Abstract
The present aim was to investigate the effects of traditional Chinese medicine prescriptions (TCM) on body temperature, blood physiological parameters, nutrient apparent digestibility and growth performance of beef cattle under heat stress conditions. Twenty-seven beef cattle were randomly divided into three groups as following; i) high temperature control (HTC), ii) traditional Chinese medicine prescriptions I+high temperature (TCM I) and iii) traditional Chinese medicine prescriptions II+high temperature (TCM II) (n = 9 per group). The results showed that the mean body temperature declined in TCM II treatment (p<0.05). Serum T3 and T4 levels with TCM I and TCM II treatments elevated (p<0.05), and serum cortisol levels of TCM I treatments decreased (p<0.05), compared with the HTC group. Total protein, albumin, globulin in TCM II treatments elevated and blood urea nitrogen levels of both TCM treatments increased, but glucose levels of both TCM treatments decreased, compared with the HTC group (p<0.05). The apparent digestibility of organic matter and crude protein with TCM I treatment increased, and the apparent digestibility of acid detergent fiber elevated in both TCM treatments (p<0.05). Average daily feed intake was not different among three groups, however average daily gain increased and the feed:gain ratio decreased with both TCM treatments, compared with the HTC group (p<0.05). The present results suggest that dietary supplementation with TCM I or TCM II improves growth performance of heat stressed beef cattle by relieving heat stress responses and increasing nutrient apparent digestibility.
Keywords: Beef Cattle, Traditional Chinese Medicines, Heat Stress, Growth Performance, Nutrient Apparent Digestibility
INTRODUCTION
Feedlot cattle finished during summer months in South China are often adversely affected by heat stress conditions (Kui et al., 2010). Summer conditions are described as above normal ambient temperature, relative humidity, and solar radiation. These conditions coupled with low wind speed can increase animal heat load, resulting in reduced growth performance, decreased animal comfort, and death (Mader et al., 2006). Heat stress as measured by increased temperature-humidity index (THI) above critical thresholds is related to decreased dry matter intake and nutrient digestibility and to reduced efficiency of growth performance (West, 2003). Several feed additives such as antibiotics have been used to relieve the negative impact of heat stress. However, a possible linkage between the use of antibiotics in animal feed and the transmission of antimicrobial resistant bacteria to humans have prompted researchers to explore other alternative approaches (Marshall et al., 1990). Along with the prohibition of antibiotics as growth promoters in the world today, traditional Chinese medicine has subsequently become increasingly interesting as alternative feed additives in recent years (Chen et al., 2007).
Traditional Chinese medicine prescriptions (TCM) were formed by a combination of Chinese medicinal herbs according to the prescription-composing principles that have an almost infinite ability to synthesize compounds that have diverse bioactive properties, which have been exploited in human and veterinary ethno-medicine for thousands of years (Chen et al., 2010). According to the TCM theory, Cortex Phellodendri, Rhizoma Atractylodes, Agastache rugosa and Gypsum Fibrosum with the following active constituents, C. Phellodendron alkaloid, R. Atractylodes Aetherolea, Herbal Agastachis Aetherolea and G. Fibrosum extract can be combined and applied to prevent or cure animal diseases caused by heat stress (Guo et al., 2011). Our previous studies also demonstrated that TCM enhanced small intestinal glucose absorption and improved growth performance of pigs in a high temperature (40°C) environment (Song et al., 2009; Song et al., 2010). While, the active constituent of Aurantii Nobilis Pericarpium (Hesperidin) provide strong cellular antioxidant protection (Wilmsen et al., 2005) and Magnolia officinalis (Magnolol) are prescribed for anxiety and stress related conditions involving elevated cortisol (COR) (Talbott et al., 2013). In addition, Astragaloside IV, a characteristic component of Astragalus membranaceus and Baicalin, a major constituent of Scutellaria baicalensis have antihypertensive and hepatoprotective activities in animal models (Chien et al., 2011). However, the effects of all previously determined TCM on heat stress responses and growth performance of beef cattle have not been clarified.
Thus, the present aims were to evaluate the effects of TCM as feed additives on body temperature, hormones, blood biochemical parameters and nutrient apparent digestibility and to determine whether TCM could be used to enhance growth performance in beef cattle under heat stress conditions.
MATERIALS AND METHODS
Animals and experimental design
Twenty-seven Jinjiang beef cattle with an average age of 10-month-old (standard error [SE]±1.8) and with a mean body weight of 210 kg (SE±20.0) were studied in a high ambient temperature environment during summer months (July to September) in South China. During the experiment, they were housed indoors in an individual pens (1.25×2 m2) with a concrete floor. The trial had a Randomized Complete Block Design. The experimental cattle were randomly allotted to three treatment groups and each group comprised nine cattle (n = 9). The experimental period lasted 60 days. All experimental protocols were approved by the Committee for the Care and Use of Experimental Animals, Jiangxi Agricultural University, Jiangxi, China.
