Cycle as a within-subjects factor. To analyze the time course of ingestion following rimonabant treatment, three-way ANOVAs on incremental food intake were performed, with Diet Schedule as a between-subjects factor and Time (time points at which food intake was recorded) and Treatment (doses of the drug), as within-subjects factors. The effects of rimonabant on the percent of time spent in the open arms and the closed arms entries were analyzed using two-way ANOVAs, with Diet Schedule and Treatment as between-subjects factors. Pairwise effects were interpreted using Fisher's least significant difference tests. The software/graphic packages used were Systat 12.0, SigmaPlot 11.0 (Systat Software Inc., Chicago, IL), InStat 3.0 (GraphPad, San Diego, CA) and Statistica 7.0 (Statsoft, Inc., Tulsa, OK).
Results
Effects of diet alternation on food intake, body weight and feed efficiency
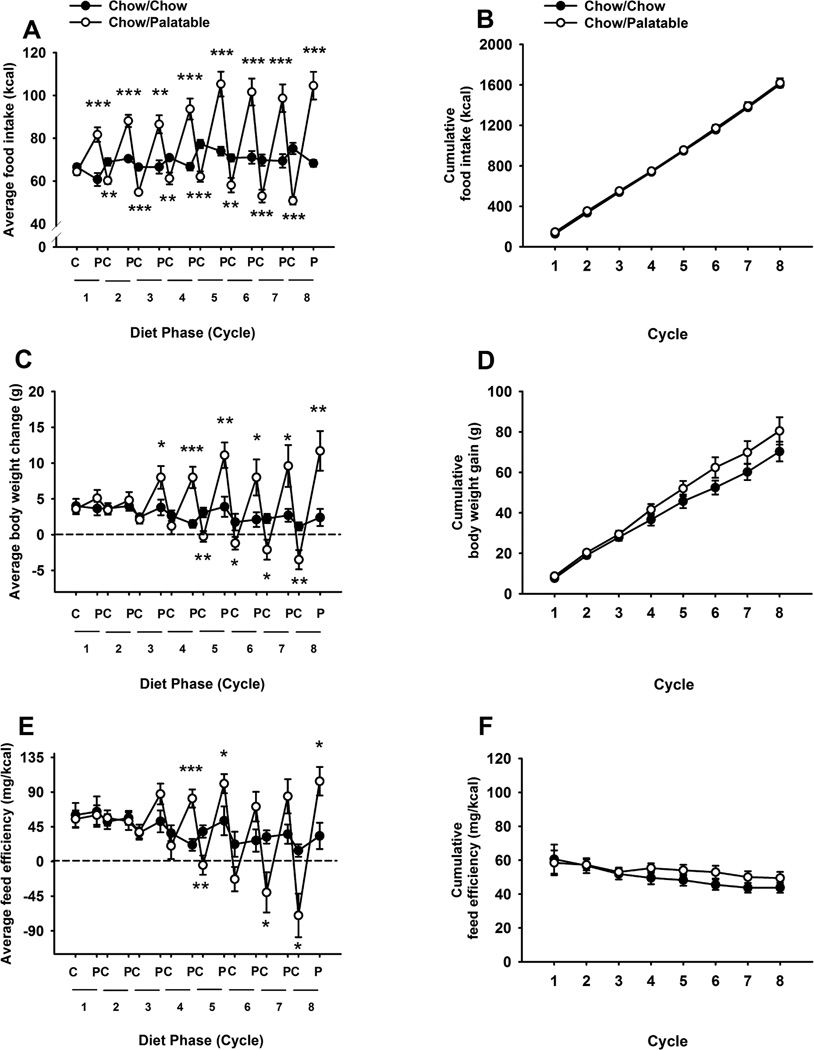
Alternating access to palatable food altered the daily food intake in a diet-specific manner [Cycle × Diet Phase × Diet Schedule: [F(7,126)=8.66, p<0.001; Figure 1A]. Following the first access to the palatable food, the Chow/Palatable group started to overeat [t(18)=4.62, p<0.001], whereas the first withdrawal from palatable food induced significant hypophagia compared to control intake [t(18)=3.66, p<0.02]. Similarly to what was observed in male rats (Dore et al, 2013b), female rats with alternating access to food cycled their intake, overeating during access to palatable diet, and undereating upon returning to the regular chow diet. Both the overeating and the hypophagia progressively increased in magnitude as a function of the number of cycles. The magnitude of overeating in the Chow/Palatable group during the P phases [+213.03 kcal, 38.92% relative to the Chow/Chow group during the 1-day P phase, across 8 cycles] was not significantly different from the hypophagia of the regular chow during the C Phases [−200.94 kcal, −18.86% relative Chow/Chow group during the 2-day C phase across eight cycles], resulting in no significant