Abstract
Objective
To determine the impact of hormonal contraception (HC) on markers of ovarian reserve, including Anti-mullerian hormone (AMH) and antral follicle count (AFC)
Design
Longitudinal, prospective cohort
Setting
University hospital
Patient(s)
Young adult female cancer survivors and healthy, similarly aged women
Intervention(s)
None
Main Outcome Measure(s)
Participants were followed annually with hormone levels and transvaginal ultrasound. Subjects who used HC within the preceding three months were considered as exposed. Linear mixed effects models were used to incorporate repeated measures and adjust for potential confounders.
Result(s)
249 women (126 survivors, 123 controls; average age 25.5) were followed for an average of 2.1 visits and over 2.15 years. After adjusting for confounders, AMH was found to be 21% lower among survivors and 55% lower among controls using HC (RR 0.79, 95% CI 0.68-0.93 & RR 0.45, 95% CI 0.30-0.68, respectively). AFC was 20% lower among survivors and controls taking HC (RR 0.80, 95% CI 0.69-0.93). When considering an individual subject, AMH was 17-35% lower when a subject had recently used HC compared to when she had not (Survivors: RR 0.83, 95% CI 0.75-0.93; Controls: RR 0.65, 95% CI 0.55-0.78), and AFC was 11% lower (RR 0.89, 95% CI 0.82-0.96). Additive HC exposure across multiple visits was not associated with differences in AMH or AFC.
Conclusion(s)
AMH and AFC are significantly lower among women with recent exposure to hormonal contraception. AMH and AFC should be interpreted with caution when measured in the setting of recent hormone use.
Keywords: contraception, anti-mullerian hormone, antral follicle count, ovarian reserve, birth control, cancer
Introduction
Decreased ovarian reserve is a common cause for female infertility, especially among cancer survivors and women of advanced reproductive age. With improved life expectancy among cancer survivors and advances in oocyte cryopreservation, more women are pursuing fertility preservation even with no infertility diagnosis.
Traditionally, fertility specialists assessed ovarian reserve with early follicular Follicle Stimulating Hormone (FSH). However, the limitations of FSH are well recognized, including its variability during the menstrual cycle and limited clinical utility during contraceptive use. Recently, other markers, including Anti-Mullerian Hormone (AMH) and ovarian antral follicle count (AFC), have been utilized for assessing the ovarian follicular pool. Produced by granulosa cells of preantral and antral follicles, AMH inhibits recruitment of primordial follicles into the follicular pool.(1) Although debate exists as to whether AMH reflects true ovarian reserve, low AMH levels have been shown to be highly specific when screening for poor response to ovarian stimulation(2), and AMH is widely used to individualize stimulation protocols for women undergoing in vitro fertilization (IVF). (3) AMH and AFC correlate with pregnancy rates and response to ovarian stimulation during IVF (4-8) and are associated with likelihood of pregnancy and time to menopause in population-based studies. (9,10)
AMH and AFC have traditionally been thought to be unaffected by hormonal contraceptive use, but recent data are contradictory. (11-16) Additionally, little data exist describing the impact of hormonal contraception on ovarian reserve markers among women exposed to gonadotoxic therapies. We sought to determine the impact of hormonal contraception (HC) on markers of ovarian reserve, including AMH and AFC, among female cancer survivors remote from cancer therapy and healthy, same-age women.
Methods
The institutional review board at the University of Pennsylvania approved this longitudinal, prospective cohort study. The trial was designed to assess changes in ovarian reserve markers in female cancer survivors and age-matched controls. All participants signed informed consent prior to enrollment.
Subjects
Adolescents and young women with a history of cancer were identified through the Abramson Cancer Center at the University of Pennsylvania, The Children's Hospital of Philadelphia, and community referrals. Postmenarchal, otherwise healthy female cancer survivors were enrolled if they were 11-39 years old, at least one year from cancer therapy, and had a uterus and at least one ovary. For each cancer survivor enrolled, a healthy, postmenarchal female within two years of age was recruited as a control. Women without exposure to chemotherapy or radiation and who had a uterus and at least one ovary were eligible. Women in both groups with the following conditions were excluded: pregnancy or lactation within three months, chronic illness limiting compliance with study protocol, medical condition associated with premature ovarian failure, or ovulatory dysfunction (i.e. polycystic ovary syndrome (PCOS)).
Data collection
Subjects were followed annually. For participation, subjects were asked to stop HC for at least four weeks and come in during their subsequent menstrual cycle. All subjects stopped HC for the initial study visit. Occasionally, subjects were unwilling to stop HC for a subsequent visit. Therefore, some participants were on HC during study visits, and some had stopped HC several weeks prior to the visit.
For subjects with regular menstrual cycles (21-35 days), assessments occurred during the early follicular phase (cycle day 1-5). Study visits were scheduled at any time during the cycle for women with irregular menses and those without withdraw bleeding six weeks after discontinuing HC.
At each visit, research personnel administered a detailed structured interview assessing medical, fertility, pregnancy, and smoking history. Subjects were specifically asked about exposure to HC, tamoxifen, gonadotropin-releasing agonists, and hormone replacement therapy (HRT). Subjects with premature ovarian failure (defined as taking HRT at enrollment) and subjects who reported using HC for HRT or menopausal symptoms were excluded. A pelvic ultrasound was performed to measure AFC, which was determined by counting all follicles 2-10 mm in average diameter. Blood samples were collected in serum separator tubes and allowed to coagulate at room temperature for 30 minutes. Tubes were then spun at 1000 RCF for 15 minutes, aliquotted into 1 mL tubes, and immediately frozen at -80°C. Samples were analyzed for AMH in batches by the Clinical Translation Research Center at the University of Pennsylvania using ELISA kits (Diagnostic Systems, Gen2, range 0.050-10.0 ng/mL, sensitivity 0.025 ng/mL, inter-assay variability <8%, intra-assay variability 5%).