Experimental treatments and rations
Experimental animals of all three treatment groups were fed the same basal concentrate, which was formulated to meet the nutrient requirements of beef cattle (NRC, 2004). The ingredient composition of the basal concentrate is shown in Table 1. The three experimental treatments were; i) high temperature control (HTC), ii) TCM I (TCM I+high temperature) and iii) TCM II (TCM II+high temperature). The animals were offered forage grass (Pennisetum hydridum) and water ad libitum, and the chemical components of forage grass was shown in Table 2.
Table 1.
Composition and nutrient levels of the basal concentrate (air dry basis)
| Items | Content (%) |
|---|---|
| Ingredient composition | |
| Corn | 66.00 |
| Wheat bran | 17.00 |
| Cottonseed cake | 10.00 |
| Rapeseed meal | 4.00 |
| Limestone | 0.80 |
| CaHPO4 | 0.60 |
| NaHCO3 | 0.30 |
| NaCl | 0.30 |
| Premix1 | 1.00 |
| Nutritional level | |
| Dry matter (DM) | 89.30 |
| Net energy (MJ/kg DM)2 | 6.32 |
| Crude protein | 13.87 |
| Neutral detergent fiber | 45.83 |
| Acid detergent fiber | 31.89 |
| Calcium | 0.47 |
| Phosphorus | 0.36 |
One kilogram of premix contained the following: 250,000 IU vitamin A; 340,000 IU vitamin D3; 1,000 IU vitamin E; 3 g Zn; 5 mg Fe; 1g Cu; 4g Mn; 50 mg I; 10 mg Se; 10 mg Co; 50 g Mg.
Calculated value.
Table 2.
The chemical components of the roughage diet (dry matter basis)
| Items | Forage grass (Pennisetum hydridum) |
|---|---|
| Dry matter | 17.18 |
| Organic matter | 92.34 |
| Crude protein | 5.64 |
| Ether extract | 2.42 |
| Neutral detergent fiber | 46.04 |
| Acid detergent fiber | 67.14 |
| Calcium | 0.24 |
| Phosphorus | 0.51 |
Preparation of TCM I and TCM II
All raw materials for TCM I and TCM II were bought from Chinese Traditional Medicine Pharmacy Tong Ren Tang (Beijing, China). TCM I was composed of six dried Chinese herbs, including Rhizoma Atractylodes, Agastache rugosa, Aurantii Nobilis Pericarpium, Magnolia officinalis, Astragalus membranaceus and Scutellaria baicalensis, which were mixed in the dry weight ratio of 1:1:1:1:0.5:0.5. TCM II was composed of four dried Chinese herbs, including Cortex Phellodendri, Rhizoma Atractylodes, Agastache rugosa and Gypsum Fibrosum were mixed in the dry weight ratio of 1:1:1:1. Composition and main active constituents of TCM I and TCM II are presented in Table 3. All dried Chinese herbs were smashed through a 2.5 mm screen sieve, then TCM I and TCM II were added into the basal concentrate of TCM I and TCM II treatment groups at a same dose of 10 g/kg, respectively.
Table 3.
Composition and main active constituents of TCM I and TCM II (air dry basis)1
| Latin name | Main active constituent | Used part | Content (%) |
|---|---|---|---|
| TCM I | |||
| Rhizoma Atractylodes | Atractylodine (≥2 mg/g) | Dried rhizome | 20 |
| Agastache rugosa | Patchoulic alcohol (≥1 mg/g) | Dried root | 20 |
| Aurantii Nobilis Pericarpium | Hesperidin (≥35 mg/g) | Dried peel | 20 |
| Magnolia officinalis | Magnolol (≥20 mg/g) | Dried bark | 20 |
| Astragalus membranaceus | Astragaloside (≥0.4 mg/g) | Dried root | 10 |
| Scutellaria baicalensis | Baicalin (≥80 mg/g) | Dried root | 10 |
| Total | 100 | ||
| TCM II | |||
| Cortex Phellodendri | Berberine (≥30 mg/g) | Dried bark | 25 |
| Rhizoma Atractylodes | Atractylodine (≥2 mg/g) | Dried rhizome | 25 |
| Agastache rugosa | Patchoulic alcohol (≥1 mg/g) | Dried leaves and stem | 25 |
| Gypsum Fibrosum | CaSO4·2H2O (≥95%) | - | 25 |
| Total | 100 | ||
TCM I, traditional Chinese medicine prescriptions I+high temperature; TCM II, traditional Chinese medicine prescriptions II+high temperature.
Main active constituents of TCM I and TCM II come from Chinese pharmacopoeia (2005).