difference in cumulative energy intake between the two groups [Diet Schedule F(1,18)=0.34, NS, Cycle × Diet Schedule: F(7,126)=0.04, NS; Figure 1B]. In addition, the Chow/Palatable group cycled their body weight and feed efficiency [average body weight gain Cycle × Diet Phase × Diet Schedule F(7,126)=6.52, p<0.001; average feed efficiency Cycle × Diet Phase × Diet Schedule F(7,126)=6.20 p<0.001; Figures 1C and 1E]. Beginning on the 3rd access to palatable food and until the 8th cycle, Chow/Palatable rats gained significantly more body weight compared to control Chow/Chow rats. In addition, Chow/Palatable rats started losing absolute body weight at the beginning of the 5th cycle. Finally, analysis of cumulative body weight [Diet Schedule F(1,18)=1.81, NS; Cycle × Diet Schedule: [F(7,126)=1.64, NS; Figure 1D] and cumulative efficiency [Diet Schedule F(1,18)=0.64, NS; Cycle × Diet Schedule: [F(7,126)=0.79, NS; Figure 1F] did not show any significant difference between groups throughout the eight cycles.
Figure 1.
Effects of intermittent, extended access to a palatable diet on (A) average daily food intake, (B) cumulative food intake, (C) average daily body weight change, (D) cumulative body weight change, (E) average daily feed efficiency, and (F) cumulative feed efficiency in female Wistar rats (N=20). Values for phase C represent the average of 2 days access to chow food. Data represent mean ±SEM. *p<0.05, **p < 0.01, ***p < 0.001, relative to the Chow/Chow group.
Effects of diet alternation on escalation of palatable food consumption
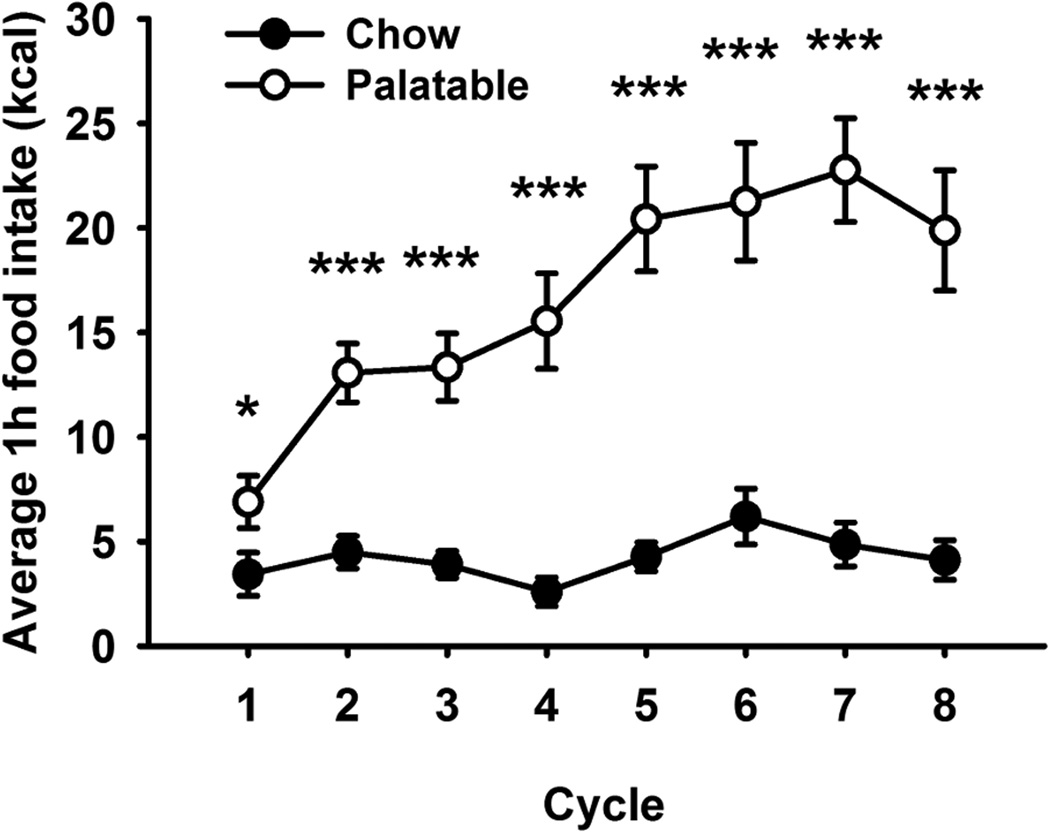
Figure 2 shows the effects of diet alternation on food intake during the first hour of access to the palatable diet. The Chow/Palatable group progressively and dramatically escalated the intake of the palatable diet during the first hour of access, as a function of the number of cycles [Cycle × Diet Schedule: F(7,126)=6.88, p<0.001]. The intake of diet-cycled rats became significantly higher than the intake of chow in control rats, starting from the first palatable diet access; by