Data Analysis
Any study visit at which subjects reported use of systemic HC during the three months preceding the visit was considered as exposed to HC for that visit, i.e. HC use was a time-varying covariate. Systemic HC included combined oral contraceptive pills (OCPs), progestin-only OCPs, contraceptive ring, contraceptive patch, or depot medroxyprogesterone acetate. Visits at which subjects were using levonorgestrel-containing intrauterine devices or data on HC use were not available were excluded. Differences in model estimates for AMH and AFC with and without HC exposure are discussed as relative risks (RR) and refer to ratios of AMH geometric means or AFC counts. Natural log transformed AMH levels were compared using multivariable linear regression models for repeated measures with robust variance estimates (GEE). (17) An interaction between HC use and cancer history (dichotomous variable indicating whether the subject previously had cancer) was tested for each outcome to assess evidence of effect modification. Interaction p-values <0.1 were considered further for multivariable models. Non-linear effects were evaluated by adding a quadratic term for age (age2) into model. Based on a priori hypotheses, the following covariates were explored further for evidence of confounding: age, age2, cancer history, BMI, tobacco use, nulliparity, race, baseline AMH/AFC, cycle day (dichotomous variable indicating whether visit occurred on cycle day 1-5). Covariates that changed the regression coefficient by less than 15% compared to a model without the covariate were dropped from the model unless they had been shown in the literature to be important confounders (i.e. age, smoking history). There was a significant interaction between cancer history and HC use; therefore, results for AMH were stratified and presented separately for cancer survivors and controls.
For AFC analyses, only visits where AFC was measured via transvaginal ultrasound were included. AFC was compared between women on HC at the visit and women not using HC via multivariable negative binomial regression models controlling for repeated measures within subject. This model allows for potentially over-dispersed Poisson counts. Cancer history was assessed for evidence of effect modification, and there was no significant interaction observed in AFC models. Therefore, the impact of HC on AFC is reported for cancer survivors and controls together.
While subjects with premature ovarian failure and history of hormone replacement therapy (HRT) use were excluded from the analysis, it is possible that some subjects may not have accurately reported HRT use. To account for the possibility that some subjects taking HC might have been using these agents for HRT, sensitivity analyses were performed excluding subjects at the highest risk for premature ovarian failure. Cut-offs were determined based on the values that represented the lowest quartile and lowest tertile for AMH and AFC among survivors at the first visit, which corresponded to AMH≤0.1ng/ml, AMH≤0.25 ng/mL, AFC≤6 and AFC≤9, respectively. All sensitivity analyses were restricted to visits occurring in the early follicular phase.
Since HC use varied over time, additional analyses were performed to estimate the impact of recent HC use on AMH and AFC within each individual. HC use was decomposed and analyzed as two variables, one representing the within individual effect of recent HC use on AMH and AFC (subject-specific effect) and the other representing the difference in ovarian reserve measures across subjects depending on additive HC exposure, estimated by the percentage of visits where the subject was exposed to HC (cross-sectional effect). (18) Covariates in these adjusted models included age, cancer history, smoking, cycle day, baseline AMH/AFC, and the interaction terms. Statistical analysis was performing using STATA version 12.1 (StataCorp, College Station, TX). A p-value <0.05 was considered statistically significant.
Results
Two hundred and forty-nine women, average age 25.5 years, met criteria for inclusion and completed 492 visits. Subjects had an average of 2.1 visits (range 1-8 visits) and were followed over 2.15 years (IQR 0.98-4.09 years). Recent HC exposure was observed in 14.6% (72/492) of visits, and 20.1% (50/249) of subjects reported recent HC exposure during at least one visit. Combined OCPs were most commonly used (79.2%), followed by transvaginal ring (11.1%), and progestin-only OCPs (9.7%).
One hundred and twenty-six survivors contributed 252 visits, and 123 controls contributed 240 visits. Survivors had an average of 2.15 visits (range 1-8 visits) and were followed for 1.01 years (IQR 0-2.96 years) compared to 2.06 visits (range 1-6 visits) over 1.04 years (IQR 0-2.66 years) among controls. Among survivors, 42 (33.3%) reported HC exposure during at least one visit, and HC exposure was observed during 61 visits (24.2%). Among controls, eight (6.5%) reported HC exposure during at least one visit, and HC exposure was observed during 11 visits (4.6%).
Baseline characteristics are presented in Table 1. There was no difference in age, years of follow-up, nulliparity, or tobacco use. Among survivors, BMI was lower among HC users compared to non-users (22.6 vs. 23.7 kg/m2, p=0.036), but BMI was not significantly different when considered across all four groups. There was also a difference in Caucasian race (HC users: survivors 97.6%, controls 75.0%; HC non-users: 90.5% survivors, controls 80.0%, p=0.008). Demographic characteristics by visit are included in Supplemental Table 1.
Table 1. Demographic data for study participants stratified by cancer history and hormonal contraceptive use.
