Abstract
Background
In mice, group 2 innate lymphoid cells (ILC2s) likely mediate helminth immunity, inflammation and tissue repair and remodeling. However, the involvement of ILC2s in human diseases, such as asthma, is not well understood.
Objective
The goals of this study were to investigate whether peripheral blood specimens can be used to monitor innate type 2 immunity in human subjects and to examine whether ILC2s are involved in human asthma.
Methods
Peripheral blood mononuclear cells (PBMCs) from subjects with allergic asthma (AA), subjects with allergic rhinitis (AR), or healthy controls (HC) were cultured in vitro with IL-25 or IL-33. Flow cytometry and cell sorting were used to identify, isolate, and quantitate ILC2s in PBMCs.
Results
Human PBMCs produced IL-5 and IL-13 when stimulated with IL-33 or IL-25 in the presence of IL-2 without antigens. In addition, IL-7 or thymic stromal lymphopoietin were able to replace IL-2. The cell population with phenotypic ILC2 characteristics, lineage−CD127+CRTH2+ cells, responded to IL-33 and produced large quantities of IL-5 and IL-13, but undetectable IL-4. PBMCs from subjects with AA produced significantly larger amounts of IL-5 and IL-13 in response to IL-25 or IL-33 compared to subjects with AR or HC. The prevalence of ILC2s in blood was greater in the AA group compared to the AR or HC groups.
Conclusion
Innate type 2 immune responses are increased in asthma but not in allergic rhinitis, suggesting potential differences in the immunopathogenesis of these diseases. Peripheral blood is useful for evaluating innate type 2 immunity in humans.
Keywords: IL-33, IL-25, IL-5, IL-13, ILC2, innate immunity, asthma, allergy
INTRODUCTION
Asthma is a chronic inflammatory disease of the respiratory tract that is characterized by airway inflammation, remodeling, hyperresponsiveness, and reversible obstruction. Asthma affects over 300 million people worldwide, and causes substantial medical and financial burdens in the United States.1 Both genetic and environmental factors are likely involved in asthma pathogenesis.2 Genome-wide association studies (GWAS) show an association between asthma and single nucleotide polymorphisms (SNPs) in several genes, including IL1RL1, IL18R, IL33, TSLP, and RORA.3, 4
CD4+ Th2 cells have a significant role in asthma.5–7 Indeed, type 2 cytokines produced by Th2 cells, such as IL-4, IL-5, and IL-13, drive many features of asthma, including IgE class switch, airway remodeling, airway hyperresponsiveness (AHR), and mucus overproduction. In addition, type 2 cytokines recruit, maintain, and activate eosinophils.8–10 However, the mechanisms involved in the development of antigen-specific Th2 cells, as well as those involved in the ongoing production of type 2 cytokines in asthmatic airways, are poorly understood.
Over the past decade, rapid progress has been made in our understanding of the mechanisms involved in the development of type 2 immunity. First, three cytokines, thymic stromal lymphopoietin (TSLP), IL-25, and IL-33, have emerged as strong candidates for the development of type 2 immunity, acting as the link(s) between innate recognition of pathogens and the resulting type 2 adaptive immune response.11, 12 Airway epithelial cells, as well as other tissues and immune cells, produce and release these cytokines upon activation of TLRs as well as via other as yet undefined mechanisms.12–14 Second, an emerging family of innate lymphoid cells (ILCs) is recognized for its crucial role in mucosal immunity, tissue repair, and remodeling.15, 16 Group 2 innate lymphoid cells (ILC2s) are a newly recognized subset of the ILC family and have been gaining attention in several scientific fields. ILC2s are identified in mesenteric fat-associated lymphoid clusters, gut-associated lymphoid tissue, mesenteric lymph nodes, spleen, liver, and lungs of mice.17–23 Murine ILC2s are characterized by ST2 expression (the IL-33 receptor, also known as IL1RL1), require RORα and IL-7Rα for their development,16, 24 and rapidly produce IL-5 and IL-13 in response IL-25 or IL-33.25
These epithelium-derived cytokines and ILC2s are involved in several aspects of lung pathology in mouse models of airway inflammation and immunity. For example, ILC2s mediate AHR, airway remodeling, and homeostasis after influenza virus infection.21, 22 Intranasal (i.n.) IL-33 or IL-25 administration causes eosinophilic airway inflammation in mice in the absence of T cells.23, 26, 27 Indeed, in the lungs of mice exposed to the protease papain or the natural aeroallergen Alternaria alternata, IL-33-responsive ILC2s provide the primary early cellular source of IL-5 and IL-13.23, 28 Furthermore, in a conventional ovalbumin (OVA)-induced adaptive immune response model, ILC2s as well as CD4+ T cells likely produce IL-13.26 Despite increasing knowledge regarding the roles of ILC2s in mice, our knowledge is limited about this novel cell type in human diseases.