the 7th access, Chow/Palatable group were able to consume approximately 5.0-fold the intake of Chow/Chow rats [M #x000B1; SEM; 22.7 ± 2.5 kcal. versus 4.9 ± 1.1 kcal respectively; t(18)=6.65, p<0.001]. This escalation was experience-dependent, as suggested by the strong fit with the sigmoidal associative learning curve (M±SEM: min: 6.7±1.7, max: 22.4±2.6, ET50: 2.9±0.6, Hill slope: 2.5±1.3, r2=0.93, p<0.001).
Figure 2.
Effects of intermittent, extended access to a palatable diet on food intake during the first hour of the P phase (when Chow/Palatable rats are fed the sugary, palatable diet) in female Wistar rats (N=20). Data represent mean ±SEM. * p < 0.05, ***p < 0.001, relative to the Chow/Chow group.
Effects of rimonabant treatment on food intake and body weight
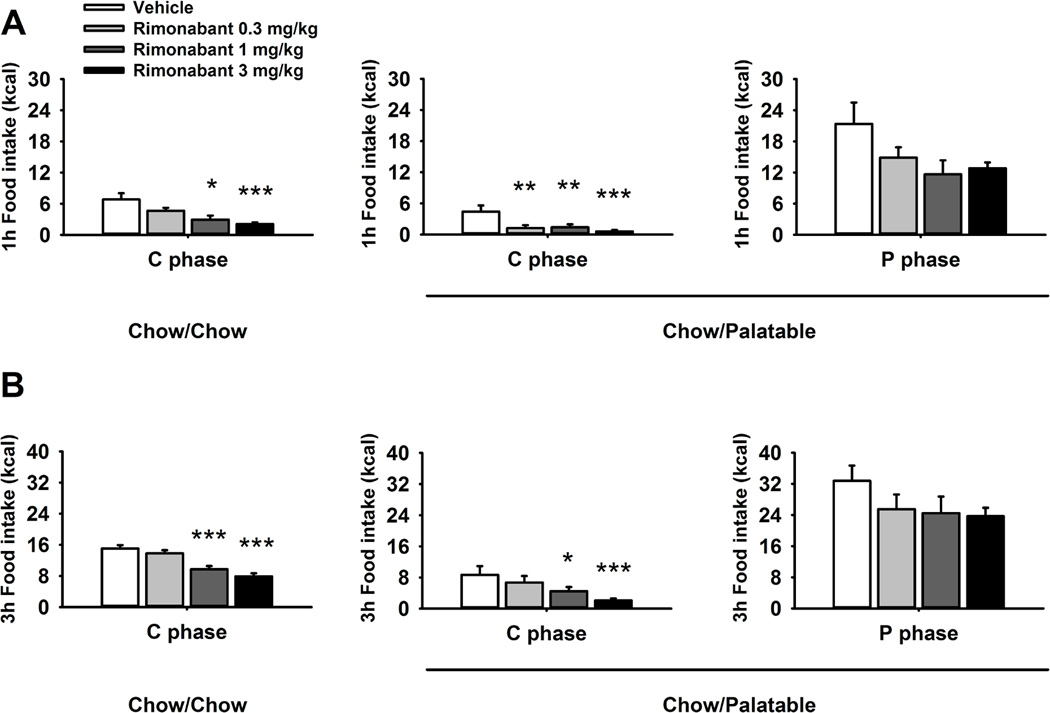
Administration of rimonabant reduced food intake in both Chow/Chow and Chow/Palatable rats regardless of the Diet Schedule [Dose: F(3,54)=21.24, p<0.001; Dose × Diet Schedule: F(3,54)=0.33, NS; Figure 3]. However, the analysis of the time course of the anorectic effect of rimonabant revealed that drug treatment reduced food intake with different potency in the Chow/Palatable and Chow/Chow groups. Indeed, rimonabant dose-dependently reduced food intake and body weight in Chow/Chow rats with a minimum effective dose of 1 mg/kg across the 24 hour measurement window. However, drug treatment resulted in increased anorexia of chow during withdrawal from palatable food in Chow/Palatable rats, reducing food intake at the lowest dose injected (0.3 mg/kg) during the first hour of treatment, and therefore more potently compared to Chow/Chow control rats. During the first hour the effect size, calculated as % of reduction compared to vehicle condition following treatment with rimonabant 0.3, 1, 3 mg/kg, was 72.03%, 68.53% and 86.01% for the Chow/Palatable group and 31.82%, 57.05%, 69.55% for the Chow/Chow group. Conversely, when rimonabant was administered in Chow/Palatable rats during the P phase, only a nonsignificant trend towards a reduction was observed.
