Abstract
Posttraumatic stress disorder (PTSD) is a mental disorder that stems from exposure to one or more traumatic events. While PTSD is thought to result from a dysregulation of emotional neurocircuitry, neurocognitive difficulties are frequently reported. Mental flexibility is a core executive function that involves the ability to shift and adapt to new information. It is essential for appropriate social-cognitive behaviours. Magnetoencephalography (MEG), a neuroimaging modality with high spatial and temporal resolution, has been used to track the progression of brain activation during tasks of mental flexibility called set-shifting. We hypothesized that the sensitivity of MEG would be able to capture the abnormal neurocircuitry implicated in PTSD and this would negatively impact brain regions involved in set-shifting. Twenty-two soldiers with PTSD and 24 matched control soldiers completed a colour–shape set-shifting task. MEG data were recorded and source localized to identify significant brain regions involved in the task. Activation latencies were obtained by analysing the time course of activation in each region. The control group showed a sequence of activity that involved dorsolateral frontal cortex, insula and posterior parietal cortices. The soldiers with PTSD showed these activations but they were interrupted by activations in paralimbic regions. This is consistent with models of PTSD that suggest dysfunctional neurocircuitry is driven by hyper-reactive limbic areas that are not appropriately modulated by prefrontal cortical control regions. This is the first study identifying the timing and location of atypical neural responses in PTSD with set-shifting and supports the model that hyperactive limbic structures negatively impact cognitive function.
Introduction
Posttraumatic stress disorder (PTSD) is a trauma-related mental disorder or injury that stems from exposure to one or more events that involved actual or threatened death or serious injury. Although the clinical presentation varies, individuals suffering from this condition experience symptoms that include re-experiencing, avoidance of triggering situations or stimuli, negative mood and cognitions, and elevated levels of arousal and reactivity. Cognitive symptoms are also very commonly reported, and include impaired concentration, affect and increased impulsivity. This condition results in significant distress as well as impairments in functioning1 (for review, see Pitman et al.2).
Structural neuroimaging results have been equivocal. The most robust finding is that the hippocampi are smaller in patients with PTSD;3,4 however, these differences may have existed before the PTSD-inducing event.2,5 Further, there have been suggestions that the volumes of the anterior cingulate6,7 and medial prefrontal cortex (PFC)8,9 are smaller in PTSD. Using diffusion tensor imaging, white matter structure was noted to be poorer in areas near the anterior cingulate, PFC and posterior angular gyrus.10 However, there is no clear consensus on the neuroanatomical changes in PTSD in the literature and no definitive structural ‘biomarker' for PTSD (for a review, see Dolan et al.11).
Functional neuroimaging studies using positron emission tomography, single-photon emission computed tomography or functional magnetic resonance imaging (fMRI) have elaborated a model of atypical neurocircuitry in PTSD. This model proposes that the amygdalae are hyper-reactive and generate a heightened fear response, whereas the medial PFC including the rostral anterior cingulate cortex, is hypo-responsive, and thus fails to appropriately inhibit the amygdala. Finally, the bilateral hippocampi are thought to be hyper-reactive within this circuit and responsible for generating intrusive memories (for reviews, see Dolan et al.11 and Hughes and Shin12). This model is supported by a meta-analysis of fMRI studies; however, these authors raise the caveat that it is difficult to understand PTSD using protocols that activate a large-scale spatially distributed neural network, and there is value in focusing on the function within a specific network.13
Although PTSD is viewed as a disorder of fear regulation, the impact on neurocognitive functions is well documented, particularly in the domains of intellectual ability and executive functioning.11,14,15 Interestingly, although the memory and attentional aspects of executive control are clearly impacted by PTSD, the effect of PTSD on the mental flexibility component of executive function is not as clear. Early behavioural studies examining PTSD and mental flexibility did not find group differences in performance.16,17 However, a more recent fMRI study reported that individuals with PTSD failed to activate the right insula when performing an affective set-shifting task,18 whereas elite military warriors without PTSD showed increased right anterior insula activation, perhaps reflecting their ability to perform well in highly stressed military situations.19 The right insula has been identified as a key hub region for monitoring and switching;20,21 we postulated that by using magnetoencephalography (MEG), a neuroimaging modality with high temporal and high spatial resolution, we could better understand the role of the right insula, as it pertains to set-shifting, within the context of the neurocircuitry of PTSD.
The ability to shift sets is an important feature underlying mental flexibility, a key executive function.22 fMRI studies have identified brain areas in prefrontal, dorsolateral frontal23, 24 and posterior cortical regions25,26 as important in the successful completion of this task. Furthermore, using MEG, we found activations in bilateral dorsolateral prefrontal cortices as early as 100 ms poststimulus onset, with recruitment of bilateral posterior parietal cortices and these activations were sustained until approximately 500 ms post stimulus.27
In the current study, we used this spatially and temporally sensitive neuroimaging approach to focus on the specifics of the neural processes underlying set-shifting in soldiers with PTSD compared with matched control soldiers. We employed a protocol with an easy (intra-dimensional) and a more difficult (extra-dimensional) shift, and applied advanced analysis methods to localize the spatiotemporal progression of activation in the two groups. We hypothesized that the sensitivity of MEG would allow us to determine whether the abnormal neurocircuitry in PTSD negatively impacted brain regions implicated in set-shifting. This is important as this may be a mechanism by which associated cognitive deficits are generated.