In humans, ILC2s have been shown to accumulate at the site of eosinophilic inflammation, including nasal polyps and sinus mucosa in subjects with chronic rhinosinusitis (CRS), skin lesions in subjects with atopic dermatitis (AD), and pleural effusions from subjects with spontaneous pneumothorax.29–32 Here, to extend this investigation and to examine whether innate type 2 immune response and/or ILC2s can be used as a biomarker of human diseases, we performed a prospective study. We addressed two specific questions: First, because blood specimens are more widely available than tissue specimens in clinical studies, we evaluated whether the innate type 2 response can be observed and whether ILC2s can be identified in peripheral blood. Second, we examined whether ILC2s are involved in allergic diseases in humans. We report that human peripheral blood mononuclear cells (PBMCs) produce type 2 cytokines, IL-5 and IL-13, but not IL-4, upon stimulation with IL-25 or IL-33 in the absence of antigens. We verified that CD3+ cells are not required for the response, and that lineage-negative (Lin−)CD127+CRTH2+ cells in PBMCs robustly produce these cytokines. Innate type 2 responses were enhanced and ILC2s were increased in subjects with allergic asthma compared to normal control subjects. Intriguingly, increased innate type 2 responses were not observed in subjects with allergic rhinitis, suggesting potential differences in the immunopathogenesis of allergic asthma and allergic rhinitis.
MATERIALS AND METHODS
Study Subjects
Peripheral blood was obtained from normal subjects (healthy control; HC), subjects with allergic rhinitis (AR), or subjects with allergic asthma (AA) outside of the allergy season. Inclusion criteria for AA were as follows: (1) a pre-bronchodilator FEV1 >60% of predicted normal with a post bronchodilator increase in flow >12% and/or a reduction in FEV1 >20% following 1–5 breaths of 25 mg/dl inhaled methacholine, (2) physician-diagnosed asthma, and (3) allergic sensitization to aeroallergens confirmed by new or existing skin testing or IgE serum immunoassays. The Mayo Clinic Institutional Review Board approved this study and informed consent was obtained from all participants. Peripheral blood from normal individuals and allergic donors was used in the experiments shown in Figure 1 and Figure 3. The study populations in Figure 2 and Figure 4 are described in detail in the legends. (See additional Methods descriptions in the Online Repository)
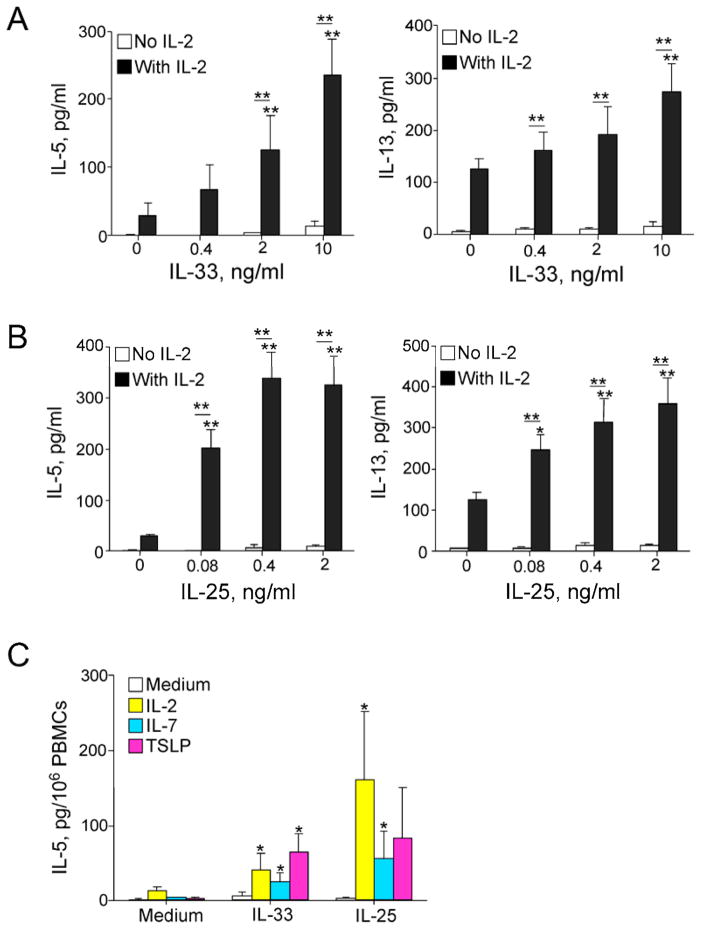
Figure 1. IL-33 or IL-25 induce antigen-independent type 2 cytokine production by human PBMCs.
PBMCs were cultured without antigen for 7 days with increasing concentrations of IL-33 (A) or IL-25 (B) in the presence or absence of 20 U/ml IL-2. IL-5 and IL-13 levels in the supernatants were determined using ELISA. Data are the mean ± SEM of 30 subjects. *p < 0.05; **p < 0.01, compared to IL-2 alone or between groups as indicated by horizontal bars. (C) PBMCs were cultured with medium, IL-33 alone (10 ng/ml), IL-25 alone (0.4 ng/ml), or in combination with IL-2 (20 U/ml), IL-7 (10 ng/ml), or TSLP (10 ng/ml). IL-5 levels in the supernatants were determined. Data are the mean ± SEM of 6 experiments. *p < 0.05 compared to IL-33 alone or IL-25 alone.