Measurement of temperature-humidity index and body temperature
Dry bulb temperature and relative humidity were measured using a hygrometer at 08:00, 14:00, and 20:00 hrs, and the average daily temperature and humidity index (THI) were calculated using the following formula: THI = db (°C)–(0.55–0.55RH)×(db–14.4), where db °C as dry bulb temperature and RH = relative humidity percentage/100. The THI classifications for heat stress are as follows: no stress, ≤74; mild stress, 74<THI<79; high stress, 79≤THI<84; and severe stress, THI≥84 (Berman, 2005). Body temperature was measured via rectum using a digital thermometer (Crison Model 637, Crison Instruments, barcelona, Spain) at 08:00 hrs on days 20, 40, 60 during the experimental period.
Hormonal and blood biochemical analysis
On days 20, 40, and 60, blood samples (15 mL each) were collected at 14:00 hrs from the jugular vein, using serum separator blood drawing tube (Sarstedt Monovete, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Serum was obtained by immediate centrifugation (10 min, 4°C, 1,500 g) and kept at −80°C until analysis. The concentrations of triiodothyronine (T3), thyroxine (T4) and COR, were determined by using radioimmunoassay kits (Zhongcheng Mechanical & Electrical Co., Hefei, China). Blood glucose (GLU), total protein (TP), albumin (ALB), globulin (GLO) and blood urea nitrogen (BUN) were determined by using spectrophotometric kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Nutrient apparent digestibility tests
Samples of concentrate and forage were collected daily during the experiment period and at the last 5 days of the experimental period; the total daily feces from each pen were weighed, totally collected and stored at −20°C until analysis. Before the analysis, the fecal samples from each pen and each single day were combined, mixed thoroughly, and 3% of the wet weight was sampled.
Feed, forage and fecal samples were dried in a forced air oven at 65°C for 72 hrs and then ground by the Wiley mill through a 1 mm screen sieve. These samples were analyzed for the dry matter, organic matter (OM), crude proteins (CP), ether extract (EE), acid detergent fiber (ADF), neutral detergent fiber (NDF) according to the AOAC (2001) method. The calcium (Ca) content was estimated by the method of ethylene diamine tetraacetic acid complexometric titration and total phosphorus (P) content was estimated by the method of visible spectrophotometer, according to GB/T 6436-2002 and GB/T 6437-2002 in China national standards. Then, the apparent digestibility of OM, CP, CF, ADF, NDF, EE, Ca and P was calculated.
Determination of the growth performance
Body weights of animals on an empty stomach were measured at 09:00 hrs on days 0, 20, 40, and 60 of the experimental period and daily feed intakes were recorded. On the basis of these data, average daily feed intake (ADFI), average daily gain (ADG) and the feed:gain (F:G) ratio were calculated.
Statistical analysis
The results are expressed as means with their standard errors. Prior to statistical calculations, data observed were tested for normal distribution with Shapiro Wilk test. Treatment effect was evaluated for significant differences with one-way analysis of variance (PROC general linear model - Univariate) according to Randomized Complete Block Design. Fisher’s least significant difference test was used to determine significant differences between groups. All statistical calculations were performed using a commercial software package (SPSS version 12.0, SPSS Inc., Chicago, IL, USA). The level of statistical significance was preset at p<0.05.
RESULTS
Temperature-humidity index and body temperature
The average daily THI values during the experimental period were higher than 79 for 55 out of 60 days. Daily changes of THI values at 08:00, 14:00, and 22:00 hrs during the experimental period are presented in Figure 1. There was no differences of body temperature between HTC and TCM I groups observed during the experiment period (p>0.05). Dietary supplementation with TCM II showed a significant decline of body temperature on day 20 (p<0.05) but no significant difference of body temperature was recorded on days 40 and 60, compared with HTC group. Changes of body temperature of all groups are presented in Figure 2.
Figure 1.
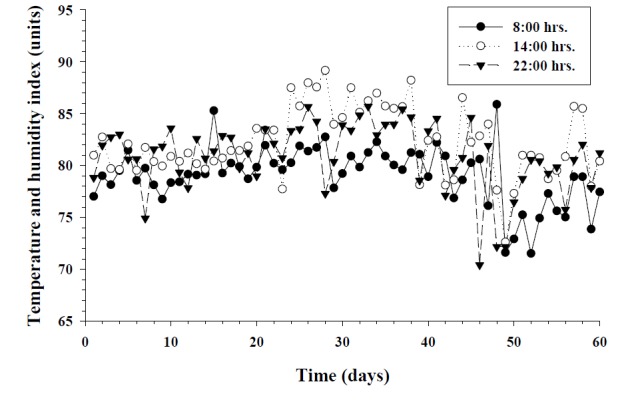
Daily changes of the temperature and humidity indexes (THI) at different hours during summer months in South China
Figure 2.