Materials and methods
Participants
Participants were active duty service members from the Canadian Armed Forces and included 22 soldiers diagnosed with PTSD (all males; mean age=33.1 years±5.9 s.d.; range 27–45 years) and 24 control soldiers (all males; mean age=37.6 years±6.8 s.d.; range 26–48 years). All participants were veterans having served in Afghanistan and/or Bosnia. For both groups, exclusion criteria included any history of seizures, traumatic brain injury, other neurological disorders and standard neuroimaging (MRI and MEG) safety exclusions. Participants taking anticonvulsant medications, benzodiazepines, or GABA antagonists were also excluded from the study. Control soldiers with an active substance use disorder were also not included.
Individuals with PTSD were diagnosed (DSM IV Axis I disorders, American Psychiatric Publishing) with a semi-structured clinical interview and psychometric testing by a psychiatrist or psychologist at a Canadian Armed Forces Operational Trauma and Stress Support Centre. These individuals were provided with information describing the study and asked to self-identify and volunteer if they wished to participate. Care was provided regardless of the participation decision. After expressing interest in volunteering for this study, potential participants were re-screened by a Canadian Armed Forces psychiatrist either in person or by teleconference and their medical records re-reviewed to confirm PTSD. For all participants in the PTSD arm, onset of PTSD was traced to an operationally related traumatic event (criterion A1). All participants were Afghanistan veterans, and >50% had participated in two or more missions. Of the participants with PTSD, 69.5% had a comorbid diagnosis of depression, 27.3% of substance abuse disorder, and 18.2% with another anxiety disorder. Control soldiers were recruited through flyers and advertisements posted at Canadian Forces bases in Ontario. Before acceptance into the study, controls were screened over the telephone using the Defense and Veterans Brain Injury Center traumatic brain injury screening tool28 to rule out traumatic brain injury and the PC-PTSD (primary care screen; www.ptsd.va.gov) to rule out PTSD. Control soldiers were matched for years of service, experience in Afghanistan and number of deployments.
All testing was conducted in the MEG Lab at the Hospital for Sick Children and received institutional ethics approvals from both the Hospital for Sick Children and Defence Research and Development Canada. All participants gave informed written consent.
Neuropsychological and clinical assessments
All participants completed a short battery of neuropsychological tests as well as brief clinical assessments. The tests, their means and standard deviations for each group are contained in Table 1. A between-groups t-test identified significant differences between the control and PTSD soldiers on measures of anxiety (GAD-7) and depression (PHQ9). Although IQ was significantly different, both groups were in the same upper normal range. Scores on the alcohol use questionnaire (Alcohol Use Disorders Identification Test) did not show any significant differences. The posttraumatic stress disorder checklist-DSM IV version S was only conducted in the PTSD group and confirmed significant PTSD symptoms in this group.
Table 1. Participant information.
|
Mean±s.d. (range) |
t-Test | ||
|---|---|---|---|
| Military controls | PTSD | ||
| n | 24 | 22 | |
| Age | 33.1±5.9 (27–45) | 37.6±6.8 (26–48) | |
| Handedness | 20 Right, 4 left | 18 Right, 4 left | |
| WASIa | 117.6±13.9 (79–137) | 108.8±13.9 (82–129) | P<0.05 |
| AUDITb | 5.8±3.6 (1–17) | 8.2±7.5 (0–26) | NS |
| GAD-7c | 2.3±2.7 (0–10) | 15.4±4.1 (6–21) | P<0.0001 |
| PHQ9d | 2±2.3 (0–10) | 17.4±4.5 (9–25) | P<0.0001 |
| PCLe | NA | 63.2±7.5 (46–77) | |
Abbreviations: NA, not applicable; NS, not significant; PTSD, posttraumatic stress disorder.
Wechsler Abbreviated Scale of Intelligence.29
Alcohol Use Disorders Identification Test.30
Generalized Anxiety Disorder 7-item Scale.31
Patient Health Questionnaire identifying depression.32
Posttraumatic Stress Disorder Checklist-DSM IV version S for ‘Specific Stressful Experience'.33
Stimuli and task
Subjects completed a version of the Intra-Extra Dimensional Set Shift Test adapted from the Cambridge Neuropsychological Test Automated Battery (CANTAB, Cambridge Cognition, Cambridge, UK34) and modified for MEG.27 The stimuli consisted of 36 images where each image could be described by two dimensions: colour and shape. The subject was required to match along one dimension. After several trials, the match parameter shifted. There were two types of shifts: intra-dimensional and extra-dimensional. Intra-dimensional shifts were easier and within the same dimension, that is, colour-to-colour; whereas extra-dimensional shifts were between dimensions, that is, colour-to-shape. Subjects completed 50 intra- and 50 extra-dimensional shifts randomly occurring within 370 total number of trials. Stimuli were presented using Presentation software (NBS, Berkeley, CA, USA). The task was self-paced with an interstimulus interval that was randomly jittered between 0.8–1.2 s.