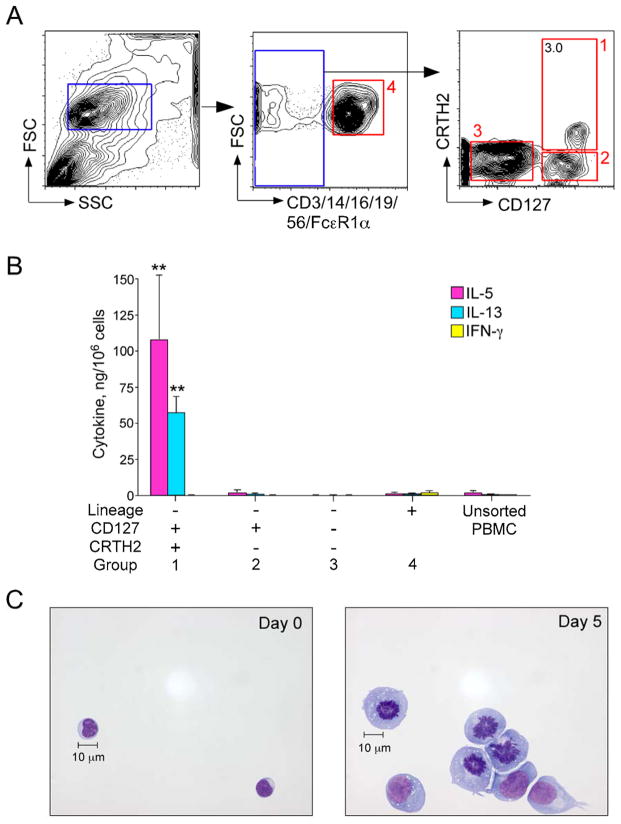
Figure 3. Circulating ILC2s in peripheral blood respond to IL-33 ex vivo.
(A) Gating strategy to isolate ILC2s and other lineage-negative (Lin−) and lineage-positive (Lin+) cell populations. Lin− lymphocytic cells were subdivided into three populations based on CD127 and CRTH2 expression. Red boxes (labeled 1–4) indicate the sorted cells. (B) Sorted cell populations from A were stimulated for 5 days with medium alone or IL-33 (10 ng/ml) plus IL-2 (20 U/ml). Levels of IL-5, IL-13, and IFNγ in cell-free supernatants were measured using Milliplex. Data shown are the mean ± SEM of 3 independent experiments. **p < 0.01 compared to medium alone. (C) ILC2s (Box 1 in A) were cultured for 5 days with IL-33 plus IL-2. Cell morphology is shown before and after culture by staining the cytospin preparations with Wright Giemsa. Scale bar = 10 μm.
Figure 2. Lineage-negative (Lin−) cells are necessary for IL-25- and IL-33-induced IL-13 production.
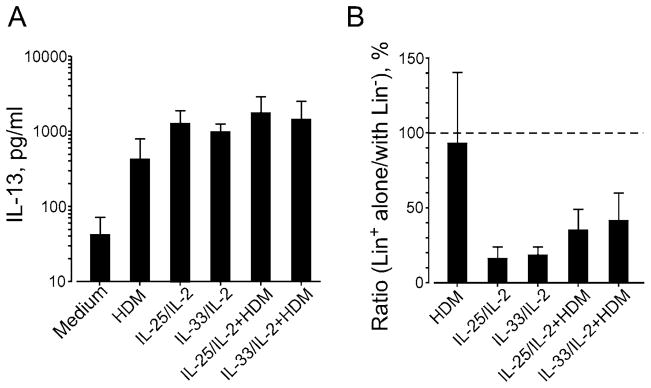
PBMCs from subjects with who had allergic asthma and were skin test-positive for house dust mite (HDM) were separated into two fractions, the Lin+ with Lin− cell fraction and the Lin+ cell alone fraction. The Lin+ with Lin− cell fraction contained 82.0% Lin+ cells and 18.0% Lin− cells; the Lin+ cell alone fraction contained 99.5% Lin+ cells and 0.5% Lin− cells. The fractions were cultured with HDM extract (25 μg/ml), IL-25 or IL-33 (10 ng/ml) plus IL-2 (20 U/ml), or combinations of HDM extract and cytokines for 7 days. (A) IL-13 levels in cell-free supernatants of the Lin+ with Lin− cell fraction were determined. (B) IL-13 levels in cell-free supernatants of the Lin+ cell alone fraction are presented as the ratio of the Lin+ cell alone fraction divided by the Lin+ with Lin− cell fraction from the same donor. Data are the mean ± SEM of 3 subjects.