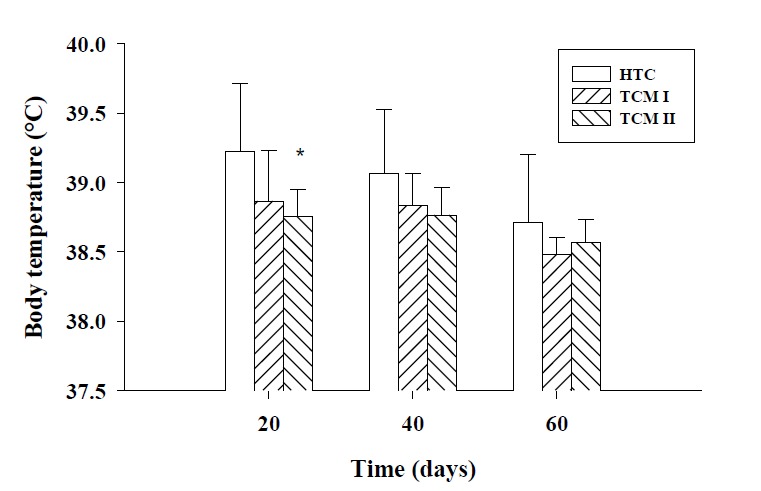
Effects of TCM I and TCM II treatments on body temperature of heat stressed beef cattle; * p<0.05, compared with HTC group. TCM I = traditional Chinese medicine prescriptions I+high temperature; TCM II = traditional Chinese medicine prescriptions II+high temperature; HTC, high temperature control.
Thyroid hormones and cortisol
The effect of TCM I and TCM II treatments on T3, T4 and COR levels are shown in Table 4. Comparing with HTC group, T3 levels of TCM I treatment on days 20, 40, and 60 were increased by 22.68% (p<0.05), 36.36% (p<0.01) and 28.57% (p<0.01), respectively but only T3 levels of TCM II treatment on days 60 were increased by 73.61% (p<0.01). Similarly, T4 levels of TCM I treatment on days 20, 40, and 60 were increased by 15.71% (p<0.05), 50.39% (p<0.01) and 15.05% (p<0.05), respectively and T4 levels of TCM II treatment on days 20, 40, and 60 were increased by 7.93% (p<0.05), 43.31% (p<0.01) and 44.95% (p<0.05), respectively. Comparing with HTC group, COR levels of TCM I treatment were decreased by 24.53% (p>0.05), 42.68% (p<0.05) and 17.05% (p<0.05) on days 20, 40, and 60, respectively and with TCM II treatment were declined by 17.92%, 20.73%, and 10.23% respectively (p>0.05).
Table 4.
Effects of two TCM treatments on thyroid hormones and cortisol of heat stressed beef cattle
| Items | Days (d) | HTC | TCM I | TCM II |
|---|---|---|---|---|
| T3 (ng/mL) | 20 | 0.97±0.03b | 1.19±0.04a | 0.96±0.04b |
| 40 | 0.88±0.54B | 1.20±0.07A | 1.13±0.09AB | |
| 60 | 0.72±0.07B | 0.90±0.06A | 1.25±0.12A | |
| T4 (μg/dL) | 20 | 6.43±0.09b | 7.44±0.67a | 6.94±0.03a |
| 40 | 5.08±0.40B | 7.64±0.58A | 7.28±0.33A | |
| 60 | 4.65±0.23c | 5.35±0.36b | 6.74±0.41a | |
| COR (μg/dL) | 20 | 1.06±0.01a | 0.80±0.20a | 0.87±0.09a |
| 40 | 0.82±0.17a | 0.47±0.08b | 0.65±0.19ab | |
| 60 | 0.88±0.03a | 0.73±0.06b | 0.79±0.06ab |
TCM, traditional Chinese medicine prescriptions; HTC, high temperature control; TCM I, traditional Chinese medicine prescriptions I+high temperature; TCM II, traditional Chinese medicine prescriptions II+high temperature; T3, triiodothyronine; T4, thyroxin; COR, cortisol; SE, standard error.
Data are mean±SE (n = 5).
Within a row, means without a common lowercase superscript are different at p<0.05.
Within a row, means without a common uppercase superscript are different at p<0.01.
Blood biochemical parameters
The effects of TCM I and TCM II treatments on blood biochemical parameters are shown in Table 5. Comparing with HTC group, blood GLU levels of TCM I and TCM II treatments on day 20 were decreased by 37.00% (p<0.01) and 18.65% (p<0.05), respectively and TP levels of TCM II treatment on days 20, 40, and 60 were increased by 7.80% (p<0.05), 7.29% (p>0.05) and 9.76% (p<0.05), respectively but no difference was noticed between the TCM I treatment and HTC group. The ALB levels with TCM II treatment on days 60 were increased by 15.59% (p<0.01) but no differences were recorded on days 20 and 40. Comparing with HTC group, GLO levels of TCM I and TCM II treatments on day 20 were increased by 9.07% (p>0.05) and 12.81% (p<0.05), respectively but no difference was noticed among groups on days 40 and 60 (p>0.05). There was no significant difference in BUN levels among groups on days 20 and 40 but there was an increase by 27.61% and 25.0% on day 60 with TCM I and TCM II treatments, respectively (p<0.05).