Figure 3.
Effects of pre-treatment (−30 min) with rimonabant (0, 0.3, 1 and 3 mg/kg, i.p.) on (A) 1 h, and (B) 3 h food intake of Chow/Chow and Chow/Palatable female Wistar rats (N=20). Drug treatment was performed in both C (first day of chow diet) and P phases. Data represent mean + SEM. Symbols denote significant difference from the group *p<0.05, **p < 0.01, ***p < 0.001, relative to the Vehicle-treated group.
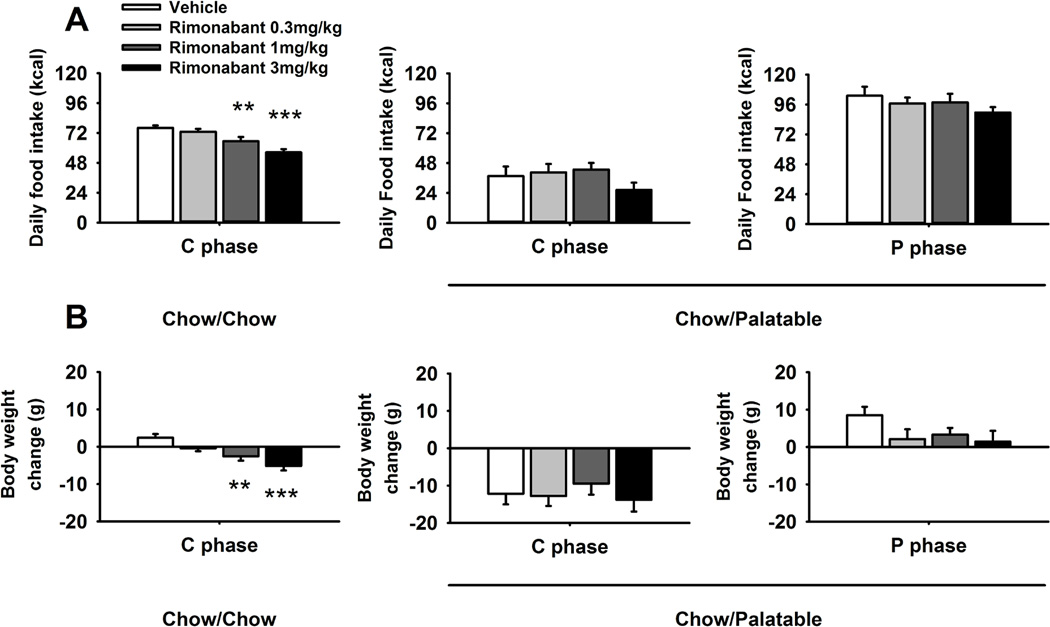
Two-way ANOVA revealed that the anorectic effect of rimonabant lasted up to 24 h [Dose: F(3,54)=7.08, p<0.001; Figure 4A]; however, at this time point, drug treatment was selective for Chow/Chow control rats as it did not affect Chow/Palatable food intake. Similar results were obtained when body weight gain was analyzed [Dose: F(3,54)=3.28, p<0.05; Figure 4B].
Figure 4.
Effects of pre-treatment (−30 min) with rimonabant (0, 0.3, 1 and 3 mg/kg,i.p.) on 24 h (A) food intake and (B) body weight change of Chow/Chow and Chow/Palatable female Wistar rats (N=20). Drug treatment was performed in both C (first day of chow diet) and P phases. Data represent mean ± SEM. **p < 0.01, ***p < 0.001 relative to the Vehicle-treated group.