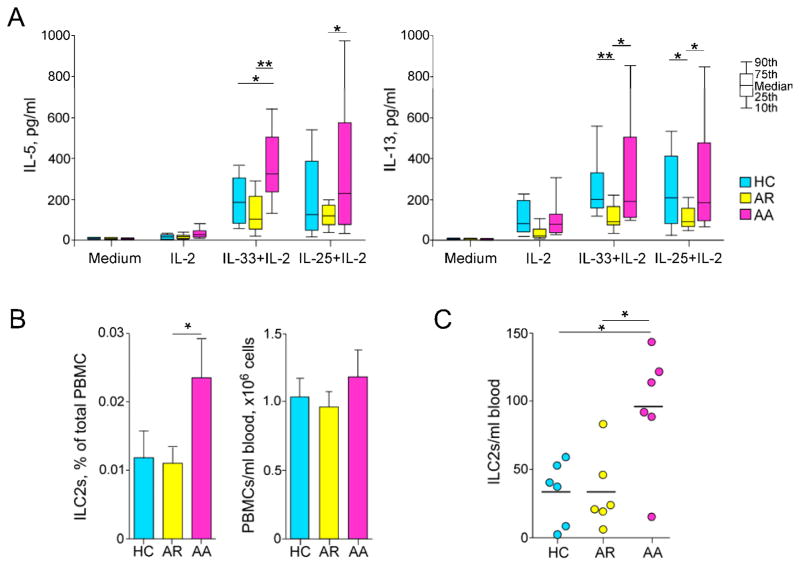
Figure 4. PBMCs from patients with allergic asthma show enhanced type 2 responses to IL-33 or IL-25.
(A) PBMCs from healthy controls (HC), subjects with allergic rhinitis (AR), or subjects with allergic asthma (AA) were cultured for 7 days with medium alone, IL-2 (20 U/ml) alone, IL-2 plus IL-33 (20 U/ml and 10 ng/ml, respectively), or IL-2 plus IL-25 (20 U/ml and 0.4 ng/ml, respectively). IL-5 (left) and IL-13 (right) levels in cell-free supernatants were determined using ELISA. Data shown are box and whisker plots (10th, 25th, 50th, 75th, and 90th percentile) of 14 or 15 subjects per group. *p < 0.05; **p < 0.01, between the groups indicated by horizontal bars. (B) ILC2s in PBMCs were identified and enumerated using flow cytometry as described in Figure 2A. Proportion of ILC2s in PBMCs and the total number of PBMCs per ml of blood are shown as the mean ± SEM (n = 6 per group). *p < 0.05, between the groups indicated by the horizontal bar. (C) Total numbers of ILC2s per ml of blood were calculated. Each dot represents one patient, and horizontal bars indicate the mean for each group.
Reagents
FITC-labeled antibodies to CD3 (SK7), CD14 (MϕP9), CD16 (NKP15), CD19 (4G7), and CD56 (NCAM16.2), PerCP-Cy5.5-labeled antibody to CD44 (G44-26), PE-labeled antibody to IL-5, and AF647-labeled antibodies to CD127 (HIL-7R-M21) and CRTH2 (BM16) were purchased from BD Biosciences (San Jose, CA). (See additional Methods descriptions in the Online Repository)
Peripheral blood mononuclear cells (PBMC) isolation and stimulation for cytokine production
Heparinized peripheral blood was layered over an equal volume of Histopaque 1077 (Sigma-Aldrich; St. Louis, MO) and centrifuged per the manufacturer’s instructions. Mononuclear cells were collected from the interface between Histopaque and plasma. PBMCs were washed and resuspended in RPMI 1640 media (Gibco/Life Technologies; Grand Island, NY) containing 10% heat-inactivated human AB serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) (Gibco/Life Technologies). Cells were cultured in 96-well round-bottom plates (1.3 to 3.3×106 cells/ml) at 37°C with 5% CO2 for 7 days with IL-33 or IL-25 at the indicated concentrations in the presence or absence of IL-2 (20 U/ml), IL-7 (10 ng/ml), or TSLP (10 ng/ml). IL-5, IL-13, and IFNγ concentrations in the cell-free supernatants were determined using ELISA kits as recommended by the manufacturer (Thermo Fisher Scientific; Rockford, IL). (See additional Methods description in the Online Repository)
FACS sorting and human peripheral blood ILC2 culture
PBMCs were stained with antibodies to lineage markers (CD3, CD14, CD16, CD19, CD56, and FceRIa) and antibodies to CD127 and CRTH2. After staining, cells were washed and fixed with 1% paraformaldehyde and analyzed using flow cytometry. For sorting experiments, lineage-negative (Lin−) cells from PBMCs of subjects with allergic asthma were enriched by depleting lineage-positive (Lin+) cells using FITC-conjugated antibodies to CD3, CD14, CD16, CD19, CD56, and FcεR1α, along with the EasySep FITC selection kit (StemCell Technologies; Vancouver, BC, Canada) as per manufacturer’s instructions. This enrichment process produced approximately 50% pure Lin− cells. Lin−-enriched cells were stained with AF647-conjugated anti-CRTH2 and PE-Cy7-conjugated anti-CD127 or appropriate isotype-matched controls and sorted using a FACSAria (BD Biosciences) into Lin+ and Lin− populations. Lin− cells were further sorted into three populations based on CRTH2 and CD127 expression, resulting in Lin−CD127−CRTH2−, Lin−CD127+CRTH2+, and Lin− CD127+CRTH2− populations. Cells were cultured at 4–10×104 cells/ml in 96-well tissue culture plates. (See additional Methods descriptions in the Online Repository)
Statistical analyses
Data are presented as the mean ± SEM for the numbers of subjects or experiments as indicated. Statistics were performed using paired and unpaired Student’s t-tests, one-way analysis of variance (ANOVA), or repeated measures ANOVA as appropriate for each set of experimental conditions. p < 0.05 was considered statistically significant.
