Abstract
Marine biodiversity currently faces unprecedented threats from multiple pressures arising from human activities. Global drivers such as climate change and ocean acidification interact with regional eutrophication, exploitation of commercial fish stocks and localized pressures including pollution, coastal development and the extraction of aggregates and fuel, causing alteration and degradation of habitats and communities. Segregating natural from anthropogenically induced change in marine ecosystems requires long-term, sustained observations of marine biota. In this review, we outline the history of biological recording in the coastal and shelf seas of the UK and Ireland and highlight where sustained observations have contributed new understanding of how anthropogenic activities have impacted on marine biodiversity. The contributions of sustained observations, from those collected at observatories, single station platforms and multiple-site programmes to the emergent field of multiple stressor impacts research, are discussed, along with implications for management and sustainable governance of marine resources in an era of unprecedented use of the marine environment.
Keywords: timeseries, sustained observations, climate change, multiple stressors, marine biodiversity, ecosystem
1. Introduction
Marine and coastal ecosystems are naturally spatially and temporally variable, but are also experiencing unprecedented changes in response to global anthropogenic forcing of atmospheric processes coupled with additional human activities operating at global, regional and local scales. Sustained observations of biota from multiple trophic levels across broad geographical scales are required to separate climate-driven signals from the noise of natural fluctuations to disentangle responses of species, communities and ecosystems to global drivers from regional and local-scale impacts [1]. Policy directives implemented to manage anthropogenic pressures in the face of such change also require knowledge of past, present and potential future ecosystem states. Thus, timeseries are the primary resource enabling tracking of the status and functioning of ecosystems while defining targets for environmental stewardship and ensuring sustainable use of natural resources.
A rich history of sustained observations of marine biota stretching back to the 1800s exists for shelf and coastal waters in the UK and Ireland. In this review, ‘sustained observations’ and ‘timeseries’ are both defined as, ‘where there is a commitment to maintain scientific research and monitoring beyond the usual length of a scientific research programme, typically three to five years’ [2]. Many broadscale and long-term observations extend across multiple decades, and in some cases, more than a century for the coastal and shelf seas. Others provide baselines at time-slices spanning the start of the industrialized fishing at sea, and the current era of accelerating anthropogenic activity and resultant pressures.
A summary of sustained biological observations within UK and Irish regional shelf and coastal seas highlights the milestones in scientific monitoring of marine biodiversity since formal scientific recording began in the mid-nineteenth century. Against this background, the applications of multi-decadal broadscale observations of the shelf seas and the contribution of near shore and coastal observatories at fixed stations to regional-scale studies are discussed. We highlight the major scientific findings and review how these timeseries are proving invaluable for forecasting future states of natural resources in response to unprecedented exposure to multiple anthropogenic stressors. The relevance of these sustained observations to national and international policy drivers is then explored to develop a prospectus for twenty-first century sustained biological observing.
2. The history of sustained biological observations in the coastal and near shore marine environment
Concerns about the sustainability of commercial fisheries were apparent by the middle of the nineteenth century and accelerated with increasing industrialization of the fishing industry. These issues prompted the establishment of the Marine Biological Association of the United Kingdom (MBA) in 1884 and the construction of its research laboratory in Plymouth, England in 1888. Investigations into physical, chemical and biological components of the Western English Channel ecosystem and at satellite laboratories in the North Sea commenced from the 1880s (see [1] for review). This expanded to include zooplankton and larval fish timeseries in the open waters of the English Channel, North Sea (from the Lowestoft Laboratory, precursor MAFF/CEFAS laboratory) and the continental shelf off southwest England as part of the UK contribution to the International Council for the Exploration of the Sea (ICES) international research programme [3].
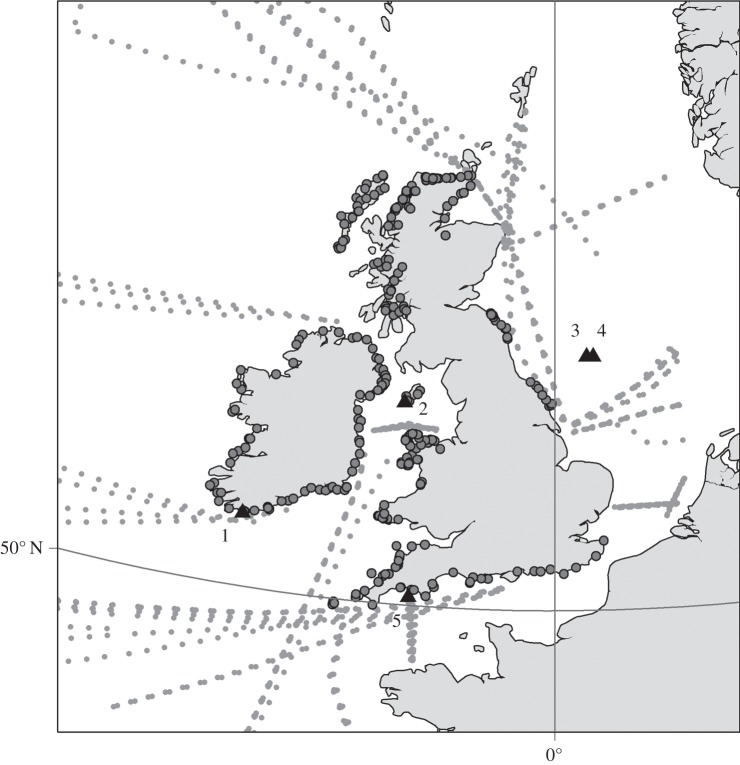
Baseline studies of subtidal benthos in the Western English Channel in the 1890s were resurveyed, including the Holme grid off Plymouth (surveyed 1958–1963) as part of a larger survey of the benthos of the English Channel [4] (figure 1). More recent evaluations of the benthic fauna have been made based on data collected in the 1970s with surveys of a reduced section of the Holme Grid continuing on a semi-regular basis since 2007 [5].