MEG data acquisition
Before entering the MEG shielded room, subjects were trained on the task. Participants were tested supine and MEG data were recorded continuously (600 Hz sampling rate, DC-100 bandpass) on a 151-channel whole-head CTF MEG (MISL, Coquitlam, BC, Canada). A T1-sagittal MPRAGE structural MR was obtained on a 3 T scanner (Siemens AG, Erlangen Germany) to allow co-registration of the MEG data to each subject's own brain anatomy.
MEG data analysis
The continuous data for each participant was epoched into trials by time-locking to stimulus onset and creating a trial length from 200 ms pre-stimulus to 1100 ms poststimulus onset. The trials were sorted into shift and non-shift trials. Non-shift trials were discarded. Retained trials were sorted into intra- and extra-dimensional shift, and only correct trials for each shift type were submitted to further analyses. Data were bandpass filtered from 1–40 Hz27 and global field power (GFP) plots (root mean squared power) were calculated across all MEG sensors for each group and shift condition. Examination of the latency and duration of the GFP peaks informed the selection of parameters for source analyses. A non-overlapping, sliding window approach was taken with five 100 ms duration windows from 75–575 ms (75–175, 175–275, 275–375, 375–475, 475–575 ms) and submitted to synthetic aperture magnetometry35 beamforming (4 mm grid reconstructed to encompass the whole brain volume with a headmodel fit to the inner skull surface) to localize neural sources active during each of these windows. The results for each subject were normalized into stereotaxic space using SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8) and averaged across subjects by conditions. These were submitted to nonparametric statistical testing (2946 permutations) and corrected for multiple comparisons using a single-threshold maximal statistic (t-max).36,37 This has been demonstrated to provide strong control for family-wise type I errors.38 Further, image contrasts for each time window were computed between groups and subjected to statistical testing.
Analysis of time course of activation
Coordinates of brain locations that showed significant activations were noted, and the time courses for these activations were reconstructed. This was accomplished by unwarping the Talairach coordinates back into each subject's brain space, calculating the time courses at the peak location of interest, then rectifying and averaging the resultant waveforms across the group. The differences between the control and PTSD groups at each time point in the time courses were permuted and tested for significance. Latencies where the difference in field strength remained significant after correction for multiple comparisons were marked on the time course. These indicated the locations and the latencies when brain activations differed significantly between the groups.
Results
Behavioural results
Reaction times for the PTSD and control groups for the intra- and extra-dimensional shifts were submitted to a 2 × 2 mixed factorial analysis of variance with group as the between-subject variable and condition as the within-subject variable. The extra-dimensional shift (668 ms±19.9 s.d.) required significantly longer (F(1,88)=6.36, P<0.02) to complete than the intra-dimensional shift (615±19.9), but there was no main effect between groups and no significant interaction.
The accuracy data also were submitted to a 2 × 2 mixed factorial analysis of variance. Performance on the extra-dimensional shift (85.6%±2.4 s.d.) was significantly less accurate (F(1,88)=8.11, P<0.01) than on the intra-dimensional shift (91.8±1.7), and soldiers with PTSD (84.6±2.9) were significantly less accurate (F(1,44)=14.34, P<0.001) than the controls (92.8±1.2).
Source analyses
Talairach coordinates, anatomical labels and Brodmann labels for significantly activated (P<0.01, corrected) brain locations for each time window in the intra-dimensional shift condition are contained in Table 2 (top half). The cells are colour coded where yellow indicates activation in dorsolateral frontal cortex, green represents posterior parietal cortex and blue signifies insula. Table 2 shows that for both control and PTSD soldiers, performance of the set-shifting task recruited bilateral insulae and left posterior parietal cortex. Further, the control soldiers recruited left dorsolateral frontal cortex, whereas the soldiers with PTSD required bilateral dorsolateral frontal cortices.
Table 2. Brain locations where significant activations were found for each set-shifting condition, at each time window, for each participant group.