Data presented per subject. Subjects with recent exposure to hormonal contraception during at least one visit were classified as hormonal contraceptive users. Demographic characteristics were compared using the Student's t test, Wilcoxon rank-sum test, and Kruskal-Wallis one-way analysis of variance for continuous variables and Pearson's Chi-squared test and Fisher's exact test for categorical variables.
| Cancer Survivors | Healthy Controls | Four way group comparison | |||||
|---|---|---|---|---|---|---|---|
| Subject Characteristics | HC Users (n=42) | HC Non-users (n=84) | P value | HC Users (n=8) | HC Non-users (n=115) | P value | P value |
| Age at enrollment (years) φ | 23 (20,27) (16-35) | 25 (20,31) (15-39) | 0.19 | 28.5 (25.5,30) (21-34) | 26 (22,29) (14-39) | 0.219 | 0.075 |
| Visits per subject, φ | 2 (1,3) (1-8) | 1 (1,3) (1-6) | 0.411 | 2 (1,3) (1-6) | 1 (1,3) (1-6) | 0.093 | 0.316 |
| Length of follow-up (years) φ | 1.2 (0,3.1) (0-7.4) | 1 (0,2.6) (0-6.2) | 0.434 | 2 (0.5,4.0) (0-4.9) | 1 (0,2.5) (0-5.5) | 0.163 | 0.471 |
| BMI at enrollment (kg/m2) φ | 22.6 (19.4,24.4) (16.8-34.7) | 23.7 (21.4,25.7) (15.2-50.6) | 0.036 | 22.2 (20.0,27.9) (19.6-43.9) | 23.3 (21.2,26.7) (16.4-54.3) | 0.704 | 0.117 |
| Caucasian Race, n (%) | 41 (97.6%) | 76 (90.5%) | 0.267 | 6 (75.0%) | 92 (80.0%) | 0.664 | 0.008 |
| Smoking at enrollment, n (%) | 2 (4.8%) | 3 (3.6%) | 1 | 0 (0.0%) | 13 (11.5%) | 0.597 | 0.186 |
| Nulligravid, n (%) | 33 (78.6%) | 53 (63.9%) | 0.094 | 5 (62.5%) | 80 (69.6%) | 0.702 | 0.37 |
| First visit conducted during early follicular phase, n (%) | 78.60% | 89.30% | 0.105 | 100% | 100% | 1 | <0.001 |
=Results reported as median, interquartile range, and range
The majority of survivors had hematologic malignancies in childhood; 31% (39/126) had lymphoma and 31% (39/126) had leukemia. Other diagnoses included sarcoma (n=22, 17.5%), Wilm's tumor (n=8, 6.4%), breast cancer (n=6, 4.8%), neuroblastoma (n=4, 3.2%), and other malignancies (n=8, 6.4%). Median time since cancer diagnosis was 10.5 years (IQR 6-17 years). Treatments included chemotherapy (n=124, 98.4%), bone marrow transplant (n=25, 19.8%), and pelvic radiation (n=6, 4.8%). Among those treated with alkylating agents (n=113), the median alkylator score was 2 (IQR 1-3).
Survivors were more likely to stop HC for the study compared to healthy women who enrolled. At the first study visit, 39.7% of survivors reported that they had used HC within the past year compared to 14.6% of controls, and 24.6% of survivors stopped HC for the purpose of participating in the study compared to 1.6% of controls (p<0.001 for both). The indications for HC use were similar between been groups (data not shown).
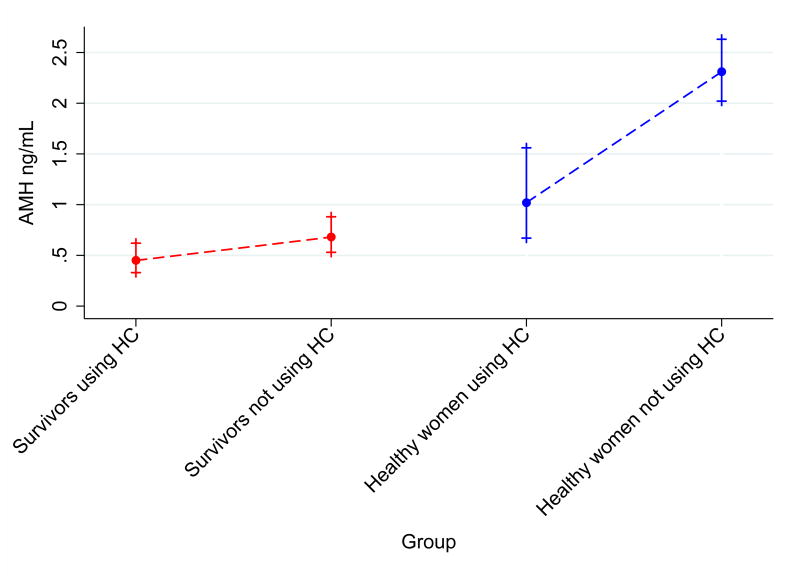
Evidence for effect modification by cancer history was observed in AMH models (p=0.083 for the interaction). Therefore, results for AMH were stratified by cancer history. Among survivors, mean AMH was 0.45 ng/mL (95% CI 0.33-0.62 ng/mL) among HC users compared to 0.68 ng/mL (95% CI 0.53-0.88) among non-HC users (Table 2). In unadjusted models, the geometric mean AMH was 44% lower among survivors using HC compared to survivors not using HC (RR 0.66, 95% CI 0.52-0.84, p=0.001). Among controls, HC users had a mean AMH of 1.02 ng/mL (95% CI 0.67-1.56) compared to 2.31 ng/mL (95% CI 2.02-2.63) among non-HC users, which represents a 56% lower AMH among healthy HC users (RR 0.44, 95% CI 0.30-0.65, p<0.001). After adjusting for confounders, the interaction between cancer history and HC use was significant (p=0.012). From this model, geometric mean AMH was 21% lower among survivors using HC compared to survivors not using HC (Adjusted RR 0.79, 95% CI 0.68-0.93, p=0.004). Among controls, mean AMH was 55% lower for HC users in the adjusted model (Adjusted RR 0.45, 95% CI 0.30-0.68, p<0.001), Figure 1A.)