Table 5.
Effects of two TCM treatments on blood biochemical parameters of heat stressed beef cattle
| Items | Days (d) | HTC | TCM I | TCM II |
|---|---|---|---|---|
| GLU (mmol/L) | 20 | 3.27±0.25aA | 2.06±0.18bB | 2.66±0.08bAB |
| 40 | 3.19±0.16a | 3.47±0.25a | 3.49±0.13a | |
| 60 | 2.18±0.06a | 2.32±0.20a | 2.20±0.18a | |
| TP (g/L) | 20 | 77.34±1.28b | 80.92±1.80ab | 83.37±2.16a |
| 40 | 75.16±0.90a | 77.68±1.89a | 80.64±1.35a | |
| 60 | 74.73±2.15b | 79.10±1.52ab | 82.02±2.59a | |
| ALB (g/L) | 20 | 35.88±0.17a | 35.70±0.35a | 36.60±0.65a |
| 40 | 33.06±1.25a | 34.94±0.67a | 35.72±0.72a | |
| 60 | 32.08±0.89bB | 33.53±0.31bAB | 37.08±1.30aA | |
| GLO (g/L) | 20 | 41.46±1.19b | 45.22±1.65ab | 46.77±1.92a |
| 40 | 45.24±3.68a | 42.74±2.12a | 44.92±1.60a | |
| 60 | 42.63±1.16a | 45.58±1.25a | 44.94±2.06a | |
| BUN (mmol/L) | 20 | 3.26±0.14a | 3.59±0.16a | 3.79±0.15a |
| 40 | 3.02±0.26a | 3.54±0.09a | 3.45±0.30a | |
| 60 | 2.68±0.26b | 3.42±0.17a | 3.35±0.26a |
TCM, traditional Chinese medicine prescriptions; HTC, high temperature control; TCM I, traditional Chinese medicine prescriptions I+high temperature; TCM II, traditional Chinese medicine prescriptions II+high temperature; GLU, glucose; TP, total protein; ALB, albumin; GLO, globulin; BUN, blood urea nitrogen; SE, standard error.
Data are mean±SE (n = 5).
Within a row, means without a common lowercase superscript are different at p<0.05.
Within a row, means without a common uppercase superscript are different at p<0.01.
Nutrient apparent digestibility
Table 6 shows the effect of TCM I and TCM II treatments on nutrient apparent digestibility of beef cattle during the experimental period. Dietary supplementation with TCM I increased the apparent digestibility of OM by 13.45% (p<0.05), CP by 16.16% (p<0.05) and ADF by 21.94% (p<0.01), but no difference was noticed on the apparent digestibility of EE, NDF, Ca and P, comparing with HTC group. Dietary supplementation with TCM II increased the apparent digestibility of ADF by 17.15% (p<0.05), but did not affect the apparent digestibility of other nutrients, comparing with HTC group. No difference about nutrient apparent digestibility was observed between TCM I and TCM II treatments.
Table 6.
Effects of two TCM treatments on nutrient apparent digestibility of heat stressed beef cattle
| Items | HTC | TCM I | TCM II |
|---|---|---|---|
| OM (%) | 69.03±1.58b | 78.32±1.45a | 72.21±3.75ab |
| CP (%) | 64.49±2.65b | 74.91±3.04a | 68.35±4.79ab |
| EE (%) | 75.73±1.26a | 77.67±1.24a | 79.03±1.59a |
| ADF (%) | 58.20±1.78bB | 70.97±2.55aA | 68.18±2.87aAB |
| NDF (%) | 72.39±2.34a | 78.23±2.08a | 75.17±1.35a |
| Ca (%) | 47.32±1.71a | 52.29±2.03a | 54.59±1.23a |
| P (%) | 41.45±1.94a | 41.46±2.42a | 40.42±1.83a |
TCM, traditional Chinese medicine prescriptions; HTC, high temperature control; TCM I, traditional Chinese medicine prescriptions I+high temperature; TCM II, traditional Chinese medicine prescriptions II+high temperature; OM, organic matter; CP, crude proteins; EE, ether extract; ADF, acid detergent fiber; NDF, neutral detergent fiber; Ca, calcium; P, total phosphorus; SE, standard error.
Data are mean±SE (n = 9).
Within a row, means without a common lowercase superscript are different at p<0.05.
Within a row, means without a common uppercase superscript are different at p<0.01.