Figure 1.
Map of UK sustained observations of biodiversity: dark circles, MarClim survey sites; pale circles, continuous plankton recorder circles 2012 surveys. Black triangles: (1) Loch Hyne; (2) Port Erin; (3) Dove plankton; (4) Dove benthos; (5) Western Channel Observatory. Figure by Dr A. McQuatters-Gollop.
A grid of sustained observing stations was set up in the western English Channel by the MBA as part of the ICES investigations in 1903 (figure 1). Station E1 (20 nautical miles from Plymouth, approx. 72 m depth, phytoplankton) and L5 (two nautical miles inshore from E1, approx. 60 m depth, zooplankton, larval fish) have been sampled on a monthly basis since 1903, but major gaps exist in both timeseries. The MBA have sampled fish in a ‘standard haul’ around station L4 (10 nautical miles from Plymouth, approx. 51 m depth) for almost 100 years to date. In response to the cessation of funding for E1 and L5 in 1987, sampling at L4 was initiated in 1988 by Plymouth Marine Laboratory (PML) and continues on a weekly basis [6].
The University of Liverpool's Port Erin Marine Laboratory on the Isle of Man maintained sustained observations at locations in the Irish Sea, measuring temperature, salinity, oxygen nutrients and chlorophyll from 1904 to provide baseline environmental data for the interpretation of biological investigations conducted at the laboratory [7]. Subtidal benthos was also monitored across several decades within the 1900s and changes linked to long-term scallop-dredging activity [8]. Following the closure of the laboratory in 2006, responsibility for the timeseries has been subsumed by the Isle of Man Government Laboratory (figure 1).
Demersal fish surveys and studies of the Plymouth herring fishery began in the 1900s providing a baseline for comparative studies a century later [9]. With the decline of the herring fishery, attention returned to mackerel [10]. Analysis of these observations combined with historical records dating back to the thirteenth century showed that switches between herring and pilchard had occurred since the Middle Ages [11]. Herring was also the subject of one of the few multi-decadal observations of marine biota initiated in Ireland by the Marine Institute between the 1960s and 1990s [12]. Both datasets showed higher growth rates in the cooler 1960s and 1970s than the warmer 1980s and 1990s, with climate rather than fishing pressure being the driver of long-term change in both regions [11,12].
Sir Alister Hardy developed the continuous plankton recorder (CPR) in the 1920s to provide spatio-temporal maps of plankton to interpret changes in English east coast herring stocks and place biological variation in the context of hydrometeorology and climate change. After trials on the Discovery Expedition to Antarctica in 1924, the CPR was deployed on merchant ships leaving from UK ports from 1931. The CPR has been based at several institutions with its current home at the Sir Alister Hardy Foundation for Ocean Science, Plymouth [13]. The CPR now covers the Atlantic Ocean and has completed 6.3 million survey miles (figure 1).
The first systematic, large-scale rocky intertidal surveys of the UK began in the English Channel in the 1930s [14] and 1950s [15], expanded around the UK and Ireland by A. J. Southward and D. J. Crisp in the 1950s and 1960s [16]. A team, led by J. R. Lewis from University of Leeds Robin Hood's Bay Marine Laboratory, North Yorkshire, studied the distribution, abundance and breeding of intertidal invertebrates (1960s–1980s) and were among the first to link climate to breeding cycles and success in endotherms [17]. The Robin Hood's Bay laboratory closed in 1983, with some data being rescued from a skip by M. A. Kendall and later digitized by Mieszkowska. The Southward, Crisp and Lewis surveys were restarted in 2002 under the MBA MarClim Project (www.marclim.co.uk; figure 1) and form the most spatio-temporally extensive rocky intertidal timeseries globally. MarClim rescued additional historical survey data for keystone species, invasives and those of current conservation importance, restarting surveys by incorporating the site locations into the MarClim UK annual survey network [18]. These data are instrumental in understanding climate impacts on marine biodiversity, ecosystem structure and functioning [19,20]. Newcastle University Dove Marine Laboratory maintains three long-term timeseries: monthly pelagic plankton station Z (approx. 6 nautical miles offshore, approx. 20 m depth) initiated by Evans in 1968, biannual benthic stations P (12 nautical miles offshore, approx. 80 m depth) and M1 (approx. 6 nautical miles offshore, approx. 55 m depth) started by Buchanan in 1971 to track marine productivity (figure 1).