Table 2. Anti-Mullerian Hormone for hormonal contraception users vs. non-users stratified by cancer history.
AMH values are presented as the geometric means and 95% confidence intervals. Differences in model estimates for AMH with and without HC exposure are presented as relative risks (RR), which refer to ratios of AMH geometric means. Adjusted relative risks were obtained from the multivariate model controlling for age, age2, cancer history, tobacco use, baseline AMH, and the interaction between HC use and cancer history.
| AMH (ng/mL) | Unadjusted RRδ | Adjusted RRδ | |||||
|---|---|---|---|---|---|---|---|
| HC users | HC non-users | All subjects | All subjects | Early follicular phase | Baseline AMH>0.1 ng/mL | Baseline AMH>0.25 ng/mL | |
| Cancer Survivors | 0.45 (0.33-0.62) | 0.68 (0.53-0.88) | 0.66 (0.52-0.84) 0.001 | 0.79 (0.68-0.93) 0.004 | 0.80 (0.65-0.98) 0.029 | 0.80 (0.65-0.98) 0.029 | 0.76 (0.59-0.98) 0.031 |
| Healthy Controls | 1.02 (0.67-1.56) | 2.31 (2.02-2.63) | 0.44 (0.30-0.65) <0.001 | 0.45 (0.30-0.68) <0.001 | 0.45 (0.31-0.64) <0.001 | 0.45 (0.31-0.64) <0.001 | 0.45 (0.31-0.63) <0.001 |
Data presented as RR with 95% CI and p value.
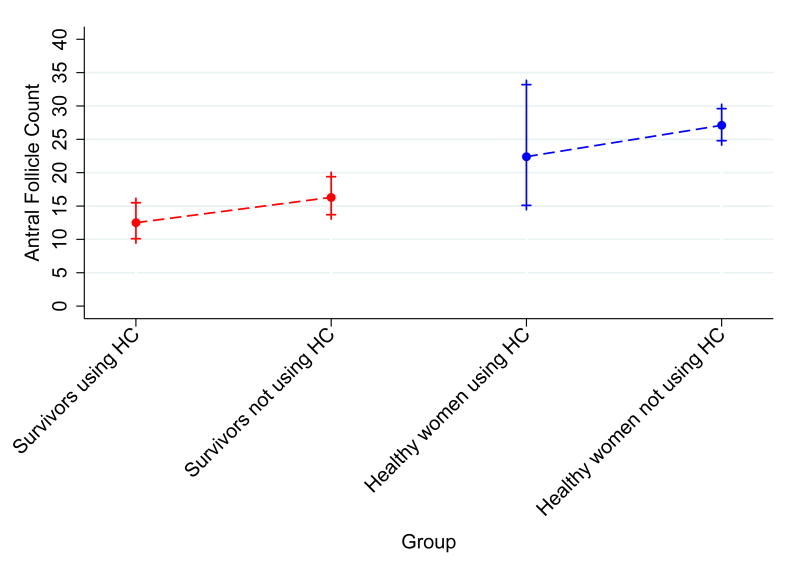
Figure 1. Anti-Mullerian Hormone levels and Antral Follicle Counts for visits in HC users vs. visits in non-users stratified by cancer history.
Data for A.) Anti-Mullerian Hormone and B.) Antral Follicle Count are presented as unadjusted geometric means and 95% confidence intervals. Graphs include data from 61 visits among survivors recently exposed to hormonal contraception, 191 visits among survivors not recently exposed to hormonal contraception, 11 visits among healthy controls recently exposed to hormonal contraception, and 229 visits among healthy controls not recently exposed to hormonal contraception.
When considering only visits that occurred during the early follicular phase, the results were similar (Adjusted RR among survivors: 0.80, 95% CI 0.65-0.98, p=0.029; Adjusted RR among controls: 0.45, 95% CI 0.31-0.64, p<0.001). Results were also consistent when the analysis was restricted to those with at least two AMH measurements (data not shown). Two sensitivity analyses were performed, one excluding subjects with baseline AMH ≤0.1 ng/mL and the other excluding subjects with baseline AMH ≤0.25 ng/mL. The results from these analyses were similar to the original estimates and remained significant (Table 2), indicating that the results cannot be explained by misclassification of HRT as HC.
AFC was also lower with HC use in survivors and controls. Since there was no significant interaction between cancer history and HC use in AFC models, effect estimates were calculated for survivors and controls together. Among survivors, mean AFC was 12.5 (95% CI 10.1-15.5) with HC use compared to 16.3 (95% CI 13.7-19.4) in the absence of HC (Table 3). Among controls, mean AFC for HC users was 22.4 (95% CI 15.1-33.2) versus 27.1 (95% CI 24.8-29.6) among non-users. AFC was 27% and 20% lower with HC exposure in unadjusted and adjusted models, respectively (Unadjusted RR 0.73, 95% CI 0.61-0.87, p<0.001; Adjusted RR 0.80, 95% CI 0.69-0.93, p=0.004, Figure 1B). The results were very similar when restricted to visits occurring in the early follicular phase (Adjusted RR 0.80, 95% CI 0.68-0.93, p=0.004) and when the analysis was restricted to those with at least two AFC measurements (data not shown). Sensitivity analyses excluding subjects with low baseline AFC levels were consistent and remained significant (Table 3), indicating that the results were not influenced by potential misclassification of HRT as HC.