Growth performance
The effects of TCM I and TCM II treatments on growth performance of beef cattle during the experimental period are presented in Table 7. No difference was noticed in the ADFI among three groups. Comparing with HTC group, dietary supplementation with TCM I increased ADG during days 1 to 20, 21 to 40, and 41 to 60 by 156% (p<0.01), 52.38% (p<0.01) and 18.75% (p>0.05), respectively and with TCM II increased ADG by 238% (p<0.01), 47.62% (p<0.01), 28.12% (p<0.05), respectively. Meanwhile, the F:G ratios on days 1 to 20, 21 to 40, and 41 to 60 with TCM I treatment were increased 50.99% (p<0.01), 26.20% (p<0.01), 16.23% (p>0.05), respectively and with TCM II treatment were increased 59.63% (p<0.01), 26.46% (p<0.05), 20.94% (p<0.05), respectively. No significant difference was noticed in the ADG or the F:G ratio between TCM I and TCM II treatments, comparing with HTC group.
Table 7.
Effects of two TCM treatments on growth performance of heat stressed beef cattle
| Items | Days (d) | HTC | TCM I | TCM II |
|---|---|---|---|---|
| Initial BW (kg) | 0 | 211.83±24.95a | 206.61±18.84a | 210.83±20.73a |
| ADFI (kg) | 1–20 | 2.84±0.53a | 2.99±0.35a | 3.02±0.16a |
| 21–40 | 3.15±0.22a | 3.15±0.35a | 3.28±0.31a | |
| 41–60 | 3.44±0.23a | 3.49±0.19a | 3.54±0.21a | |
| ADG (kg) | 1–20 | 0.16±0.05B | 0.41±0.11A | 0.54±0.21A |
| 21–40 | 0.42±0.09B | 0.64±0.19A | 0.62±0.15A | |
| 41–60 | 0.64±0.16b | 0.76±0.13ab | 0.82±0.18a | |
| F:G (kg/kg) | 1–20 | 15.73±3.19A | 7.71±1.97B | 6.35±1.98B |
| 21–40 | 7.90±1.67a | 5.83±1.06b | 5.81±2.00b | |
| 41–60 | 5.73±1.36a | 4.80±1.03ab | 4.53±0.83b | |
| Finish BW (kg) | 60 | 236.22±18.68b | 242.78±20.52ab | 249.06±24.51a |
TCM, traditional Chinese medicine prescriptions; HTC, high temperature control; TCM I, traditional Chinese medicine prescriptions I+high temperature; TCM II, traditional Chinese medicine prescriptions II+high temperature; BW, body weight; ADFI, average daily feed intake; ADG, average daily gain; F:G, the feed:gain ratio; SE, standard error.
Data are mean±SE (n = 9).
Within a row, means without a common lowercase superscript are different at p<0.05.
Within a row, means without a common uppercase superscript are different at p<0.01.
DISCUSSION
Temperature-humidity index, calculated by temperature and relative humidity is used to indicate heat stress conditions in animals (Mujibi et al., 2010). The critical values for minimum, mean and maximum THI were 74, 79, and 84, respectively (Mader et al., 2006). In this trial, the daily THI values during most of the time period were in excess of 79 and this indicated that the experimental beef cattle in South China were substantially affected by heat stress during summer months. Body temperature is an excellent indicator of animal’s susceptibility to heat load. Rectal temperature is generally considered as a good index of core body temperature even though there is considerable variation among different parts of the core body and at different times of the day (Srikandakumar et al., 2004). In this trial, dietary supplementation with TCM I and TCM II numerically declined rectal temperature, compared with HTC group but a significant decrease of rectal temperature was recorded in TCM II treatment. Similarly, our previous studies demonstrated that TCM II improved growth performance and declined body temperature or kept normal temperature of the pigs under high temperature environment (Song et al., 2009).
According to the traditional Chinese medicine theory, high ambient temperature stressors can cause hot illness in the body and use of some traditional Chinese medicines for clearing away heat and eliminating dampness usually help to prevent and cure the sickness. TCM I was composed of six dried herbs; Agastache rugosa, Rhizoma Atractylodes, Aurantii Nobilis Pericarpium, Magnolia officinalis, Astragalus membranaceus, and Scutellaria baicalensis. A. rugosa and R. Atractylodes are generally well-known for dissipating dampness. While, A. Nobilis Pericarpium and M. Officinalis are invigorate spleen drugs, as well, A. Nobilis Pericarpium presents strong cellular antioxidant protection (Wilmsen et al., 2005) and M. officinalis is used for treatment of anxiety and stress related conditions involving elevated COR, such as sleep disturbances and restlessness in humans (Talbott et al., 2013). A. membranaceus and S. baicalensis also provide antihypertensive and hepatoprotective activities in animal models (Chien et al., 2011). TCM II was composed of four dried herbs; Cortex Phellodendri, Gypsum Fibrosum, Rhizoma Atractylodes, and Agastache Rugosa. Among these herbs, C. Phellodendron and G. Fibrosum play an important role on clearing heat (Lu et al., 2006; Ikarashi et al., 2013), as well as A. Rugosa and R. Atractylodes are damp-clearing medicines (Yan et al., 2006). It was also reported that the effects of these TCM decoctions in vitro enhanced the antioxidant status and reduced lipid peroxidation in heat-stressed intestinal crypt-like epithelial cells (Guo et al., 2011). As well, the effects of these herbal extracts may recover splenic lymphocytes from the immunosuppression induced by heat stress (Zhu et al., 2011).