Annual surveys of organisms within Lough Hyne, Cork, Ireland, since 1980 have detected the arrival and proliferation of invasive non-native and climate indicator species and long-term changes in the abundance of invertebrates leading to restructuring of intertidal and shallow subtidal communities [21] (figure 1). The sustained observations of rocky shore species carried out by staff at Sherkin Island Marine Station, Cork (1975–1990s) have also documented fluctuations in sessile invertebrates and macroalgae related to climate [22].
Some of the most extensive sustained observations of marine mammals are maintained by the Sea Mammal Research Unit (SMRU) at St Andrews, Scotland. SMRU has been recording grey seal pup abundances annually since 1960 and counts of moulting common seals since 1988 around the UK. Surveys investigating small cetacean abundances were conducted in European Atlantic waters and the North Sea (1994–2007).
Sustained observations have also been established by government agencies and government funded organizations in recent decades in the form of surveillance and compliance monitoring to track changes in ecosystem or protected area status, report against policy targets and develop status indicators [23]. The Clean Seas Environment Monitoring Programme cruise was initiated in the 1980s as part of a drive to coordinate UK marine monitoring. As part of this cruise, fish and benthos samples are taken at 25 fixed timeseries stations in intermediate and open sea areas around England and Wales.
The UK Seabird Monitoring Programme, led by the Joint Nature Conservation Committee in partnership with regional government authorities and ornithological trusts, has been tracking seabird populations in the UK and Ireland since 1969 [24]. The Irish Scheme for Cetacean Observation and Public Education started in 2003 to promote public volunteer recording of whales, dolphins and porpoises in Irish waters [25]. Observations of cetaceans by volunteers from 16 groups across Wales have been coordinated by Natural Resources Wales (née Countryside Council for Wales) since 1990 [26]. Similar schemes run by the UK Wildlife Trusts represent the contribution that citizen science can make to sustained observations of marine species.
3. Insights from sustained observations into ecological patterns and process-based responses to environmental and anthropogenic change in UK regional seas
(a). Shelf-wide surveys
CPR tows have generated over 250 000 samples and represent the largest marine biological dataset in the world. As early as 10 years into the timeseries Hardy was detecting links between fluctuations in plankton and herring abundances [27]. Data from CPR tows have been used to show how zooplankton in the North Atlantic and North Sea have undergone the largest detected biogeographic range shifts in any natural system (greater than 10° latitude polewards) in response to climate warming [28]. Changes in planktonic community structure have become especially notable after the 1983–1988 ‘regime shift’ with a shift in dominance from the Arctic-Boreal Calanus finmarchicus to the Lusitanian Calanus helgolandicus [29]. These changes have also impacted higher trophic levels, negatively affecting Northeast Atlantic cod, salmon and sandeel during warm periods throughout the twentieth century via match/mismatch of zooplankton food resources for larval and juvenile fish and apex predators [30–32].
The Centre for Environment, Fisheries and Aquaculture Science (CEFAS) is responsible for sustained observations of fish across several UK regional seas. The annual groundfish survey of the North Sea was initiated in 1977 and since 1991 has formed part of the International Bottom Trawl Survey series, which is coordinated by ICES. Recording of the North Sea young fish survey for inshore coastal waters of the central and southern North Sea began in the 1960s and intensified in the 1970s, with annual surveys from 1981 to 2010, when the surveys finished. These data show abundance of small fish and demersal species with a low maximum length have steadily increased in absolute numbers over large parts of the North Sea during the past 30 years. Although fishing appears to be the major driver of these changes it cannot fully explain the observed trend [33].
Analyses of a standard survey covering the whole of ICES Divisions VIIa and VIIf since 1993, with additional tows northeast of Division VIIg have revealed an increase in the proportion of mature fish coinciding with rising SST and a decline in recruitment and stock biomass at high rates of fishing mortality [34]. A detailed study of demersal fish in ICES Division VIIe of the Western English Channel (1989–2011) charts fluctuations in abundance and size classes for plaice, sole, lesser spotted dogfish, blonde and thornback rays and declines in spotted ray and spurdog abundances [35]. The data from the quarter 4 westerly groundfish survey (2002–2011) comprising 107 tows demonstrated a decline in length–frequency distribution of fish in both target and non-target categories in response to commercial exploitation [36].
CEFAS data showed a slow but progressive decline in the trophic level of the demersal community (1982–2000) [37]. Assessment of a more extensive segment of the timeseries found that change had only occurred during the past two decades and that effects of fishing on the trophic structure of fish communities were more complex than previously assumed. A heterogeneous latitudinal response to warming detected within the dataset reflects a northward shift in the mean latitude of abundant, widespread thermal specialists. By contrast, a southward shift of small, abundant warm water species with limited occupancy and a northern range boundary in the North Sea was observed [38].