Table 3. Antral Follicle Count for hormonal contraception users vs. non-users.
AFCs are presented as geometric means and 95% confidence intervals. Differences in model estimates for AFC with and without HC exposure are presented as relative risks (RR), which refer to ratios of AFC geometric means. Adjusted estimates were obtained from the multivariate model controlling for age, cancer history, tobacco use, cycle day, and baseline AFC.
| Antral Follicle Count | Unadjusted RRδ | Adjusted RRδ | |||||
|---|---|---|---|---|---|---|---|
| HC users | HC non-users | All subjects | All subjects | Early follicular phase | Baseline AFC>6 | Baseline AFC>9 | |
| Cancer Survivors | 12.5 (10.1-15.5) | 16.3 (13.7-19.4) | 0.73 (0.61-0.87) <0.001 | 0.80 (0.69-0.93) 0.004 | 0.80 (0.68-0.93) 0.004 | 0.80 (0.68-0.95) 0.010 | 0.81 (0.68-0.96) 0.017 |
| Healthy Controls | 22.4 (15.1-33.2) | 27.1 (24.8-29.6) | |||||
Data presented as RR with 95% CI and p value.
Because individual women contributed information while on and off of HC, our data afforded a unique opportunity to determine the within-person impact of HC use on AMH and AFC. Comparing each subject's AMH values over time, we observed that AMH was significantly lower when a subject was recently exposed to HC compared to visits when she was not exposed. In unadjusted analyses, AMH within a cancer survivor over time was 20% lower when she took HC compared to when she did not. For a control, there was a 35% reduction in a subject's AMH with recent HC use. (Survivors: RR 0.80, 95% CI, 0.71-0.90, p<0.001; Controls: RR 0.65, 95% CI 0.54-0.78, p<0.001). The results were similar after adjusting for confounders (Survivors: 17% lower, RR 0.83, 95% CI 0.75-0.93, p=0.001; Controls: 35% lower, RR 0.65, 95% CI 0.55-0.78, p<0.001). In a cross sectional analysis of data, additive HC exposure over multiple visits was not significantly associated with AMH levels when adjusting for within woman HC use.
Similar trends were observed for AFC. In unadjusted analyses, repeated measurements of AFC for a subject taking HC were 12% lower than that same woman's AFC when not taking HC (RR 0.88, 95% CI 0.81-0.96, p=0.005). Adjustment for confounders yielded similar results (RR 0.89, 95% CI 0.82-0.96, p=0.004). In the cross-sectional analysis, additive HC exposure over multiple visits was not associated with AFC after adjusting for within woman HC use.
To illustrate the potential impact of HC use on an individual, plots of AMH and AFC levels over time were generated for subjects who were exposed to HC for at least one visit and not exposed for at least one visit (Supplemental Figure 1). Of the 21 subjects who met these criteria, 81% and 71% had similar plots to those shown in the figure for AMH and AFC, respectively.
Discussion
In this analysis, we observed that AMH and AFC are decreased among cancer survivors and healthy controls with recent HC use. Our findings, while preliminary, suggest that HC may alter markers of ovarian reserve in healthy women and after gonadotoxic injury. The findings were robust after controlling for important confounders. Furthermore, the effect estimates were similar and remained significant when participants with low baseline AMH or AFC were excluded and when considering only visits that occurred during the early follicular phase.
Additionally, we observed that an individual woman's AMH was 17-35% lower and her AFC was 11% lower when she had recently used HC compared to when she had not. Equally as important, additive HC exposure across multiple visits was not associated with differences in AMH and AFC after adjusting for confounders and within woman changes in HC use. These findings, while preliminary, suggest that the decline in AMH and AFC that occurs with HC use is transient and does not cause permanent alterations in ovarian reserve.
Our results are consistent with previous studies examining the impact of HC on AMH. A recent prospective contraceptive trial compared AMH levels in healthy women before contraceptive start and nine weeks later and observed that AMH declined approximately 50%. (11) These results were consistent across delivery methods (combined OCPs: 51%, transdermal patch: 49%, vaginal ring: 47%). Additionally, a cross-sectional study of 256 young Danish women reported lower AMH in HC users compared to non-HC users (2.5 vs. 2.9 ng/mL, p=0.01). (12) A similar cross-sectional analysis of 732 Danish women demonstrated that after adjusting for age, AMH levels were 30% lower among women using HC compared to non-users. (19) In comparison, we observed a 55% lower AMH among healthy HC users after adjustment for confounders.