Stress responses of animals are excited mainly by the activation of hypothalamic-pituitary-adrenal (HPA) axis and the adrenocorticotrophic nervous system (Ishii et al., 1997). HPA axis is the stress response modulator, which transformed the nerve, endocrine and the cell factor information as the physiological reaction. Heat stress activates HPA axis of animals and causes a series of physiological and metabolic changes, and changed metabolic status elicited by elevated COR and reduced thyroid hormones (T3 and T4) levels. The present data showed that COR levels with TCM I and TCM II treatments had a significant decrease but T3 and T4 levels were increased, compared with the HTC group. These results clearly indicated that both TCM I and TCM II played an important role in relieving heat stress responses of the experimental beef cattle. As well as, there was a marked increase in TP and BUN with TCM I and TCM II treatments, and the same result was found in the nutrient apparent digestibility of CP. These changes may be associated with the increased levels of thyroid hormones, which play an important role in the animal’s adaptation to environmental changes, and T3 also promotes glucose absorption and utilization of animals.
Several investigators also reported that hot environment (26 to 40°C) impaired the ADFI and ADG of pigs (Sutherland et al., 2006) and dairy cattle (Nonaka et al., 2008). In the present results, dietary supplementation with TCM I or TCM II increased ADG and decreased the F:G ratio of heat stressed beef cattle, but no difference of ADFI was observed. Therefore, the contributions of TCM I or TCM II to growth performance of heat stressed beef cattle were different to the changes of metabolic and stress-related hormones, blood biochemical parameters and the apparent digestibility of OM, CP, and ADF. A possible explaining is that TCM I plays an important role in improving the nutrient digestibility and growth performance by invigorating spleen and stomach and improving antioxidant status, but TCM II may alleviate heat stress by keeping constant normal body temperature of cattle in hot environments. Several studies also indicated that traditional Chinese medicines provide anti-heat stress effects by regulating biochemical parameters and improving antioxidant status of dairy and beef cattle (Jin et al., 2008; Zhao et al., 2008). It was also confirmed that heat stress syndrome is the combination of reactions from endocrine and immune and digestive systems and traditional Chinese medicinal compounds improved heat stress reactions by their functions on regulating all over animals body.
CONCLUSION
Summer environment in South China induced heat stress in the experimental beef cattle. Dietary supplementation with TCM I and TCM II improved growth performance in heat stressed beef cattle by relieving heat stress responses and increasing nutrient apparent digestibility.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 31201823), International Science and Technology Cooperation Program of China (No. 20-609J), the China Agricultural Research System (CARS) Program sponsored by Ministry of Agriculture of China (No. CARS-38). The authors appreciate all the helps from our colleagues and collaborators.
REFERENCES
- Berman A. Estimates of heat stress relief needs for Holstein dairy cows. J Anim Sci. 2005;83:1377–1384. doi: 10.2527/2005.8361377x. [DOI] [PubMed] [Google Scholar]
- Chen Q, Shen SF, Liu PH, Cui L, Jiang FM, Hua XG. Study on Chinese traditional and herbal drugs on blood biochemical parameters and milk performance of heat stress cow. China Dairy Cattle. 2007;11:10–13. (in Chinese, with English abstract) [Google Scholar]
- Chen YY, Xu GZ, Zhang KC. Effects of Chinese Herbal Drugs as Additives on yield performance and physiological parameters of heat stress cow. J Dairy Sci. 2010;1:39–42. [Google Scholar]
- Chien C, Wu Y, Tsai T. Biological analysis of herbal medicines used for the treatment of liver diseases. Biomed Chromatogr. 2011;25:21–38. doi: 10.1002/bmc.1568. [DOI] [PubMed] [Google Scholar]
- Guo KJ, Xu SF, Yin P, Wang W, Song XZ, Liu FH, Xu JQ, Zoccarato I. Active components of common traditional Chinese medicine decoctions have antioxidant functions. J Anim Sci. 2011;89:3107–3115. doi: 10.2527/jas.2010-3831. [DOI] [PubMed] [Google Scholar]
- Ikarashi N, Ogiue N, Toyoda E, Nakamura M, Kon R, Kusunoki Y, Aburada T, Ishii M, Tanaka Y, Machida Y, Ochiai W, Sugiyama K. Elucidating the mechanisum by which Gypsum Fibrosum, a traditional Chinese medicine, maintains cutaneous water content. Biol Pharm Bull. 2013;36:1615–1621. doi: 10.1248/bpb.b13-00494. [DOI] [PubMed] [Google Scholar]