Effects of diet alternation and rimonabant treatment on anxiety-like behavior
Chow/Palatable rats were first tested for spontaneous anxiety-like behavior induced by palatable food withdrawal during the first day of the C phase of the 12th cycle. As shown in Figure 5A, diet-cycled rats did not show any spontaneous anxiety-like behavior when compared to control Chow/Chow rats [% open arm time, t(18)=0.07, NS; closed arm entries, t(18)=0.14, NS].
Figure 5.
Effects of pretreatment with rimonabant (30 min pretreatment, 0, 3 mg/kg, i.p.) on anxiety-like behavior in female Wistar rats (N=20) withdrawn from chronic, intermittent access to palatable diet. (A) Spontaneous anxiety-like behavior in the first day of Phase C during the 12th cycle, measured using the elevated plus-maze test. (B) Rimonabant-induced anxiety-like behavior in the Chow/Palatable group, in the first day of Phase C during the 38th cycle, measured using the elevated plus-maze test. Data represent mean ±SEM. *p<0.05 versus Chow/Chow vehicle-treated group; # p < 0.05 versus Chow/Palatable vehicle-treated group.
Chow/Palatable rats were also tested for rimonabant-induced anxiety-like behavior later in the cycling procedure, during the first day of the C phase of the 38th cycle. As shown in Fig. 5B, a two-way ANOVA revealed that rimonabant administration selectively reduced the percentage of time spent in the open arms, i.e. showing an anxiogenic-like effect, in Chow/Palatable rats, but not in Chow/Chow rats [Dose: F(1,15)=6.31, p<0.05]. No significant differences between groups were detected in the number of closed arm entries, an index of locomotor activity [Dose: F(1,15)=1.39, NS]
Discussion
The results of the present study show that female rats, undergoing a protocol of shortened intermittent access to a palatable diet recently established in our laboratory (Dore et al, 2013b), developed feeding behavior adaptations characterized by cycles of overeating and spontaneous rejection of the otherwise acceptable chow diet. Our results are similar to those observed in significantly longer diet-cycling procedures (Cottone et al, 2009b). The present findings also show that blockade of CB1 receptor resulted in increased anorexia of regular chow diet in palatable food withdrawn female rats, without affecting the intake of palatable food. In addition, cycled female rats withdrawn from the palatable diet did not show any sign of spontaneous anxiety-like behavior, but revealed a markedly increased vulnerability to the precipitating anxiogenic effects of CB1 receptor blockade.
In this study, we show that female rats undergoing the shortened diet alternation procedure displayed a pattern of spontaneous cycling of food intake, body weight and feed efficiency which resembles the food adaptations observed during longer food alternation procedures (Cottone et al, 2009a,b; Iemolo et al, 2012). Similarly to what has been observed in male rats (Dore et al, 2013b), incremental food intake, body weight and feed efficiency of female rats dramatically cycled across the eight cycles of observation; however, cumulatively, these variables did not differ between groups. This represents a major advantage of the task, as potential energy-homeostasis confounding factors can then be excluded.
In this study, female rats dramatically escalated food intake during the first hour of access to the palatable diet throughout the eight cycles of the study. The escalated intake peaked during the 7th access to the palatable diet where Chow/Palatable rats ate ~5 fold the intake of Chow/Chow rats. Interestingly, the intake during the first hour of renewed access increased to a much greater extent (Chow/Palatable first hour intake as % increase compared to Chow/Chow: Cycle 1, ~+100%; Cycle 8, ~+378%), compared to the moderate increase in daily palatable food intake (Chow/Palatable daily intake as % increase compared to Chow/Chow: Cycle 1, ~+34%; Cycle 8, ~+53%), Therefore, the mechanism behind the escalation of palatable diet intake was mostly induced by a gradual shift of the intake towards the beginning of the renewed access, rather than an overall increase in feeding behavior. “Escalation” is a phenomenon observed with extended access to many different drugs of abuse, such as cocaine and heroin (Ahmed et al, 2000; Park et al, 2013). It is believed that escalation towards uncontrolled taking is a key factor and a hallmark of drug addiction (Koob and Kreek, 2007).