While there are no prior studies investigating differences in AMH among cancer survivors taking HC, one group found that among women of advanced reproductive age, age-adjusted AMH was 34% lower among HC users compared to non-users (0.42 ng/mL vs. 0.64 ng/mL, p=0.001). (13) These mean AMH values and the effect estimates are similar to the observations we report for cancer survivors. Reductions in AMH with HC use have also been observed in PCOS patients. (20,21)
Few studies have evaluated the impact of HC use on ovarian follicle counts. In a large cross-sectional study, researchers observed significantly lower AFC among healthy women taking OCPs compared to non-users (12.0 vs. 15.5, p<0.001). (12) A comparable analysis reported that after adjusting for age, AFC values were 30% lower among those using HC. (19) Similarly, in a prospective cohort study comparing AFC among 34 women taking HC for one year to 36 women with no HC exposure, total AFC was significantly lower among HC users (AFC 17.1 vs. 23.4; p=0.007). (16) Interestingly, the observed difference among HC users was attributable to fewer follicles measuring 6-10mm (2.65 vs. 6.04, p<0.001), but a similar number of smaller antral follicles. A longitudinal study of 45 women with AFC assessed before HC and after 6 months of use demonstrated a significant decline in AFC among healthy controls and subjects with PCOS. (14)
Contrary to our findings, other authors have reported stable AMH levels after initiation of HC. Streuli and colleagues assessed serial AMH levels after initiation of HC in 24 healthy volunteers who had not used HC in the past three months. While no significant differences in AMH were observed with HC compared to non-HC (15), the study was small and subjects were exposed to HC for less than three weeks. Similarly, researchers in Hong Kong did not detect significant changes in AMH 3-4 months after initiation of HC in 95 healthy women. However, the results were not adjusted for age, which ranged from 26-50 years, and the exposure included agents not included in our analysis (combined injectable contraceptive and levonorgestrel-containing intrauterine device). Furthermore, the subgroup treated with combined OCPs (n=23) had a decline in AMH from 27.1 pmol/L (3.79 ng/mL) at baseline to 17.1 pmol/L (2.39 ng/mL) after initiation of OCPs, but significance tests were not reported among this subgroup.(22)
The observed differences in AMH and AFC with HC use have strong biologic plausibility. AMH is secreted by granulosa cells of preantral and antral follicles, which are sensitive to FSH. HC causes down-regulation of FSH, and decreased FSH stimulation of granulosa cells may result in reduced AMH secretion. When FSH is suppressed, one would expect other alterations to occur, including decreased follicle number, decreased follicle diameter, and decreased ovarian volume. All of these findings have been reported with HC use. (12,13,16)
Our study adds to the growing body of literature suggesting that AMH is more variable than originally reported. In addition to HC use, there is evidence that AMH varies during the menstrual cycle. Wunder and colleagues measured AMH in healthy, ovulatory women every 2-4 days and noted significant differences throughout the cycle, with peak levels in the late follicular phase and lower levels in the luteal phase. (23) A similar study revealed two patterns of AMH variability. Women in the lowest AMH quartile (mean early follicular AMH of 0.67 ng/mL) had stable levels during the cycle while increased variability was observed among women in the upper three quartiles. (24) A study of 12 healthy women aged 25-43 years undergoing natural cycle monitoring observed that AMH levels were 20% higher in the mid-follicular phase and 20% lower during the late luteal phase. (25) Furthermore, AMH variation was clinically significant since over half of women had repeat AMH values that crossed clinic-specific cut-offs used to predict hyperresponse or poor response.
This study has several strengths. First, the longitudinal study design enabled us to have repeated measures for many subjects, thus improving the statistical power. Additionally, our observation that HC use suppresses two markers of ovarian reserve adds to the strength of our finding. These effects were observed in two populations with varying baseline measures of AMH and AFC. Since data were collected prospectively, we were able to thoroughly assess covariates for confounding and control for important confounders. Furthermore, the fact that most subjects underwent ovarian reserve testing during the early follicular phase is a unique aspect of this study and a major strength. For those few subjects not measured in the early follicular phase, we were able to either control for timing of the visit or restrict the analysis to only those visits that occurred during the early follicular phase.
Some may question whether the lower AMH values observed among survivors taking HC resulted from confounding since women with premature ovarian failure would be more likely to use hormonal agents. We took several measures to exclude this possibility. First, all women with history of premature ovarian failure were excluded. Additionally, we performed sensitivity analyses excluding women with the lowest baseline AMH and AFC levels, and the effect estimates remained robust. Finally, we observed lower AMH and AFC levels in healthy controls as well as in survivors, making it unlikely that the observed effect in survivors resulted from confounding.
One weakness is that we were unable to examine differences in AMH and AFC depending on when subjects stopped HC prior to the study visit. We would expect AMH and AFC to be lower among those who continued using HC during their study visit compared to those who stopped HC several weeks before the visit. Since subjects were asked to stop HC at least four weeks prior to study visits, data for those on HC during the visit were limited, and we lacked the statistical power needed to analyze those visits separately. Additionally, while we collected detailed information regarding the types of contraceptives used and the length of time that subjects had been off of HC, we did not systematically collect data on how long subjects had been on HC prior to stopping. Therefore, we were unable to examine differences in AMH and AFC based on length of HC use. Further studies are needed to examine the impact of length of HC use on AMH and AFC and to determine the rate of recovery after discontinuation of HC.
Another limitation of this study is that the group of cancer survivors was heterogeneous. Survivors had a variety of cancer types and treatment regimens, and the age at diagnosis ranged from 4 months to 33 years. Further studies are needed to confirm these results among specific groups of survivors. Additionally, selection bias may have occurred since healthy women who were using HC prior to the onset of the study may have been less likely to enroll compared to survivors using HC. To minimize potential bias, we assessed for differential effects of HC use in survivors and controls, controlled for important confounders, and stratified the analysis when there was evidence of effect modification. Finally, the number of women exposed to HC was relatively small, and the results require validation in larger cohorts.