RESULTS
IL-33 and IL-25 stimulate type 2 cytokine production by PBMCs without antigens
In vitro stimulation of murine tissue cells (adipose tissues, lung and bone marrow) with IL-33 results in IL-5 and IL-13 production, and ILC2s are predominantly responsible for the response.17, 22, 23, 28, 33 ILC2s were also identified in human peripheral blood.29 To determine whether human peripheral blood, in particular PBMCs, display similar antigen-independent type 2 cytokine responses, we cultured PBMCs with IL-33 in the presence or absence of IL-2. No antigen was added to the culture. When cultured with IL-33 alone, PBMCs produced minimal amounts of IL-5 (Figure 1A, left panel). IL-2 alone induced modest IL-5 production. When combined with IL-2, IL-33 induced significant IL-5 production in a concentration-dependent manner (p<0.01). In addition, IL-33 alone induced minimal IL-13 production (Figure 1A, right panel). In the presence of IL-2, IL-33 induced concentration-dependent production of IL-13.
To examine whether PBMCs can be stimulated with another pro-type 2 cytokine, we performed parallel experiments using IL-25. Similar to IL-33, IL-25 alone induced minimal production of IL-5 or IL-13 in PBMCs (Figure 1B). In the presence of IL-2, IL-25 significantly induced IL-5 and IL-13 production in a concentration-dependent manner (p<0.01). A comparison of the dose-response curves in panels A and B showed that IL-25 is approximately 25 times more potent than IL-33. Because IL-2 belongs to the common cytokine receptor γ-chain (γc) family and activates the STAT5 pathway,34 we questioned whether other cytokines in the same family are capable of replacing IL-2 and stimulating PBMCs. Indeed, although IL-7 or TSLP alone induced minimal IL-5 or IL-13 production, they synergized with IL-33 or IL-25 and significantly enhanced IL-5 and IL-13 production by PBMCs (Figure 1C, p<0.05).
To verify that the type 2 responses that we detected in PBMCs are independent of T cells, we depleted PBMCs of CD3+ cells prior to culture using immunomagnetic beads. CD3+ cell depletion did not decrease but rather enhanced PBMC IL-5 and IL-13 production when stimulated with IL-25 plus IL-2 (see Figure E1 in this article’s Online Repository).
Finally, to compare antigen- and IL-25/IL-33-dependent type 2 cytokine production, PBMCs from subjects with allergic asthma who were sensitive to house dust mite (HDM) were divided into two fractions: those with both Lin+ cells and Lin− cells and those with Lin+ cells alone. The fraction with Lin+ cells and Lin− cells clearly produced IL-13 when stimulated with HDM extract or IL-25 or IL-33 in the presence of IL-2 (Figure 2A). IL-25- and IL-33-induced IL-13 production was nearly abolished in the fraction with Lin+ cells alone (Figure 2B); in contrast, HDM-induced IL-13 production was not affected in this fraction. Taken together, these findings suggest that, similar to mouse mucosal tissues, human PBMCs produce type 2 cytokines when stimulated with IL-33 or IL-25 in the presence of IL-2 and other γc-family cytokines, and that the response is likely mediated by innate immune cells.
Functional ILC2s are present in human peripheral blood
In antigen-dependent systems, the predominant source of IL-5 and IL-13 are CD4+ Th2 cells.7 In 2010, several laboratories independently identified a murine Lin− lymphoid cell type that responds to IL-25 and/or IL-33 and produces type 2 cytokines.17–19 These cells were subsequently termed group 2 innate lymphoid cells or ILC2s by a consensus report.25 A homologous population of innate immune cells is beginning to be identified in human mucosal organs and peripheral blood.29–32 We hypothesized that ILC2s are the source of IL-5 and IL-13 in PBMC cultures. Therefore, we freshly isolated cells from human PBMCs using sorting with flow cytometry. Because there is no established protocol for identifying human ILC2s, we followed the strategy used by Mjosberg et al. 25, 29 We used a lineage cocktail of antibodies to CD3, CD14, CD16, CD19, CD56, and FcεRIα to identify T cells, monocytes, neutrophils, B cells, NK cells, mast cells and basophils. Lin− cells, which are negative for these lineage markers, were further subdivided and sorted based on CD127 and CRTH2 expression (Figure 3A). Finally, the Lin+ cell population as well as three populations of Lin− cells (Figure 3A, identified by red boxes 1–4) were cultured with IL-33 plus IL-2 for 5 days. The Lin− and CD127/CRTH2 double-positive cell population (i.e., Lin−CD127+CRTH2+) produced a large quantity of IL-5 and IL-13 (Figure 3B, please note the scale of the y-axis), whereas Lin−CD127+CRTH2+ cells cultured with medium alone did not produce detectable IL-5 or IL-13 (data not shown). Lin+ cells as well as the Lin−CD127+CRTH2− population produced small amounts of IL-5 and IL-13, and no cytokine was detected in the Lin−CD127−CRTH2− population. Minimal IFNγ was produced by Lin+ cells but not by any of the three Lin− populations. No or minimal amounts of IL-4 (<4 pg/106 cells) or IL-17A (107 pg/106 cells) were produced by Lin−CD127+CRTH2+ cells stimulated with IL-33 plus IL-2. In addition, in a separate experiment, large quantities of IL-5 (79 ng/106 cells) and IL-13 (121 ng/106 cells) were produced by Lin−CD127+CRTH2+ cells stimulated with IL-33 plus IL-7.