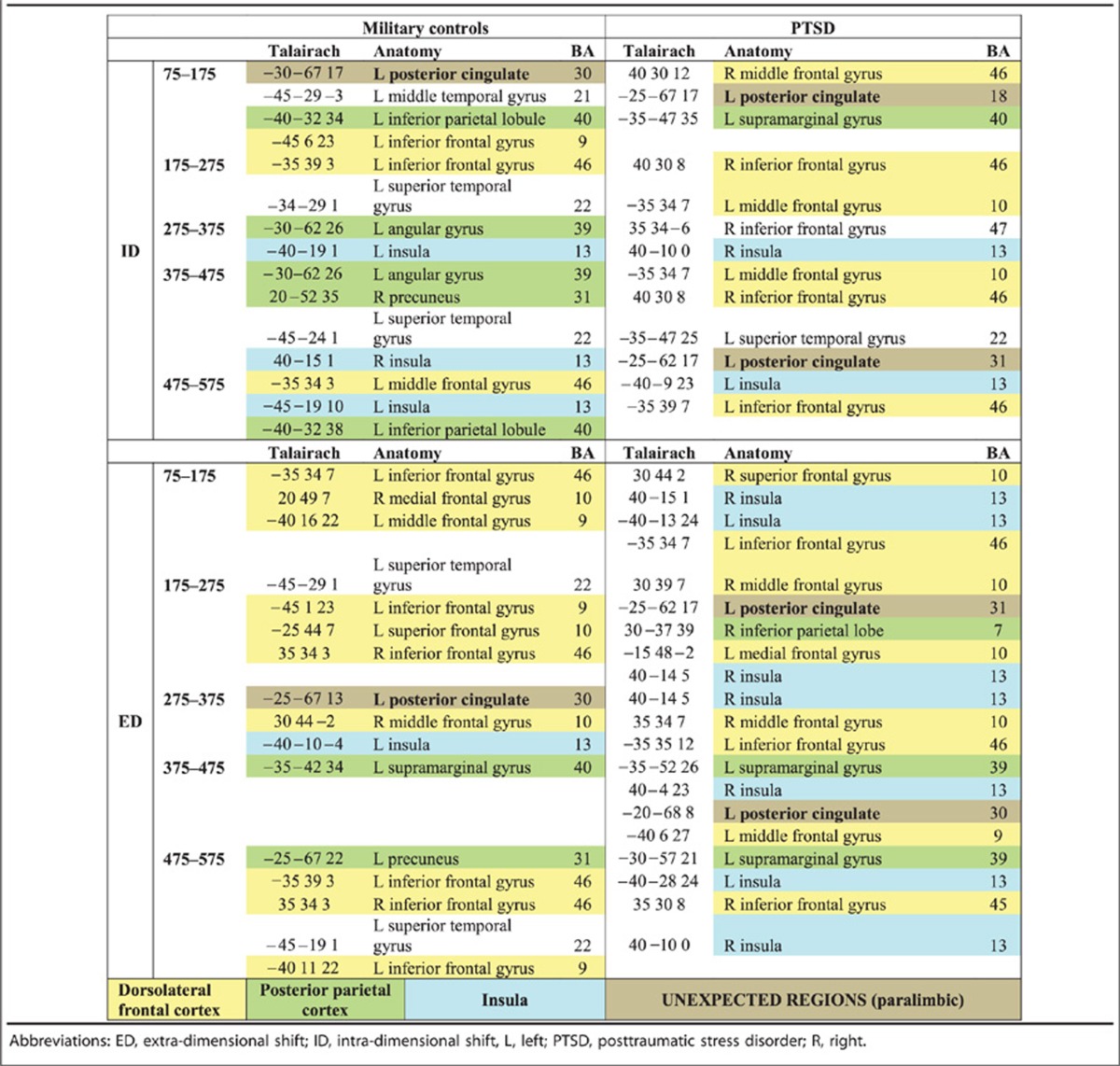
Brain regions involved in extra-dimensional shifting are listed in the bottom half of Table 2, using the same colour-coding scheme. Immediately, it is clear that the harder extra-dimensional shifts required a greater number of brain regions for both groups, although the expected regions are seen. For the control soldiers, activated areas included the left insula, left posterior parietal cortex and bilateral dorsolateral frontal cortices, whereas the individuals with PTSD required bilateral insulae, left posterior parietal regions and bilateral dorsolateral frontal cortices.