The spontaneous and progressive under-eating of the otherwise acceptable chow diet has been demonstrated to be independent from energy-homeostasis mechanisms (Cottone et al, 2008a) and it has been proposed to result either from a devaluation process due to the recent experience with the more rewarding alternative (Flaherty and Rowan, 1986; Iemolo et al, 2013) or from the aversive state induced by a palatable food withdrawal, similarly to what is observed with drugs of abuse (Parylak et al, 2011).
A major difference between the findings of the present study and the results obtained using the same procedure in male rats (Dore et al, 2013b) is related to the intake of palatable food during the first access. While diet-cycled female rats significantly overate during the first hour of access to the palatable diet compared to chow control rats, male rats ate similarly to chow fed rats (Dore et al, 2013b). A possible explanation for the observed difference could be related to sex differences in neophobia (Cooke et al, 2007), an adaptive response that protects individuals from the possible harmful post-ingestive consequences of unfamiliar tastants (Corey, 1978; Birch, 1999).
In the present paper, we show that female rats withdrawn from the highly palatable diet did not differ from controls in the percent of open arm time in the elevated plus-maze test. This finding contrasts with the observation that female rats, which underwent an extended diet-cycling procedure, show spontaneous anxiety-like behavior when withdrawn from the highly palatable diet (Cottone et al, 2009b), and suggests that a shorter diet-cycling procedure is not sufficient to produce a spontaneous negative emotional state. However, when palatable food withdrawn female rats were systemically treated with rimonabant, a dramatic anxiogenic-like effect was observed, selectively in diet-cycled rats. Furthermore, rimonabant, administered during withdrawal from palatable food, also resulted in increased anorexia during the first hour of treatment (Blasio et al, 2013).
It is important to highlight that the primary goal of this study was to investigate the negative emotional state during abstinence from a highly palatable diet following rimonabant treatment rather than possible physical signs of withdrawal (i.e. wet-dog shakes, forepaw flutter, ptosis, etc.) (Gellert and Holtzman, 1978). The reason behind this choice is that extensive literature has highlighted the contribution to the addiction process of anxiety, irritability and dysphoria, when access to the drug is prevented (Koob and Le Moal, 2001; Koob and Volkow, 2010). Indeed, it has been argued that the development of such a negative affective state contributes to compulsivity through negative reinforcement mechanisms (Koob et al, 2001; Koob et al, 2010). Future studies will be needed to clarify whether rimonabant can also precipitate physical signs of withdrawal when access to palatable food is prevented.
Contrarily to what was observed with the regular chow diet, when the effects of rimonabant were evaluated on the overeating of the palatable diet, only a nonsignificant trend towards a reduction was observed. This result is consistent with previous observations showing that intermittent access to palatable diet decreases the effectiveness of anorectic effects of CB1 receptor blockade (Parylak et al, 2012; Blasio et al, 2013). Indeed, treatment with the CB1 receptor antagonist, SR147778, was less effective in reducing binge eating of a sweet fat diet in female rats (Parylak et al, 2012). Moreover, rimonabant was ineffective in reducing overeating of a palatable diet in female rats that underwent a longer palatable food cycling procedure (Blasio et al, 2013). On the other hand, we have previously shown that when using the same intermittent access procedure as used here in male rats, excessive palatable food intake is blocked with an increased potency compared to chow. Therefore, a possible explanation for the divergent effects observed could be due to the different sex tested, since female rats undergoing intermittent access to palatable food develop overeating, which is generally less sensitive to the anorectic effects of CB1 receptor blockade.
In summary, we have shown the consummatory and emotional effects of a shortened diet alternation procedure in female rats. The procedure described here in female rats has the advantage of being considerably faster than previously established protocols of palatable diet alternation (Hagan et al, 1997; Cifani et al, 2009; Cottone et al, 2009b; Rossetti et al, 2013). In addition, our results show that withdrawal from alternating access to the palatable diet makes individuals vulnerable to the anxiogenic effects of rimonabant. More generally our findings support the hypothesis that the emergence of severe psychiatric side-effects following rimonabant treatment in obese patients (Akbas et al, 2009; Moreira et al, 2009) could result from a worsening negative emotional state in a subpopulation of individuals abstaining from palatable foods in the attempt to lose weight.
Acknowledgments
We thank Anika Begum and Alyssa C. DiLeo for the technical assistance. This publication was made possible by grant numbers MH091945, MH093650 and DA030425 from the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA) and by the Peter Paul Career Development Professorship (P.C.) and by Boston University's Undergraduate Research Opportunities Program (UROP). A portion of this work was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism (NIAAA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosures
The authors declare no conflict of interest.