Meta-analyses of fisheries-independent data from the FishBase dataset (http://www.fishbase.org) of sustained observations including those collected by CEFAS, Defra and UK scientific institutes examined the impacts of climate change for over 100 million individual fish sampled over 1.2 million km2 from 1980 to 2008 across the continental shelf seas around the UK and Ireland. The findings demonstrated responses to warming in 72% of common species, with three times more species increasing in abundance than declining. These trends are reflected in international commercial landings [39]. Profound reorganization of the relative abundance of species in local communities occurred despite decadal stability in the presence–absence of species, with the majority of common northeast Atlantic fishes responding significantly to warming [39]. Climate warming has also driven vertical distributional shifts in demersal fish in the North Sea with the whole demersal fish assemblage deepening by approximately 3.6 m following the thermal gradient to cooler waters [38].
(b). Nearshore observatories
The Western Channel Observatory, run by PML and the MBA, forms a central hub for long-term sampling including the E1, L4 and L5 observing stations. Decadal-scale changes in phytoplankton, zooplankton and larval fish abundance, with subsequent changes in fish stocks and pelagic community composition, across the twentieth century have been linked to cyclical environmental patterns (‘Russell cycle’) [40] changes in weather and pervasive global warming [41].
Between 1953 and 1972, the MBA completed over 1500 trawls for the standard haul in the waters off Plymouth [1], with trawls continuing on a quasi-monthly basis to date. The data show how climatic warming between (1970s–2000s) has driven changes in inshore fish assemblages of the English Channel towards dominance by ‘warmwater’ species [9]. Dichotomous size-dependent responses of species to climate change and commercial fishing over a century scale included rapid responses of small-bodied species to the prevailing thermal environment, suggesting their life-history traits predisposed populations to respond quickly to changing climates. Larger species declined in abundance and size, reflecting expectations from sustained size-selective overharvesting [42]. Climate change has also influenced the phenology of the squid Loligo forbesi, with migration shifting earlier between the warmest and coldest years (1953–1972) in the Western English Channel [43]. Temperature has a contrasting influence on the migration phenology of the Boreal flounder, Platichthys flesus that migrates from estuarine habitat to spawning grounds at sea earlier in cooler years [44].
Benthic faunal samples taken at various locations near the Plymouth L4 station and Eddystone lighthouse since 1895 have demonstrated significant differences in community composition between the early-, mid- and late-1900s, with a reduction in echinoderm diversity, decreased abundance of large molluscs and increased small-bodied mollusc and amphipod abundances [5]. The study area is an important beam trawling and scallop-dredging ground and the observed differences are concomitant with changes associated with disturbance from demersal fishing activities.
The benthic time-series sites maintained by the Dove Laboratory are used to investigate multi-decadal dynamics of the North Sea ecosystem. Analyses of these data have established links between the zooplankton and benthos abundances with periods of cooling and warming [45]. Links to changes in benthic assemblages and in some instances extirpation of species in the central North Sea have also been made [46].
A 25 year study at Hinkley Point, Bristol Channel from 1980 showed a striking stability in the population dynamics of the crustacean Crangon crangon, [47], whereas sole recruitment in this nursery area was positively correlated with water temperatures and growth was positively correlated with the winter North Atlantic oscillation (NAO). The results of this long-term monitoring show how climate change impacts can vary with species and trophic level.
(c). Coastal surveys and timeseries
The MBA-led Marine Biodiversity and Climate Change Project (MarClim) has carried out annual rocky intertidal surveys at 120 sites around the UK since 2002 and at 63 sites during 2003 in Ireland. MarClim data have shown some of the fastest shifts in biogeographic range limits for any species in response to pervasive climate change, with poleward extensions of northern boundaries for Lusitanian gastropods Phorcus (Osilinus) lineatus and Gibbula umbilicalis, the macroalga Bifurcaria bifurcata, barnacle Perforatus perforatus and limpet Patella depressa [18,48]. By contrast, Boreal species such as the barnacle Semibalanus balanoides and the kelp Alaria esculenta have declined in abundance in response to warming [18]. These species track both periods of previous warming and cooling in the UK climate and the onset of pervasive warming in the mid-1980s, demonstrating their importance as sensitive climate indicator species [18]. Rates of change are species-specific, with resultant changes to biodiversity and ecosystem functioning [20]. The long-term impact of extreme weather events is also tracked by MarClim. The reef building polychaete Sabellaria alveolata suffered severe mortalities throughout Wales during the cold winter of 1962/1963 but has recolonized locations close to its northern range limit during the period of recent climate warming [49,50].
Species-specific responses to climate change result in changes to community and ecosystem structure. The MarClim Cellar Beach timeseries (annual since 1952) shows a tight relationship to local SST with Lusitanian Chthamalid barnacles being more abundant after warmer years, and the Boreal S. balanoides more abundant after cooler years [16,51]. In contrast to the strong influences detected for plankton, no significant relationship to the larger climate indices of the NAO or the AMO is evident [52].