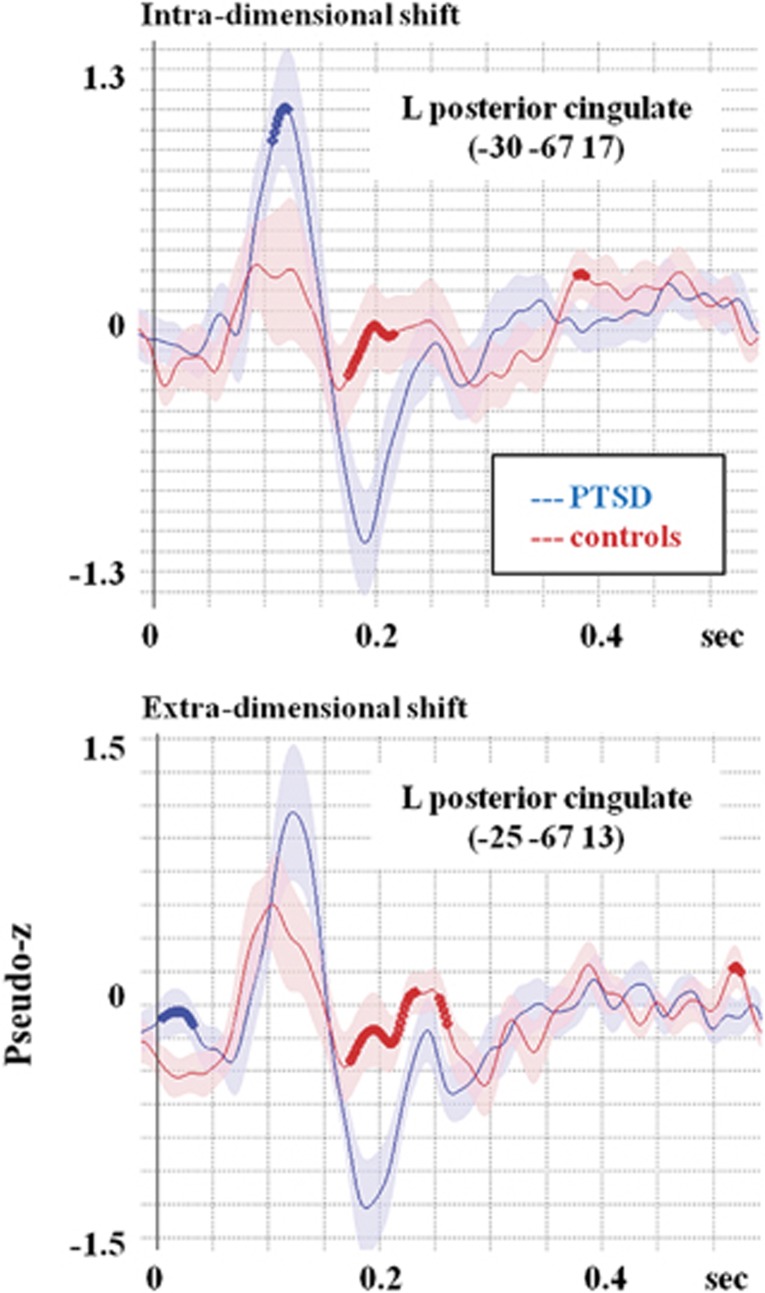
In Table 2, there are a number of cells coded with the colour brown. These cells indicate neural regions that are not typically seen in a set-shifting task. Specifically, the left posterior cingulate showed prominent and significant activation in both groups for both types of set shifting. To explore the involvement of the left posterior cingulate, the time course of activation in this location was reconstructed for the two groups and tested for differences. Figure 1 reveals that the posterior cingulate was activated earlier and to a greater extent in the PTSD as compared with control soldiers.
Figure 1.
Reconstructed time courses from the left posterior cingulate, an area that was identified as active in this set-shifting task, although not typically seen on this kind of protocol. For intra-dimensional shifting, the PTSD group shows significantly greater activation in an early time window. For the extra-dimensional shifting, the between-groups difference is no longer significant due to the increased activation in this area in the military controls. Possibly, this increased activation reflects the increasing difficulty of the extra-dimensional shift, which is manifest as a stress-related increase in paralimbic regions for the controls. PTSD, posttraumatic stress disorder.
Between-groups image contrast
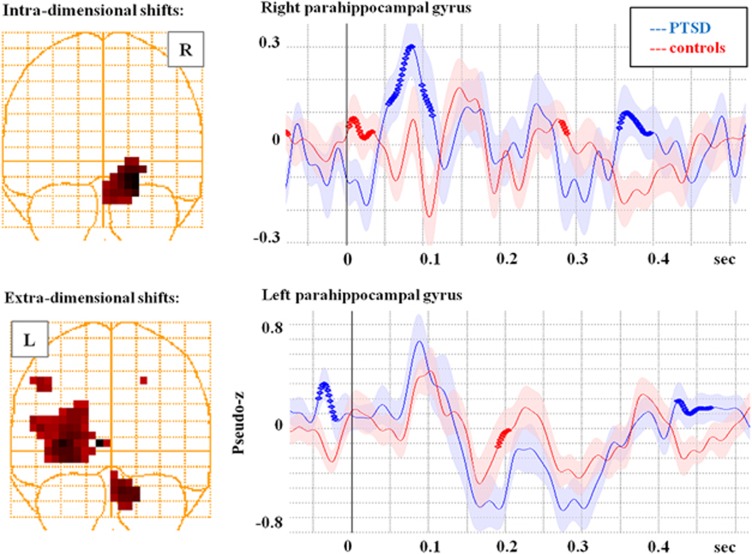
To directly compare the differences in neural activation between the control and PTSD groups for the two conditions, we submitted the source localization results to an image contrast where significant differences (P<0.01, corrected) between the images in each of the time windows were identified. For the intra-dimensional shift, there were three regions with significant group differences: the right insula (BA13), the left inferior frontal gyrus (BA 47) and the right parahippocampal gyrus (BA35). For the extra-dimensional shift, only two regions showed significant differences between the PTSD and control soldiers. This was the left posterior cingulate (BA 30/31) and the left parahippocampal gyrus (BA 19). As mentioned above, the posterior cingulate is not typically seen in a set-shifting task, nor are the parahippocampal gyri. To explore the involvement of these paralimbic structures, the time course of activation for the right and left parahippocampal gyri were reconstructed and shown in Figure 2. It is clear from this figure that the parahippocampal gyrus is more activated in the PTSD compared with the control group. Interestingly, the between-groups differences are more pronounced with intra- rather than extra-dimensional shifting.