Stimulation of Lin−CD127+CRTH2+ cells with IL-33 plus IL-2 also dramatically changed their cell morphology. Prior to culture, Lin−CD127+CRTH2+ cells were approximately 10 μm in diameter with a high nucleus to cytoplasm ratio, and were indistinguishable from common lymphocytes (Figure 3C, left panel). Culture with IL-33 plus IL-2 caused marked increases in cellular size, the development of Golgi bodies and endoplasmic reticulum, and a decreased nucleus to cytoplasm ratio (Figure 3C, right panel). In addition, cells with apparent mitotic nuclei were frequently observed. We also used intracellular cytokine staining to verify production of IL-5 by ILC2s within the PBMCs. To circumvent technical problems associated with intracellular staining, ILC2s were gated as the Lin−CD127+CD44hi population (see Figure E2 in this article’s Online Repository). This cell population uniformly expressed the IL-33 receptor ST2 and produced IL-5 when PBMCs were stimulated with IL-33 plus IL-2. Taken together, these results indicate that within human PBMCs, the Lin−CD127+CRTH2+ population, which is compatible with the published phenotypic characteristics of human ILC2s, responds vigorously to IL-33 and produces large amounts of IL-5 and IL-13, but not IL-4. Other Lin− cell populations or Lin+ cells do not appear to have similar capabilities.
Innate type 2 responses are enhanced in the peripheral blood of patients with asthma
To investigate the utility of PBMC analyses of innate type 2 responses and to investigate the association between ILC2s and human asthma, we performed a prospective study. We examined PBMCs from subjects with allergic asthma (AA) and recruited subjects with allergic rhinitis (AR) and healthy controls (HC) as disease and normal controls, respectively. These three groups did not differ by age or sex (Table 1). However, as expected, FEV1 was significantly lower in the AA group compared to the AR group. Inhaled corticosteroid and short-acting bronchodilator use was significantly greater in AA compared to AR or HC. No apparent differences in allergen sensitizations were observed between AA and AR subjects.
Table 1.
| Healthy Controls (HC) (n = 18) | Allergic Rhinitis (AR) (n = 16) | Allergic Asthma (AA) (n = 18) | P values | |
|---|---|---|---|---|
| Age (y), mean +/− SEM | 43.4 +/− 2.9 | 47.7 +/− 3.0 | 45.6 +/− 3.3 | .5781 |
| Sex, female/male | 14/4 | 9/7 | 9/9 | .2960 |
| Asthma | 0 | 0 | 18 | |
| Allergic Rhinitis | 0 | 16 | 18 | |
| CRS | 0 | 0 | 0 | |
| Ever smoked (%) | 11.1 | 25.0 | 0 | .0746 |
| Current smoker (%) | 0 | 12.5 | 0 | .0963 |
| Ever had immunotherapy (%) | 0 | 0 | 5.6 | .3818 |
| FEV1 (% predicted, mean) (subjects tested) | 112 (1) | 105 (9) | 85 (16) | .0004* |
| Medications | ||||
| ICS (subject number) | 0 | 2 | 10 | 0.0087*, 0.0002¶ |
| LABA (subject number) | 0 | 0 | 2 | ns |
| SABD (subject number) | 0 | 0 | 18 | <0.0001*¶ |
| LTM (subject number) | 0 | 0 | 3 | ns |
| Skin Test, positive/subjects tested | 0/5 | 16/16 | 15/15 | <0.0001ঠ|
| Trees (%) | 0 | 62.5 | 55.6 | |
| Grass (%) | 0 | 50.0 | 38.9 | |
| Ragweed (%) | 0 | 81.3 | 72.2 | |
| Mold (%) | 0 | 50.0 | 38.9 | |
| Dust mite (%) | 0 | 31.3 | 50.0 | |
| Pet (%) | 0 | 31.3 | 61.1 | |
| Cockroach (%) | 0 | 6.3 | 16.7 | |
P values;
AR vs AA,
HC vs AA,
HC vs AR
Abbreviations: HC, Healthy control; AR, Allergic rhinitis; AA, Allergic asthma; FEV1, Forced expiratory volume in 1 second; ICS, inhaled corticosteroids; LABA, long-acting beta agonists; SABD, short-acting bronchodilators; LTM, leukotriene modifiers; n.s., not significant
Fresh PBMCs from donors were stimulated with IL-33 plus IL-2 or IL-25 plus IL-2 without antigens, and the concentrations of IL-5 and IL-13 in cell-free supernatants were analyzed as an indicator of the innate type 2 response. As described earlier (Figure 1), IL-2 alone induced minimal IL-5 production from PBMCs of all three groups (Figure 4A, left panel). When stimulated with IL-33 plus IL-2, PBMCs from the HC group produced modest amounts of IL-5, suggesting that innate type 2 responses exist in normal individuals. Importantly, IL-5 production by PBMCs stimulated with IL-33 plus IL-2 was significantly greater in AA subjects compared to HC subjects (Figure 4A, p<0.05). In contrast, PBMCs from the AR group produced comparable amounts of IL-5 compared to PBMCs from the HC group and significantly lower amounts compared to the AA group (Figure 4A, p<0.01). When stimulated with IL-25 plus IL-2, PBMCs from the AA group produced significantly higher amounts of IL-5 compared to PBMCs from the AR group (p<0.05). When IL-13 levels in the same specimens were analyzed, PBMCs from the AA group produced significantly more IL-13 than those from the AR group (Figure 4A, right panel, p<0.05). Furthermore, considerable individual variability in the PBMC responses to IL-33 plus IL-2 or IL-25 plus IL-2 was noted in the AA group.