Long-term timeseries of bivalve populations in Dublin Bay, Ireland, have yielded valuable information about the decline in bivalve populations during the 1980s and 1990s but little change in overall biodiversity [53,54]. The majority of species showed a decline in numbers in the decade 1985–1995 with Cerastoderma edule becoming locally extinct. Older records suggest that these low numbers may not be exceptional, and may also be tied in to a 6–7 year cycle. Between 1995 and 2001, there was a recovery in most species per sites, and a spectacular 10-fold increase in the numbers of Tellina tenuis [53].
4. Impact of sustained observations on the global research field of climate impacts
Major advances in our understanding of how marine biodiversity have been impacted by local and regional-scale pressures have been made from analyses of the individual datasets from sustained observations. Long-term data have demonstrated the importance of factors underlying recruitment fluctuations in commercial species, periodic failures and switches in pelagic fisheries [55] and the influence of fluctuating climate driving marine ecosystems [17] including phenological shifts in migrations [43,44], and phenology of plankton including larval stages [42]. The importance of potential mismatches between the phenology of food and larval fish in a period of climate change has been demonstrated [56], providing substantive evidence for Cushing's long-espoused theory on match–mismatch [57].
The multi-decadal timespan and wide spatial coverage of MarClim data has allowed the development and testing of predictive ecological climate models that have been instrumental in forecasting changes to individual species and intertidal biodiversity that have wider temperate implications and have been cited globally. These include dynamic species distribution models to predict population abundances of intertidal species at specific sites based on SST and wave fetch and the relative roles of local environmental temperature versus basin-scale oscillations [52,58].
The UK Marine Environmental Change Network (MECN), established in 2003 as a focal centre for long-term marine data from around the British Isles and Ireland, aims to separate global, regional and local anthropogenic impacts from natural fluctuations. Over 300 timeseries of biological sustained observations were brought together by MECN to detect the existence of potential regime shifts across benthic and pelagic foodwebs. State-space models detected a step-change consistent with a regime shift in the central and southern North Sea in 1989 and again in 1994, whereas pervasive long-term trends were observed in the northern and southern North Sea [59].
Global meta-analyses of the rates of species range shifts in response to climate change have also heavily used the UK sustained observations datasets. Poloczanska et al. [60] calculated average global rates of species range shifts of 72 km per decade using a timescale between 1960 and 2009. These long-scale assessments feed into important global assessments such as the Intergovernmental Panel for Climate Change Fifth Assessment Report [61].
5. Science-policy applications
Marine policy for UK regional seas was initiated in 1969 with the publication of, ‘Conservation policy in the shallow seas’ by the Natural Environment Research Council (NERC), and the then Nature Conservancy Council (NCC) [62]. At the time, a suitable evidence base on environmental change was lacking and therefore little could be done to address issues raised. The impetus towards a formal marine conservation policy for the UK came from an NCC/NERC working party convened in 1975 that recommended ‘a study of the many factors regulating the natural fluctuations in marine communities should be seen as a basic and continuing contribution to a marine conservation programme’ [63].
The establishment of new legislation in the 1980s increased environmental awareness, and the requirement for basic data on the distribution of habitats and species became paramount. This led to a vast expansion in data collecting activities in the 1980s through projects such as the Marine Nature Conservation Review [64]. While the need for data was never greater, funding for many long-term marine sustained observations was withdrawn, with approximately 40% of timeseries across Europe stopped [65]. Since the 1980s, significant legislation has been developed supporting the sustainable use of marine ecosystems. The 1992 UN Convention on Biological Diversity prompted a worldwide response to address the crisis of a sustained biodiversity loss and underpins current legislation.
The UK marine monitoring and assessment community made a commitment to provide coordinated advice on the state of the UK seas and climate change impacts using high-quality, robust and peer-reviewed evidence. The UK Marine Science Strategy was established to summarize how marine ecosystem state and trends relate to the governmental vision of having ‘clean, healthy, safe, productive and biologically diverse oceans and seas within one generation’, prompting the Marine and Coastal Access Act 2009 with high-level priorities, including sustained long-term monitoring to underpin the provision of this evidence [66].
The European Marine Strategy Directive was created to more sustainably use the marine environment across Europe and achieve Good Environmental Status (GES) of Europe's seas by 2020 through the provision of 11 descriptors [67]. CPR data have been used to develop UK and OSPAR pelagic indicators of the state of planktonic communities [68], sustained observations of North Sea fish have been instrumental for fish stock indicators [69] and MarClim datasets have been used in development of several UK benthic indicators of GES for Descriptors 1 Biodiversity, four Foodwebs and six Seabed Integrity [70].
Sustained observations are also proving instrumental in the communication of climate change impacts through the Marine Climate Change Impacts Partnership (MCCIP) Annual Report Cards [71], Charting Progress State of the UK Seas assessments [72], the National Ecosystem Assessment [73], including MarClim, CPR, MBA and CEFAS fish and benthic timeseries, SMRU, British Trust for Ornithology and the UK Seabird Monitoring Programme.