Figure 2.
Reconstructed time courses from the right and left parahippocampal gyri, regions that were identified as significantly different between groups on an image contrast. For intra-dimensional shifts, the right parahippocampal gyrus shows a significantly greater response in the PTSD group, whereas for the extra-dimensional shifting, both groups show an increased response, although the increase is greater in the controls such that they reach a similar level as the PTSD. Possibly, this reflects the reaction of the paralimbic structures to the stress of completing this more difficult condition of the task. PTSD, posttraumatic stress disorder.
Exploring the insula with reconstructed time course data
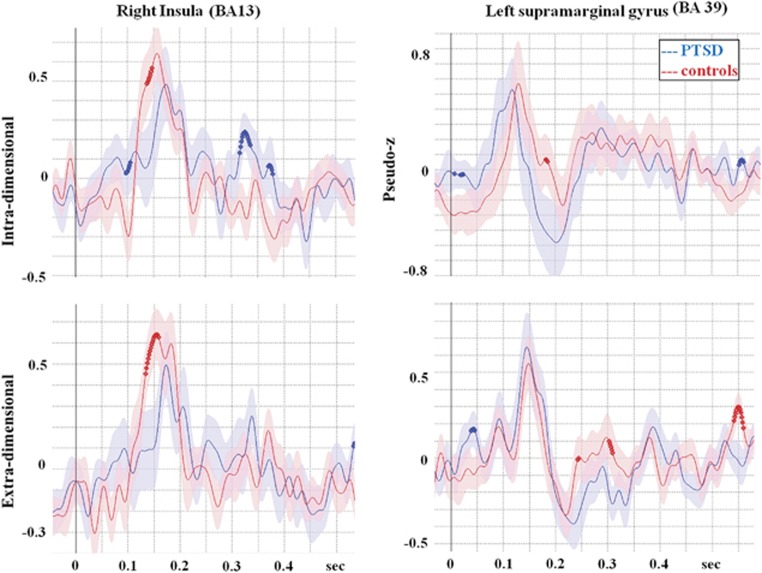
To test the hypothesis proposed by Simmons et al.19 that elite soldiers without PTSD show increased right insula activation, we reconstructed time courses in the right insula and statistically compared them between groups. To ensure that this was not due to an overall greater activation in one group, we also reconstructed time courses for both groups in the left posterior parietal cortex, the other key hub region for set-shifting. These time courses are contained in Figure 3 and show that the right insula is more activated in the control than the PTSD group, although both groups showed similar neural involvement. Further, it is clear that the suppressed involvement of right insula in the PTSD group is specific to right insula and is not seen in the left posterior parietal area, the other canonical set-shifting region.
Figure 3.
Reconstructed time courses from the right insula and left supramarginal gyrus to test the hypothesis of whether the insula is specifically recruited in unaffected individuals to maintain task performance. As the supramarginal gyrus does not show significant differences between PTSD and controls, this suggests that the increased activation in the controls is localized to the insula and is not a general global increase. PTSD, posttraumatic stress disorder.
Discussion
In this study, using the excellent temporal and spatial resolution of MEG, we determined significant differences in the brain regions recruited during performance of a set-shifting task between soldiers with PTSD and matched military controls. Our behavioural data demonstrated that although soldiers with PTSD had reaction times that were comparable with matched controls, their performance was significantly less accurate. This represents the classic speed accuracy trade-off where increased task difficulty forces the participant to choose between maintaining speed or accuracy. In this case, soldiers with PTSD found the extra-dimensional shift sufficiently more difficult that they traded accuracy for maintenance of reaction time.
Initial source analysis in the two groups identified similar areas of activation that proceeded from dorsolateral frontal cortex to insula to posterior parietal cortices. These areas are consistent with both fMRI and MEG reports in the literature for set-shifting23, 24, 25, 26 and our work in civilian adults.27
However, both our source analyses of the spatiotemporal progression of set-shifting and our analysis of the between-groups contrast revealed significant activations in the paralimbic cortex, an area not typically activated for set-shifting. Specifically, we saw activations in posterior cingulate, parahippocampal gyri and regions in the temporal lobes. This finding was both surprising and reassuring. Surprising in that the set-shifting task is not an emotional, affective or traumatic task. The stimuli consisted of simple shapes with colours and are entirely innocuous. These findings are reassuring in that our identification of these regions fits well with proposed models of the neurocircuitry of PTSD. For example, reviews of Hughes and Shin12 and Patel et al.13 concur that the amygdalae and hippocampi are hyper-reactive, whereas the medial PFC is hypo-responsive and fails to inhibit the limbic structures. Further, diffusion tensor imaging studies reported reductions in white matter volume in the cingulum and superior longitudinal fasciculus in PTSD.39 In our case, we did not find amygdalae responses, which fit with our presentation of nonemotional innocuous stimuli. However, we found a dissociation of the response in the paralimbic structures—specifically, we found increased activation in the cingulate and parahippocampal cortex in the group with PTSD, whereas the medial PFC, particularly the insula, were significantly less active. As suggested by this model, posterior parietal regions are not substantively different in PTSD.