References
- Adamec R, Shallow T. Effects of baseline anxiety on response to kindling of the right medial amygdala. Physiology & behavior. 2000;70(1–2):67–80. doi: 10.1016/s0031-9384(00)00247-x. [DOI] [PubMed] [Google Scholar]
- Adamec R, Shallow T, Burton P. Anxiolytic and anxiogenic effects of kindling--role of baseline anxiety and anatomical location of the kindling electrode in response to kindling of the right and left basolateral amygdala. Behavioural brain research. 2005;159(1):73–88. doi: 10.1016/j.bbr.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Aggarwal AKEIAP-IISASNMP, Sarin GSEIAP-IISASNMP, Srinivasan CVEIAP-IISASNMP, Wadhwa LEIAP-IISASNMP. Ind-Swift Laboratories Limited SCONSENACMC, editor. AN IMPROVED PROCESS FOR THE PREPARATION OF RIMONABANT. 2008 WO. Vol IN2007/000546. [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22(4):413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Akbas F, Gasteyger C, Sjodin A, Astrup A, Larsen TM. A critical review of the cannabinoid receptor as a drug target for obesity management. Obes Rev. 2009;10(1):58–67. doi: 10.1111/j.1467-789X.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Sugar bingeing in rats. Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al] 2006;Chapter 9(Unit9):23C. doi: 10.1002/0471142301.ns0923cs36. [DOI] [PubMed] [Google Scholar]
- Berner LA, Bocarsly ME, Hoebel BG, Avena NM. Pharmacological interventions for binge eating: lessons from animal models, current treatments, and future directions. Current pharmaceutical design. 2011;17(12):1180–1187. doi: 10.2174/138161211795656774. [DOI] [PubMed] [Google Scholar]
- Birch LL. Development of food preferences. AnnuRev Nutr. 1999;19:41–62. doi: 10.1146/annurev.nutr.19.1.41. [DOI] [PubMed] [Google Scholar]
- Blasio A, Iemolo A, Sabino V, Petrosino S, Steardo L, Rice KC, et al. Rimonabant Precipitates Anxiety in Rats Withdrawn from Palatable Food: Role of the Central Amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifani C, Polidori C, Melotto S, Ciccocioppo R, Massi M. A preclinical model of binge eating elicited by yo-yo dieting and stressful exposure to food: effect of sibutramine, fluoxetine, topiramate, and midazolam. Psychopharmacology (Berl) 2009;204(1):113–125. doi: 10.1007/s00213-008-1442-y. [DOI] [PubMed] [Google Scholar]
- Cooke LJ, Haworth CM, Wardle J. Genetic and environmental influences on children's food neophobia. Am J Clin Nutr. 2007;86(2):428–433. doi: 10.1093/ajcn/86.2.428. [DOI] [PubMed] [Google Scholar]
- Corey DT. The determinants of exploration and neophobia. Neuroscience & Biobehavioral Reviews. 1978;2(4):235–253. [Google Scholar]
- Corwin RL, Grigson PS. Symposium overview--Food addiction: fact or fiction? J Nutr. 2009;139(3):617–619. doi: 10.3945/jn.108.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104(1):87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. FG 7142 specifically reduces meal size and the rate and regularity of sustained feeding in female rats: evidence that benzodiazepine inverse agonists reduce food palatability. Neuropsychopharmacology. 2007;32(5):1069–1081. doi: 10.1038/sj.npp.1301229. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. American journal of physiology. 2008a;295(4):R1066–R1076. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008b;33(3):524–535. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, et al. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009a;106(47):20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2009b;34(1):38–49. doi: 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Wang X, Park JW, Valenza M, Blasio A, Kwak J, et al. Antagonism of sigma-1 receptors blocks compulsive-like eating. Neuropsychopharmacology. 2012;37(12):2593–2604. doi: 10.1038/npp.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacology, biochemistry, and behavior. 1994;49(1):171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51(8):1356–1367. doi: 10.1007/s00125-008-1048-2. [DOI] [PubMed] [Google Scholar]
- DiPatrizio NV, Piomelli D. The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends in neurosciences. 2012;35(7):403–411. doi: 10.1016/j.tins.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V. CRF Mediates the Anxiogenic and Anti-Rewarding, but not the Anorectic Effects of PACAP. Neuropsychopharmacology. 2013a doi: 10.1038/npp.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore R, Valenza M, Wang X, Rice KC, Sabino V, Cottone P. The inverse agonist of CB receptor SR141716 blocks compulsive eating of palatable food. Addiction biology. 2013b doi: 10.1111/adb.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacology, biochemistry, and behavior. 1996;54(1):31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Rowan GA. Successive, simultaneous, and anticipatory contrast in the consumption of saccharin solutions. J Exp Psychol Anim Behav Process. 1986;12(4):381–393. [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. The Journal of pharmacology and experimental therapeutics. 1978;205(3):536–546. [PubMed] [Google Scholar]
- Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord. 1997;22(4):411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hay PJ, Mond J, Buttner P, Darby A. Eating disorder behaviors are increasing: findings from two sequential community surveys in South Australia. PloS one. 2008;3(2):e1541. doi: 10.1371/journal.pone.0001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Valenza M, Tozier L, Knapp CM, Kornetsky C, Steardo L, et al. Withdrawal from chronic, intermittent access to a highly palatable food induces depressive-like behavior in compulsive eating rats. Behav Pharmacol. 2012;23(5–6):593–602. doi: 10.1097/FBP.0b013e328357697f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Blasio A, St Cyr SA, Jiang F, Rice KC, Sabino V, et al. CRF-CRF1 receptor system in the central and basolateral nuclei of the amygdala differentially mediates excessive eating of palatable food. Neuropsychopharmacology. 2013;38(12):2456–2466. doi: 10.1038/npp.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, et al. The prevalence and correlates of binge eating disorder in the world health organization world mental health surveys. Biol Psychiatry. 2013;73(9):904–914. doi: 10.1016/j.biopsych.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC. Cannabinoids and appetite: food craving and food pleasure. International review of psychiatry (Abingdon, England) 2009;21(2):163–171. doi: 10.1080/09540260902782810. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. The American journal of psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Diaz M, Rueda-Orozco PE, Ruiz-Contreras AE, Prospero-Garcia O. The endocannabinoid system modulates the valence of the emotion associated to food ingestion. Addiction biology. 2012;17(4):725–735. doi: 10.1111/j.1369-1600.2010.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Grieb M, Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best practice & research. 2009;23(1):133–144. doi: 10.1016/j.beem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Oakley Browne MA, Wells JE, Scott KM, McGee MA. Lifetime prevalence and projected lifetime risk of DSM-IV disorders in Te Rau Hinengaro: the New Zealand Mental Health Survey. The Australian and New Zealand journal of psychiatry. 2006;40(10):865–874. doi: 10.1080/j.1440-1614.2006.01905.x. [DOI] [PubMed] [Google Scholar]
- Park PE, Schlosburg JE, Vendruscolo LF, Schulteis G, Edwards S, Koob GF. Chronic CRF receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia. Addiction biology. 2013 doi: 10.1111/adb.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Koob GF, Zorrilla EP. The dark side of food addiction. Physiol Behav. 2011;104(1):149–156. doi: 10.1016/j.physbeh.2011.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Cottone P, Sabino V, Rice KC, Zorrilla EP. Effects of CB1 and CRF1 receptor antagonists on binge-like eating in rats with limited access to a sweet fat diet: lack of withdrawal-like responses. Physiol Behav. 2012;107(2):231–242. doi: 10.1016/j.physbeh.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivy J, Herman CP. Dieting and binging. A causal analysis. The American psychologist. 1985;40(2):193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- Preti A, Girolamo G, Vilagut G, Alonso J, Graaf R, Bruffaerts R, et al. The epidemiology of eating disorders in six European countries: results of the ESEMeD-WMH project. Journal of psychiatric research. 2009;43(14):1125–1132. doi: 10.1016/j.jpsychires.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Rossetti C, Spena G, Halfon O, Boutrel B. Evidence for a compulsive-like behavior in rats exposed to alternate access to highly preferred palatable food. Addiction biology. 2013 doi: 10.1111/adb.12065. [DOI] [PubMed] [Google Scholar]
- Stoving RK, Andries A, Brixen K, Bilenberg N, Horder K. Gender differences in outcome of eating disorders: a retrospective cohort study. Psychiatry research. 2011;186(2–3):362–366. doi: 10.1016/j.psychres.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Velazquez-Sanchez C, Ferragud A, Moore CF, Everitt BJ, Sabino V, Cottone P. High Trait Impulsivity Predicts Food Addiction-Like Behavior in the Rat. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature protocols. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]