To investigate whether the observed differences in innate type 2 responses among the groups can be explained by the numbers of ILC2s, we used flow cytometry to quantitate ILC2s in peripheral blood. Using the same gating strategy outlined in Figure 3A, we identified ILC2s as Lin−CD127+CRTH2+ cells. ILC2s generally consisted of a small fraction, approximately 0.01~0.03%, of total PBMCs in peripheral blood (Figure 4B). The proportion of ILC2s in PBMCs (Figure 4B) and the number of ILC2s in a given volume of peripheral blood (Figure 4C) were significantly increased in the AA group compared to the AR or HC groups (p<0.05). In addition, within the AA group, heterogeneity in the number of ILC2s was observed (Figure 4C). There were no apparent differences in the expression levels of IL-33 receptor ST2 by ILC2s between the AA group and the HC group (see Figure E3 of this article’s Online Repository). These findings suggest that the innate type 2 immune response as well as the number of ILC2s is increased in patients with allergic asthma, but not in patients with allergic rhinitis.
DISCUSSION
The Lin−CD127+CD161+CRTH2+ST2+ cell population was initially identified in human fetal gut, adult peripheral blood, and nasal polyp tissues.29 These cells produced IL-13 in response to IL-25, suggesting that they are the human counterpart of murine ILC2s. In this study, we verified these previous observations using cell sorting. Sorted human Lin−CD127+CRTH2+ cells produced IL-5 and IL-13, but not IL-4 or IFN-γ upon in vitro stimulation with IL-33 (Figure 3). They proliferated vigorously and developed prominent Golgi bodies and endoplasmic reticulum structures, consistent with their robust ability to produce cytokine proteins (Figure 3). In addition, IL-2, as well as IL-7 and TSLP, acted synergistically with IL-25 or IL-33 (Figure 1). Taken together, the phenotypic data as well as the functional and morphologic characteristics strongly suggest the Lin−CD127+CRTH2+ cells in human peripheral blood are ILC2s.
In the current study, we used flow cytometry and found ILC2s in the PBMCs of healthy control subjects as well as in subjects with allergic asthma or allergic rhinitis. Thus, the presence of ILC2s per se is unlikely to be unique to a specific disease. Consistently, PBMCs from healthy control subjects responded to IL-33 or IL-25 stimulation and produced type 2 cytokines. Importantly, however, such innate type 2 responses were significantly upregulated in patients with allergic asthma. Furthermore, ILC2s were more abundant in peripheral blood from patients with allergic asthma, providing a plausible explanation for the increased cytokine production by PBMCs. However, it is also possible that ILC2s from subjects with allergic asthma are optimally primed for IL-33 or IL-25 responsiveness or enhanced for cytokine production, and our results cannot rule out this possibility. Biochemical characterization, such as expression and phosphorylation of GATA3 and p38 MAPK, may be necessary to explain the potential functional differences in ILC2s from different diseases because these molecules may provide critical regulatory signals in murine ILC2 functions.35 Nonetheless, our study demonstrates that human fresh peripheral blood cells, namely PBMCs, can be used to monitor functional innate type 2 responses and to quantitate ILC2 numbers.
One of the major findings in this study is the clear difference in the innate type 2 responses between patients with allergic asthma and patients with allergic rhinitis (Figure 4A). Therefore, increased innate type 2 immunity and ILC2 numbers are unlikely to be markers for Th2-type immune responses in general. We speculate that ILC2s and their products, including IL-5, IL-13, and tissue growth factors, play a more prominent role in allergic asthma than in allergic rhinitis. Indeed, a GWAS identified associations between asthma and SNPs in IL1RL1/IL18, IL33, and RORA,3, 4 thus implicating both ILC2s and IL-33 in the pathophysiology of asthma. RORA is a key transcription factor for ILC2 development.24, 36 In the same GWAS, associations between serum IgE levels and FCER1A, IL13, STAT6, and IL4R/IL21R SNPs were detected,4 but no overlap was observed between the key SNPs associated with serum IgE and those associated with asthma.37 It is also intriguing to note that ILC2s have the capacity to regulate basal eosinophilopoiesis and tissue eosinophil accumulation through constitutive and stimulated production of IL-5 in mice.38 Thus, the immunopathogenesis of asthma and allergy is likely different, and asthma may be affected by type 2 innate immunity as well as adaptive immunity whereas allergy may be affected predominantly by type 2 adaptive immunity.