Although findings in the literature suggest that increased anterior cingulate cortex activity is a biomarker reflecting a familial risk of developing PTSD after trauma,2 our data do not support this as we did not see greater anterior cingulate activation in the PTSD compared with the controls that would have differentiated the groups. In fact, both groups showed posterior cingulate involvement, and this was more pronounced in PTSD. However, the studies used different tasks, thus, it would be important to replicate the findings with similar tasks. Also, these prior studies were conducted with fMRI and thus do not have the same temporal resolution as MEG. Therefore the reported effects may be very slow or late whereas MEG recordings can capture fast and early neurophysiological activity.
Studies using fMRI, by Simmons et al.,18, 19 showed that individuals with PTSD failed to appropriately and adequately activate the right insula when performing a set-shifting task. Further, these authors proposed that the right insula was a key region for resilience against PTSD and maintenance of performance during stress situations. We specifically tested this hypothesis by re-constructing time courses in the right insula as well as in the left supramarginal gyrus. We chose the left supramarginal gyrus as this is another established hub area for set-shifting, but it has not been implicated in PTSD. We found a significant difference with a greater and earlier activation in the right insula in the control soldiers for both intra- and extra-dimensional shifting; we did not find significant differences between groups in the left supramarginal gyrus suggesting that the control soldiers specifically recruited the insula, and this is not simply due to widespread, greater activation in the controls.
There are a few caveats to consider when interpreting our findings. One is with regard to our exclusion of some, but not all, medications. We excluded participants who were taking anti-convulsants, benzodiazepines and GABA antagonists; however, participants were not free from all medications. We acknowledge that this is not ideal, and we point the reader to a discussion of the benefits and costs when deciding the exclusions around participant medications.40 On a similar note, all participants in the PTSD arm of our study were in active or maintenance psychotherapy. Again, though more ideal, testing individuals with PTSD who had not undergone some therapy would not have been feasible. Finally, 70% of our cohort had comorbid major depressive disorder; thus, it could be suggested that our findings captured cognitive dysfunction associated with depressive symptomology and not specifically PTSD. However, a classic study using single-photon emission computed tomography demonstrated significantly reduced regional blood flow to paralimbic structures in clinical depression with underactivation in this condition.41 Although blood flow does not correlate directly with the MEG neurophysiological response, it is unlikely that underactivity in single-photon emission computed tomography translates into an excessive overactivity in MEG. Thus we think that we have captured cognitive dysfunction associated with PTSD and not depression; however, this needs to be directly confirmed.
Finally, a recent study demonstrated that, to some extent, the neuropsychological impairments induced by PTSD can be improved with treatment.42 One factor that motivated our study of the neural underpinnings of set-shifting in PTSD is the fact that set-shifting, and mental flexibility, is a core component of executive functions. Individuals who struggle with mental flexibility may find that their cognitive difficulty with shifting interacts with other executive control domains, for example, memory processing and inhibition. If we can elucidate the brain regions underlying abnormal set-shifting in PTSD, monitoring the impact of therapy on their neural responses may serve as a biomarker for tracking therapy efficacy.
In conclusion, by capitalizing on the high spatial and temporal resolution of MEG, we have determined that the core brain regions underlying set-shifting are comparable between soldiers with PTSD and controls; however, the individuals with PTSD demonstrate a significant and atypical involvement of paralimbic regions. This may be one mechanism that impedes performance on a set-shifting task in PTSD; possibly, this contributes to difficulties with mental flexibility, which may underlie deficits in other cognitive executive functions.
Acknowledgments
This work was supported by funding from Defence Research and Development Canada (Contract No: W7719-135182/001/TOR) to MJT and EWP and the Canadian Forces Health Services. We thank Marc Lalancette for assistance with data collection.
The authors declare no conflict of interest.