The values reported for AMH in this study should be interpreted with caution. The majority of our samples were analyzed using the original Gen2 assay, which may produce lower than expected AMH values. (26) It is unlikely that this issue impacted our results since the effect would be non-differential with respect to HC use and would therefore bias the results toward the null. Also, when AMH values from the original and revised protocols were compared using pooled serum from subjects in this study, we observed no difference in the AMH levels.
To our knowledge, this study is the first to report the impact of HC on markers of ovarian reserve in female cancer survivors. These results have important implications for patient counseling. AMH and AFC are frequently assessed in female cancer survivors as indicators of reproductive potential after cancer therapy. Often, survivors seek assessment of ovarian reserve before they are ready to conceive and are likely to be using contraception. Some cancer survivors, and health care providers, may mistake low AMH levels for infertility instead of subfertility, which increases the likelihood that survivors will stop using contraception and will have an unintended pregnancy. The current analysis suggests that for female cancer survivors using HC, ovarian reserve and potential for future fertility may be better than expected. Indeed, our group previously reported that female cancer survivors are able to achieve spontaneous pregnancy at a rate similar to unaffected women despite significant impairments in markers of ovarian reserve. (27) Thus, cancer survivors utilizing HC should be counseled carefully regarding future fertility and chance of unintended pregnancy. These results are also important when determining stimulation protocols and doses among those undergoing fertility treatment or fertility preservation therapies.
In conclusion, our data suggest that AMH and AFC are significantly lower among women using HC and should be interpreted with caution when measured in the setting of recent hormone use. Further studies are needed to confirm these findings and, if corroborated, to determine the length of time required for ovarian reserve measures to return to normal after discontinuation of HC.
Supplementary Material
Supplemental Table 1. Demographic data for study visits stratified by cancer history and hormonal contraceptive use. Data presented per visit. A visit was classified as occurring in the setting of hormonal contraceptive use if the subject had been exposed to hormonal contraception within three months preceding the visit. Demographic characteristics were compared using the Student's t test, Wilcoxon rank-sum test, and Kruskal-Wallis one-way analysis of variance for continuous variables and Pearson's Chi-squared test and Fisher's exact test for categorical variables.
Supplemental Figure 1. Anti-Mullerian Hormone levels and Antral Follicle Counts among individuals over time. Plots of Anti-Mullerian Hormone (AMH) levels and Antral Follicle Counts (AFC) over time for individual subjects illustrate the decline in ovarian reserve markers observed in the setting of recent hormonal contraceptive use. Data points labeled “HC” indicate that the subject had taken hormonal contraception within three months of the study visit and “no HC” indicates no exposure to hormonal contraception in the preceding three months.
A) AMH and B) AFC for an 18-year-old woman who was diagnosed with Wilm's, Tumor at age 8.
C) AMH and D) AFC for a 24 year old woman who was diagnosed with Acute, Lymphocytic Leukemia at age 2.
E) AMH and F) AFC for a 29 year old woman diagnosed with Hodgkin's Lymphoma at age 20.
G) AMH and H) AFC for a healthy woman who enrolled as a control at age 30.
Acknowledgments
Financial support: Supported by NIH Grants K01 L:1-CA-133839-03 (CG), 1R01HD062797 (CG, MS), and T32 HD007440 (LJ).
Footnotes
Financial disclosures Lauren N. C. Johnson, MD has nothing to disclose.
Mary D. Sammel, ScD is a consultant for Swiss Precision Diagnostics.
Katherine E. Dillon, MD has nothing to disclose.
Lara Lechtenberg, BA has nothing to disclose.
Allison Schanne, BA has nothing to disclose.
Clarisa R. Gracia, MD, MSCE has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Mullerian Hormone Inhibits Initiation of Primordial Follicle Growth in the Mouse Ovary. Endocrinology. 2002;143(3):1076–84. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 2.The Practice Committee for the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2012;98(6):1407–15. doi: 10.1016/j.fertnstert.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 3.Nelson SM. Biomarkers of ovarian response: current and future applications. Fertil Steril. 2013;99(4):963–9. doi: 10.1016/j.fertnstert.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 4.Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77(3):468–71. doi: 10.1016/s0015-0282(01)03201-0. [DOI] [PubMed] [Google Scholar]
- 5.van Rooij IAJ, Broekmans FJM, Velde te ER, Fauser BJC, Bancsi LF, de Jong FH, et al. Serum anti-mullerian hormone levels: a novel measure of ovarian reserve. Human Reproduction. 2002;17(12):3065–71. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 6.Kwee J, Schats R, McDonnell J, Themmen A, de Jong FH, Lambalk C. Evaluation of anti-Müllerian hormone as a test for the prediction of ovarian reserve. Fertil Steril. 2008;90(3):737–43. doi: 10.1016/j.fertnstert.2007.07.1293. [DOI] [PubMed] [Google Scholar]