A question remains regarding why ILC2s are increased in peripheral blood from subjects with allergic asthma. ILC2s, similar to other ILC family members, arise from common lymphoid progenitors in the bone marrow and require the transcriptional inhibitor Id2 and transcription factors RORα and GATA3 for their development.24, 35, 36, 39, 40 Like natural killer cells, ILC2s are recruited directly to mucosal tissues through peripheral blood circulation.16 Systemic IL-25 or IL-33 administration into the peritoneal cavity of mice increases the number of ILC2s in the lungs.26 Human ILC2s respond to IL-33 or IL-2529, 30 as well as lipid mediators, such as prostaglandin D241 and leukotriene D442 in vitro. Indeed, increased IL-33 protein expression is observed in respiratory mucosa of patients with severe asthma.43 IL-33 was also readily induced by exposing airway epithelial cells from patients with nasal polyps to fungal antigens in vitro.30 Thus, increased numbers of ILC2s in human peripheral blood may reflect increased production and/or release of IL-33 and other innate inflammatory mediators in respiratory mucosa.
In summary, we have described the connections between innate type 2 immune responses and allergic asthma. However, one limitation of this study is its relatively small sample size, which precludes us from performing subgroup analyses. Several questions remain to be answered. For example, do the number or activity of circulating ILC2s correlate with disease severity or exacerbation? Clearly, clinical studies with different study designs and larger sample sizes are necessary to address such questions. Nonetheless, this report demonstrates that the innate type 2 immune response and ILC2s can be monitored using PBMCs from patients with allergic diseases as well as from normal individuals. These data provide a novel research tool and evidence for its feasibility in addressing the roles of innate type 2 immunity and ILC2s in human diseases.
Supplementary Material
KEY MESSAGES.
Human PBMCs demonstrate innate type 2 immune responses to IL-33 and IL-25 in the presence of IL-2.
Group 2 innate lymphoid cells (ILC2s) isolated from PBMCs produce large quantities of IL-5 and IL-13, but not IL-4, when stimulated with IL-33.
Innate type 2 responses are enhanced and ILC2s are increased in the peripheral blood of subjects with allergic asthma, but not in subjects with allergic rhinitis.
Acknowledgments
Supported by NIH Grant AI34486 and the Mayo Foundation.
We thank Ms. LuRaye Eischens for secretarial help and Ms. Kay Bachman for recruitment of study subjects.
ABBREVIATIONS
- AA
allergic asthma
- AD
atopic dermatitis
- AHR
airway hyperresponsiveness
- APC
allophycocyanin
- AR
allergic rhinitis
- CRS
chronic rhinosinusitis, DCs, dendritic cells
- ELISA
enzyme-linked immunosorbent assay
- FITC
fluorescein isothiocyanate
- GWAS
genome-wide association studies
- HDM
house dust mite
- IL
interleukin
- ILCs
innate lymphoid cells
- i.n
intranasal
- HC
healthy control
- Lin+
lineage-positive
- Lin−
lineage-negative
- PBMC
peripheral blood mononuclear cells
- PerCP
peridinin chlorophyll protein complex
- SEM
standard error of the mean
- SNP
single nucleotide polymorphism
- TLR
toll-like receptor
- TSLP
thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.From the Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2012 Available from: http://www.ginasthma.org/
- 2.Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis. 1990;142:1351–8. doi: 10.1164/ajrccm/142.6_Pt_1.1351. [DOI] [PubMed] [Google Scholar]
- 3.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–7. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 4.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faul JL, Demers EA, Burke CM, Poulter LW. The reproducibility of repeat measures of airway inflammation in stable atopic asthma. Am J Respir Crit Care Med. 1999;160:1457–61. doi: 10.1164/ajrccm.160.5.9812027. [DOI] [PubMed] [Google Scholar]
- 6.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 7.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 8.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 9.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 11.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–90. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol. 2012;143:222–35. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–87. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 16.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–7. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 17.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of Th2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 18.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–6. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–8. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage−CD25+CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–36. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 26.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–8. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 27.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–16. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 28.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 30.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–9. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon BI, Hong S, Shin K, Choi EH, Hwang JJ, Lee SH. Innate type 2 immunity is associated with eosinophilic pleural effusion in primary spontaneous pneumothorax. Am J Respir Crit Care Med. 2013;188:577–85. doi: 10.1164/rccm.201302-0295OC. [DOI] [PubMed] [Google Scholar]
- 33.Brickshawana A, Shapiro VS, Kita H, Pease LR. Lineage(−)Sca1(+)c-Kit(−)CD25(+) cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J Immunol. 2011;187:5795–804. doi: 10.4049/jimmunol.1102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furusawa J, Moro K, Motomura Y, Okamoto K, Zhu J, Takayanagi H, et al. Critical role of p38 and GATA3 in natural helper cell function. J Immunol. 2013;191:1818–26. doi: 10.4049/jimmunol.1300379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–59. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–8. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–48. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein Wolterink RGJ, Serafini N, van Nimwegen M, Vosshenrich CAJ, de Bruijn MJW, Pereira DF, et al. Essential, dose-dependent role for the transcription factor GATA3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proc Natl Acad Sci USA. 2013;110:10240–5. doi: 10.1073/pnas.1217158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra26. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–13. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–4. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.