References
- American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders (4th edn, text rev) American Psychological Association: Washington, DC, USA; 2000. [Google Scholar]
- Pitman PK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges DW, Woon FL. Premordid brain volume estimates and reduced total brain volume in adults exposed to truama with or without posttraumatic stress disorder: a metaanalysis. Cogn Behav Neurol. 2010;23:124–129. doi: 10.1097/WNN.0b013e3181e1cbe1. [DOI] [PubMed] [Google Scholar]
- Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- Gilbertson M, Paulus L, Williston S, Gurvits TV, Lasko NB, Pitman RK, et al. Neurocognitive function in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. J Abnorm Psychol. 2006;115:484–495. doi: 10.1037/0021-843X.115.3.484. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A, et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S, et al. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro P, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci USA. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, et al. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: An MRI study. Neuroimage. 2011;54:S62–S68. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan S, Martindale S, Robinson J, Kimbrel NA, Meyer EC, Kruse MI, et al. Neuropsychological sequelae of PTSD and TBI following war deployment among OEF/OIF veterans. Neuropsychol Rev. 2012;22:21–34. doi: 10.1007/s11065-012-9190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KC, Shin LM. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev Neurother. 2011;11:275–285. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Kleiner JS, Vasterling JJ, Field AP. Memory for emotionally neutral information in posttraumatic stress disorder: a meta-analytic investigation. J Abnorm Psychol. 2007;116:448–463. doi: 10.1037/0021-843X.116.3.448. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Verfaellie M. Posttraumatic stress disorder: A neurocognitive perspective. J Int Neuropsychol Soc. 2009;15:826–829. doi: 10.1017/S1355617709990683. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr, Sutker PB, et al. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Lasko NB, Schacter SC, Kuhne AA, Orr SP, Pitman RK, et al. Neurological status of Vietnam veterans with chronic posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci. 1993;5:183–188. doi: 10.1176/jnp.5.2.183. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo IA, Matthews SC, Paulus MP, Stein MB, et al. Initial evidence of a failure to activate right anterior insula during affective set shifting in posttraumatic stress disorder. Psychosom Med. 2009;71:373–377. doi: 10.1097/PSY.0b013e3181a56ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Fitzpatrick S, Strigo IA, Potterat EG, Johnson DC, Matthews SC, et al. Alterered insula activation in anticipation of changing emotional states: neural mechanisms underlying cognitive flexibility in special operations forces personnel. Neuroreport. 2012;23:234–239. doi: 10.1097/WNR.0b013e3283503275. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, et al. At the heart of the ventral attention system: The right anterior insula. Hum Brain Mapp. 2009;30:2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Picton TW, Alexander MP, Gillingham S, et al. Mapping task switching in frontal cortex through neuropsychological group studies. Front Neurosci. 2008;2:79–85. doi: 10.3389/neuro.01.013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A, et al. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Andrews C, Grasby PM, Brooks DJ, Robbins TW, et al. Contrasting cortical and subcortical activations produced by attentional-set-shifting and reversal learning in humans. J Cogn Neurosci. 2000;12:42–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Konishi S, Hayashi T, Uchida I, Kikyo H, Takahashi E, Miyashita Y, et al. Hemispheric asymmetry in human lateral prefrontal cortex during cognitive set shifting. Proc Natl Acad Sci USA. 2002;99:7803–7808. doi: 10.1073/pnas.122644899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie CH, Specht K, Marshall JC, Frink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage. 2006;30:1038–1049. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Oh A, Vidal J, Taylor MJ, Pang EW. Neuromagnetic correlates of intra- and extra-dimensional set-shifting. Brain Cogn. 2014;86:90–97. doi: 10.1016/j.bandc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Schwab KA, Baker G, Ivins B, et al. The brief traumatic brain injury screen (BTBIS): Investing the validity of a self-report instrument for detecting traumatic brain injury (TBI) in troops returning from deployment in Afghanistan and Iraq Neurology 200666(Supp.2):A234 [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Pearson Education: San Antonio, TX, USA; 1999. [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG.AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care, 2nd edition. World Health Organziation, Department of Mental Health and Substance Dependence. Document No. WHO/MSD/MSB/01.6a2001
- Spitzer RL, Kroenke K, Williams JBW, Lowe B. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behav· Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four-to-eight year-old children. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25:249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Chau W, McIntosh AR, Schulz M, Pantev C. Improving permutation test power for group analysis of spatially filtered MEG data. Neuroimage. 2004;23:983–996. doi: 10.1016/j.neuroimage.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Singh KD, Barnes GR, Hillebrand A. Group imaging of task-related changes in cortical synchronization using nonparametric permutation testing. Neuroimage. 2003;19:1589–1601. doi: 10.1016/s1053-8119(03)00249-0. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JK, Lamke JP, Gaebler M, Scheel M. White matter integrity and its relationship to PTSD and childhood trauma – a systematic review and meta-analysis. Depress Anxiety. 2013;30:207–216. doi: 10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Brewin CR, Bremner JD, Daniels JK, Friedman MJ, Liberzon I, et al. Does neuroimaging research examining the pathophysiology of posttraumatic stress disorder require medication-free patients. J Psychiatry Neurosci. 2010;35:80–89. doi: 10.1503/jpn.090047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lewis PJ, Regenold W, Wagner HR., Jr Paralimbic hypofusion in unipolar depression. J Nucl Med. 1994;35:929–934. [PubMed] [Google Scholar]
- Walter KH, Palmierir PA, Gunstad J. More than symptom reduction: changes in executive function over the course of PTSD treatment. J Trauma Stress. 2010;23:292–295. doi: 10.1002/jts.20506. [DOI] [PubMed] [Google Scholar]