6. Strategy for sustained observations in the twenty-first century
Many timeseries are extremely vulnerable with threats to funding. Some have ceased and others run on an ad hoc funding basis with insufficient funds for sample processing and data analysis. MCCIP published the results of a survey on knowledge gaps for climate impacts research and policy in the UK and Ireland in 2012 [74]. This noted that both UK and Scottish strategy documents include ‘responding to climate change and its interaction with the marine environment’ as one of three high-level priorities. Therefore, there is a clear requirement for the policy community to understand the current state of marine climate change research, much of which stems from sustained observations.
MECN acted as a hub for UK sustained observations between 2002 and 2012. No funding currently exists for networking activities between the organizations responsible for the timeseries. A more linked approach to funding sustained observations of marine biodiversity between the government agencies and departments and the UK Research Councils would ensure extant timeseries continue with a long-term financial commitment to funding as opposed to the current annual applications and renewal of funding.
Nationally accredited marine biodiversity data centres including the Data Archive for Seabed Species and Habitats DASSH, Fisheries Data Archive Centre FishDAC and the National Biodiversity Network are Medin-accredited data archives. Establishing DOI status for sustained observations held by these data archive centres would greatly assist with the demonstrations of how widely used the data are in scientific research programmes and policy-related assessments.
As financial resources become squeezed, data collection by Citizen Science programmes could become crucial to fill in gaps. Citizen Science projects have a long history in the UK, developing from the rise of the amateur naturalist in the late 1800s and the establishment of Natural History Societies. For example, over 40 000 volunteer birdwatchers have contributed to long-term data collected by BTO since 1933 and made significant contributions to the scientific literature on bird ecology. Seasearch established in 1981 has collected more than 300 000 species records from volunteer divers around the UK and Ireland. The data have recently informed the recommendations of Marine Conservation Zones around the UK. Many other projects are in their infancy, for example OPAL, The Big Sea Survey, ClimateWatch and Marine Metre Squared and could be used to fill existing data gaps.
7. Concluding comments
Sustained observing needs sustained funding. Such funding along with associated networks is essential to maximize coverage to disentangle local and regional change from global trends. Long-term observations also generate hypotheses for experimental testing in the laboratory and field; they enable calibration and validation of models providing predictive power and therein exploration of future scenarios. They also show nonlinear responses of complex marine systems to multiple stressors leading to regime shifts. Such scientific insights are essential for not only better understanding of the oceans, but also for their sustainable management in a rapidly changing world.
Acknowledgements
Louise Allcock, Simon Berrow, Silvana Birchenough, Deirdre Brophy, Mark Costello, Michael Guiry, David Johns, Mark Johnson, Robert Kennedy, Christine Maggs, T. Kieran McCarthy, Abigail McQuatters-Gollop, Anne-Marie Power, Glenn Nolan, John Pinnegar, Robin Raine, David Reid, Matthew Service, Joe Silke, Ben Wigham and Jim Wilson for information on timeseries. Moira MacLean for additional assistance.
Data accessibility
Many of the sustained observations datasets can be accessed via the websites of the responsible institutes or organizations, and many of the Plymouth timeseries from the Western Channel Observatory website (http://www.westernchannelobservatory.org.uk/). Several including the MarClim dataset are held on the National Biodiversity Network Marine Recorder Database and in the DASSH.
Funding statement
N.M. was supported by a Marine Biological Association Research Fellowship, Natural England and Natural Resources Wales for MarClim related activities. N.M. and S.J.H. were funded by the NERC Velocity of Climate Change Research NE/J024082/1. N.M., H.S., L.F. and S.J.H. were supported by the SWAT network.