- Ishii T, Nakamura M, Yagi H, Soga H, Kayaba S, Gotoh T, Satomi S, Itoh T. Glucocorticoid-induced death in the murine thymus: The effect at later stages. Arch Histol Cytol. 1997;60:65–78. doi: 10.1679/aohc.60.65. [DOI] [PubMed] [Google Scholar]
- Jin LM, Wu QL, Jin BF, Fang GY, Liu HB, Lin ZP, Wang MH, Ma GM, Dai H. Influence of anti-heat stress Chinese herbal medicine additive on amount of milk and blood biochemical parameter s in dairy cow. Chinese J Vet Sci. 2008;28:852–857. (in Chinese, with English abstract) [Google Scholar]
- Kui H, Alateng SH, Turigen BY. Effect of dietary supplementation potassium chloride on the production performance and blood indicators of dairy cows in the condition of heat stress. China Anim Husb Vet Med. 2010;37:10–13. (in Chinese, with English abstract) [Google Scholar]
- Lu YN, Qiu QY, Wang Y. Effects of phellodendron and its main components on the cell membrane fluidity. Chinese Journal of Pathophysiology. 2006;22:156–159. (in Chinese, with English abstract) [Google Scholar]
- Mader TL, Davis MS, Brown-Brand T. Environmental factors influencing heat stress in feedlot cattle. J Anim Sci. 2006;84:712–719. doi: 10.2527/2006.843712x. [DOI] [PubMed] [Google Scholar]
- Marshall B, Petrowski D, Levy SB. Inter- and intarspecies spread of Escherichia coli in a farm environment in the absence of antibiotic usage. Proc. Natl. Acad. Sci USA. 1990;87:6609–6613. doi: 10.1073/pnas.87.17.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujibi FDN, Moore SS, Nkrumah DJ, Wang Z, Basarab JA. Season of testing and its effect on feed intake and efficiency in growing beef cattle. J Anim Sci. 2010;88:3789–3799. doi: 10.2527/jas.2009-2407. [DOI] [PubMed] [Google Scholar]
- Nonaka I, Takusari N, Tajima K, Suzuki T, Higuchi K, Kurihara M. Effects of high environmental temperatures on physiological and nutritional status of prepubertal Holstein heifers. Livest Sci. 2008;113:14–23. [Google Scholar]
- Song XZ, Xu JQ, Wang T, Liu FH. Chinese medicine granule affects the absorption and transport of glucose in porcine small intestinal brush border membrane vesicles under heat stress. Asian Austral J Anim. 2009;22:246–253. [Google Scholar]
- Song XZ, Xu JQ, Wang T, Liu FH. Anti-stress Chinese medicine decoction enhance growth performance and intestinal glucose absorption in heat-stressed pigs by up-regulating the expression of SGLT1, GLUT2 mRNA. Livest Sci. 2010;128:75–81. [Google Scholar]
- Srikandakumar A, Johnson EH. Effect of heat stress on milk production, rectal temperature, respiratory rate and blood chemistry in Holstein, Jersey and Australian milking zebu cows. Trop Anim Health Prod. 2004;36:685–692. doi: 10.1023/b:trop.0000042868.76914.a9. [DOI] [PubMed] [Google Scholar]
- Sutherland MA, Niekamp SR, Rodriguez-Zas SL, Salak-Johnson JL. Impacts of chronic stress and social status on various physiological and performance measures in pigs of different breeds. J Anim Sci. 2006;84:588–596. doi: 10.2527/2006.843588x. [DOI] [PubMed] [Google Scholar]
- Talbott SM, Talbott JA, Pugh M. Effect of Magnolia officinalis and Phellodendron amurense(Relora®) on cortisol and psychological mood state in moderately stressed subjects. J Int Soc Sports Nutr. 2013;10:37. doi: 10.1186/1550-2783-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JW. Effects of heat-stress on production in dairy cattle. J Dairy Sci. 2003;86:2131–2144. doi: 10.3168/jds.S0022-0302(03)73803-X. [DOI] [PubMed] [Google Scholar]
- Wilmsen PK, Spada DS, Salvador M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J Agric Food Chem. 2005;53:4757–4761. doi: 10.1021/jf0502000. [DOI] [PubMed] [Google Scholar]
- Yan MC, Chen J, Chou GX. Research review on chemical constituent and pharmacological action of Rhizoma Atractylodes. Acta Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai. 2006;20:95–98. (in Chinese, with English abstract) [Google Scholar]
- Zhao Z, Chen XW, Li SJ, Lin ZH, Yao GS. Effects of Chinese herbal feed additive on anti-heat stress of Holstein. Agricultural Sciences in Guangxi. 2008;39:377–379. (in Chinese, with English abstract) [Google Scholar]
- Zhu X, Cheng G, Liu F, Yu J, Wang Y, Yu T, Xu J, Wang M. Taguchi approach for anti-heat stress prescription compatibility in mice spleen lymphocytes in vitro. Arch Pharm Res. 2011;34:1125–1133. doi: 10.1007/s12272-011-0710-2. [DOI] [PubMed] [Google Scholar]