- 7.Hendriks DJ, Mol BWJ, Bancsi LFJMM, Velde te ER, Broekmans FJM. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: A meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril. 2005;83(2):291–301. doi: 10.1016/j.fertnstert.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Broer SL, Mol BWJ, Hendriks D, Broekmans FJM. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91(3):705–14. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, et al. Antimüllerian Hormone as a Predictor of Natural Fecundability in Women Aged 30–42 Years. Obstetrics & Gynecology. 2011;117(4):798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-Mullerian Hormone as a Predictor of Time to Menopause in Late Reproductive Age Women. Journal of Clinical Endocrinology & Metabolism. 2012;97(5):1673–80. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimullerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305–10. doi: 10.1016/j.fertnstert.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen SL, Ramlau-Hansen CH, Andersen CY, Ernst E, Olsen SF, Bonde JP, et al. The association between circulating levels of antimullerian hormone and follicle number, androgens, and menstrual cycle characteristics in young women. Fertil Steril. 2012;97(3):779–85. doi: 10.1016/j.fertnstert.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Shaw CM, Stanczyk FZ, Egleston BL, Kahle LL, Spittle CS, Godwin AK, et al. Serum antimüllerian hormone in healthy premenopausal women. Fertil Steril. 2011;95(8):2718–21. doi: 10.1016/j.fertnstert.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-mullerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2007;134:196–201. doi: 10.1016/j.ejogrb.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimüllerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008;90(2):395–400. doi: 10.1016/j.fertnstert.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Deb S, Campbell BK, Pincott-Allen C, Clewes JS, Cumberpatch G, Raine-Fenning NJ. Quantifying effect of combined oral contraceptive pill on functional ovarian reserve as measured by serum anti-Müllerian hormone and small antral follicle count using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2012;39(5):574–80. doi: 10.1002/uog.10114. [DOI] [PubMed] [Google Scholar]
- 17.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 18.Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22(16):2591–602. doi: 10.1002/sim.1524. [DOI] [PubMed] [Google Scholar]
- 19.Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, Friis-Hansen L, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reproductive BioMedicine Online. 2012;25(6):612–9. doi: 10.1016/j.rbmo.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Panidis D, Georgopoulos NA, Piouka A, Katsikis I, Saltamavros AD, Decavalas G, et al. The impact of oral contraceptives and metformin on anti-Müllerian hormone serum levels in women with polycystic ovary syndrome and biochemical hyperandrogenemia. Gynecol Endocrinol. 2011;27(8):587–92. doi: 10.3109/09513590.2010.507283. [DOI] [PubMed] [Google Scholar]
- 21.Fábregues F, Castelo-Branco C, Carmona F, Guimerá M, Casamitjana R, Balasch J. The effect of different hormone therapies on anti-müllerian hormone serum levels in anovulatory women of reproductive age. Gynecol Endocrinol. 2011;27(4):216–24. doi: 10.3109/09513590.2010.487595. [DOI] [PubMed] [Google Scholar]
- 22.Li HWR, Wong CYG, Yeung WSB, Ho PC, Ng EHY. Serum anti-müllerian hormone level is not altered in women using hormonal contraceptives. Contraception. 2011;83(6):582–5. doi: 10.1016/j.contraception.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Wunder DM, Bersinger NA, Yared M, Kretschmer R, Birkhäuser MH. Statistically significant changes of antimüllerian hormone and inhibin levels during the physiologic menstrual cycle in reproductive age women. Fertil Steril. 2008;89(4):927–33. doi: 10.1016/j.fertnstert.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 24.Sowers M, McConnell D, Gast K, Zheng H, Nan B, McCarthy JD, et al. Anti-Mullerian hormone and inhibin B variability during normal menstrual cycles. Fertil Steril. 2010;94(4):1482–6. doi: 10.1016/j.fertnstert.2009.07.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadlow N, Longhurst K, McClements A, Natalwala J, Brown SJ, Matson PL. Variation in antimullerian hormone concentration during the menstrual cycle may change the clinical classification of the ovarian response. Fertil Steril. 2013;99(6):1791–7. doi: 10.1016/j.fertnstert.2013.01.132. [DOI] [PubMed] [Google Scholar]
- 26.Cundy S. Urgent field safety notice: AMH Gen II ELISA (REF A79765) [Accessed March 20, 2014]; http://wwwmhragovuk/home/groups/fsn/documents/fieldsafetynotice/con297532pdf.
- 27.Dillon KE, Sammel MD, Ginsberg JP, Lechtenberg L, Prewitt M, Gracia CR. Pregnancy after cancer: Results from a prospective cohort study of cancer survivors. Pediatr Blood Cancer. 2013;60:2001–6. doi: 10.1002/pbc.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Demographic data for study visits stratified by cancer history and hormonal contraceptive use. Data presented per visit. A visit was classified as occurring in the setting of hormonal contraceptive use if the subject had been exposed to hormonal contraception within three months preceding the visit. Demographic characteristics were compared using the Student's t test, Wilcoxon rank-sum test, and Kruskal-Wallis one-way analysis of variance for continuous variables and Pearson's Chi-squared test and Fisher's exact test for categorical variables.
Supplemental Figure 1. Anti-Mullerian Hormone levels and Antral Follicle Counts among individuals over time. Plots of Anti-Mullerian Hormone (AMH) levels and Antral Follicle Counts (AFC) over time for individual subjects illustrate the decline in ovarian reserve markers observed in the setting of recent hormonal contraceptive use. Data points labeled “HC” indicate that the subject had taken hormonal contraception within three months of the study visit and “no HC” indicates no exposure to hormonal contraception in the preceding three months.
A) AMH and B) AFC for an 18-year-old woman who was diagnosed with Wilm's, Tumor at age 8.
C) AMH and D) AFC for a 24 year old woman who was diagnosed with Acute, Lymphocytic Leukemia at age 2.
E) AMH and F) AFC for a 29 year old woman diagnosed with Hodgkin's Lymphoma at age 20.
G) AMH and H) AFC for a healthy woman who enrolled as a control at age 30.