References
- 1.Southward AJ, et al. 2005. Long-term oceanographic and ecological research in the western English Channel. In Advances in marine biology, pp. 1–105. San Diego, CA: Academic Press. [DOI] [PubMed] [Google Scholar]
- 2.Frost MT, Jefferson R, Hawkins SJ. 2006. The evaluation of time series: their scientific value and contribution to policy needs. Report prepared by the Marine Environmental Change Network (MECN) for the Department for Environment, Food and Rural Affairs (DEFRA). Contract CDEP 84/5/311. 22, p. 94. Marine Biological Association Occasional Publications Plymouth, UK: Marine Biological Association. [Google Scholar]
- 3.Rozwadowski HM. 2003. The sea knows no boundaries. A century of marine science under ICES. Seattle, WA: University of Washington Press [Google Scholar]
- 4.Allen EJ. 1899. On the fauna and bottom deposits near the thirty-fathom line from the Eddystone grounds to start point. J. Mar. Biol. Assoc. UK 5, 365–542. ( 10.1017/S0025315400043526) [DOI] [Google Scholar]
- 5.Capasso E, Jenkins SR, Frost M, Hinz H. 2010. Eddystone benthic communities: investigation into the extent of change over a century-wide scale. J. Mar. Biol. Assoc. UK 90, 1161–1172. ( 10.1017/S0025315409991020) [DOI] [Google Scholar]
- 6.Smyth TJ, Fishwick JR, Lisa AM, Cummings DG, Harris C, Kitidis V, Rees A, Martinez-Vicente V, Woodward EMS. 2010. A broad spatio-temporal view of the Western English Channel observatory. J. Plankton Res. 32, 585–601. ( 10.1093/plankt/fbp128) [DOI] [Google Scholar]
- 7.Evans GL, Hardman-Mountford NJ, Hartnoll RG, Kennington K, Mitchelson-Jacob EG, Shammon T, Williams PJlB. 2003. Long-term environmental studies in the Irish Sea: a review. Defra Contract CDEP 84/5/311. Scientific Report 2 London, UK: Defra. [Google Scholar]
- 8.Hill AS, Veale LO, Pennington D, Whyte SG, Brand AR, Hartnoll RG. 1999. Changes in Irish Sea benthos: possible effects of 40 years of dredging. Estuar. Coast. Shelf Sci. 48, 739–750. ( 10.1006/ecss.1999.0476) [DOI] [Google Scholar]
- 9.Genner MJ, Sims DW, Wearmouth VJ, Southall EJ, Southward AJ, Henderson PA, Hawkins SJ. 2004. Regional climatic warming drives long-term community changes of British marine fish. Proc. R. Soc. Lond. B 271, 655–661. ( 10.1098/rspb.2003.2651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steven GA, Corbin PG. 1939. Mackerel investigation at Plymouth: preliminary report. Rapport et. Process verbaux des reunions. J. Conseil/Conseil Perm. Int. pour l'Exploration de la Mer 111, 1–18. [Google Scholar]
- 11.Southward AJ, Boalch GT, Maddock L. 1988. Fluctuations in the herring and pilchard fisheries of Devon and Cornwall linked to change in climate since the 16th century. J. Mar. Biol. Assoc. UK 68, 423–445. ( 10.1017/S0025315400043320) [DOI] [Google Scholar]
- 12.Lynch D. 2011. Biological changes in Celtic Sea and southwest of Ireland herring based on a long-term data archival project. MSc thesis, Trinity College.
- 13.Reid PC, Colebrook JM, Matthews JBL, Aiken J. 2003. The continuous plankton recorder: concepts and history from plankton indicators to undulating recorders. Prog. Oceanogr. 58, 117–173. ( 10.1016/j.pocean.2003.08.002) [DOI] [Google Scholar]
- 14.Moore HB, Kitching JA. 1939. The biology of Chthamalus stellatus (Poli). J. Mar. Biol. Assoc. UK 23, 521–541. ( 10.1017/S0025315400014053) [DOI] [Google Scholar]
- 15.Crisp DJ, Southward AJ. 1958. The distribution of intertidal organisms along the coasts of the English Channel. J. Mar. Biol. Assoc. UK 37, 157–208. ( 10.1017/S0025315400014909) [DOI] [Google Scholar]
- 16.Southward AJ, Crisp DJ. 1954. The distribution of certain intertidal animals around the Irish coast. Proc. Royal Irish Acad. 57, 1–29 [Google Scholar]
- 17.Lewis JR. 1986. Latitudinal trends in reproduction, recruitment and population characteristics of some rocky littoral molluscs and cirripedes. Hydrobiologia 142, 1–13. ( 10.1007/BF00026742) [DOI] [Google Scholar]
- 18.Mieszkowska N, Kendall MA, Hawkins SJ, Leaper R, Williamson P, Hardman-Mountford NJ, Southward AJ. 2006. Changes in the range of some common rocky shore species in Britain: a response to climate change?. Hydrobiologia 555, 241–251. ( 10.1007/s10750-005-1120-6) [DOI] [Google Scholar]
- 19.Simkanin C, Power AM, Myers A, McGrath D, Southward AJ, Mieszkowska N, Leaper R, O'Riordan R. 2005. Using historical data to detect temporal changes in the abundances of intertidal species on Irish shores. J. Mar. Biol. Assoc. UK 85, 1329–1340. ( 10.1017/S0025315405012506) [DOI] [Google Scholar]
- 20.Hawkins SJ, et al. 2009. Consequences of climate-driven biodiversity changes for ecosystem functioning of North European rocky shores. Mar. Ecol. Prog. Ser. 396, 245–259. ( 10.3354/meps08378) [DOI] [Google Scholar]
- 21.Trowbridge CD, Little C, Pilling GM, Stirling P, Miles A. 2011. Decadal-scale changes in the shallow subtidal benthos of an Irish marine reserve. Bot. Mar. 5, 419–506 [Google Scholar]
- 22.Bishop G. 2003. The ecology of the rocky shores of Sherkin Island: a twenty-year perspective, p. 305 Brussels, Belgium: Sherkin Island Marine Station [Google Scholar]
- 23.Hardman-Mountford NJ, Allen JI, Frost MT, Hawkins SJ, Kendall MA, Mieszkowska N, Richardson KA, Somerfield PJ. 2005. Diagnostic monitoring of a changing environment: an alternative UK perspective. Mar. Pollut. Bull. 50, 1463–1471. ( 10.1016/j.marpolbul.2005.06.022) [DOI] [PubMed] [Google Scholar]
- 24.JNCC. 2013. Seabird population trends and causes of change: 1986–2012. London, UK: Joint Nature Conservation Committee; See http://www.jnccdefra.gov.uk/page-3201 [Google Scholar]
- 25.Berrow S, Whooley P, Firth L, Knights A. 2010. Review of inshore cetacean monitoring programme. Final Report of ISCOPE II. National Parks and Wildlife Service. [Google Scholar]
- 26.Baines ME, Evans PGH. 2012. Atlas of the marine mammals of Wales. CCW Monitoring Report No. 68, p. 139, 2nd edn Cardiff, UK: CCW. [Google Scholar]
- 27.Hardy AC, Lucas CR, Henderson GTD, Fraser JR. 1936. The ecological relations between the herring and the plankton investigated with the plankton indicator. I, II, III and IV. J. Mar. Biol. Assoc. UK 21, 147–291. ( 10.1017/S0025315400011267) [DOI] [Google Scholar]
- 28.Beaugrand G, Reid PC, Ibanez F, Lindley JA, Edwards M. 2002. Reorganisation of north Atlantic marine copepod biodiversity and climate. Science 296, 1692–1694. ( 10.1126/science.1071329) [DOI] [PubMed] [Google Scholar]
- 29.Beaugrand G. 2004. The North Sea regime shift: evidence, causes, mechanisms and consequences. Prog. Oceanogr. 60, 245–262. ( 10.1016/j.pocean.2004.02.018) [DOI] [Google Scholar]
- 30.Beaugrand G, Reid PC. 2003. Long-term changes in phytoplankton, zooplankton and salmon related to climate. Glob. Change Biol. 9, 801–817. ( 10.1046/j.1365-2486.2003.00632.x) [DOI] [Google Scholar]
- 31.Beaugrand G, Brander KM, Lindley JA, Souissi S, Reid PC. 2003. Plankton effect on cod recruitment in the North Sea. Nature 426, 661–663. ( 10.1038/nature02164) [DOI] [PubMed] [Google Scholar]
- 32.Kirby RR, Beaugrand G. 2009. Trophic amplification of climate warming. Proc. R. Soc. B 276, 4095–4103. ( 10.1098/rspb.2009.1320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daan N, Gislason H, Pope JG, Rice JC. 2005. Changes in the North Sea fish community: evidence of indirect effects of fishing?. ICES J. Mar. Sci. 62, 177–188. ( 10.1016/j.icesjms.2004.08.020) [DOI] [Google Scholar]
- 34.Armstrong MJ, Gerritsen HD, Allen M, McCurdy WJ, Peel JAD. 2004. Variability in maturity and growth in a heavily exploited stock: cod (Gadus morhua L.) in the Irish Sea. ICES J. Mar. Sci. 61, 98–112. ( 10.1016/j.icesjms.2003.10.005) [DOI] [Google Scholar]
- 35.Burt GJ, Ellis JR, Harley BF, Kupschus S. 2013. The FV Carhelmar beam trawl survey of the western English Channel (1989–2011): history of the survey, data availability and the distribution and relative abundance of fish and commercial shellfish. In Science Series Technical Report, p. 139 CEFAS. [Google Scholar]
- 36.Rogers SI, Ellis JR. 2000. Changes in the demersal fish assemblages of British coastal waters during the 20th century. ICES J. Mar. Sci. 57, 866–881. ( 10.1006/jmsc.2000.0574) [DOI] [Google Scholar]
- 37.Jennings S, Greenstreet S, Hill L, Piet G, Pinnegar J, Warr KJ. 2002. Long-term trends in the trophic structure of the North Sea fish community: evidence from stable-isotope analysis size-spectra and community metrics. Mar. Biol. 141, 1085–1097. ( 10.1007/s00227-002-0905-7) [DOI] [Google Scholar]
- 38.Dulvy NK, Rogers SI, Jennings S, Stelzenmüller V, Dye SR, Skjoldal HR. 2008. Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. J. Appl. Ecol. 45, 1029–1039. ( 10.1111/j.1365-2664.2008.01488.x) [DOI] [Google Scholar]
- 39.Simpson SD, Jennings S, Johnson MP, Blanchard JL, Schön PJ, Sims DW, Genner MJ. 2011. Continental shelf-wide response of a fish assemblage to rapid warming of the sea. Curr. Biol. 21, 1565–1570. ( 10.1016/j.cub.2011.08.016) [DOI] [PubMed] [Google Scholar]
- 40.Russell FS, Southward AJ, Boalch GT, Butler EI. 1971. Changes in biological conditions in the English Channel off Plymouth during the last half century. Nature 234, 468–470. ( 10.1038/234468a0) [DOI] [Google Scholar]
- 41.Hawkins SJ, Southward AJ, Genner MJ. 2003. Detection of environmental change in a marine ecosystem: evidence from the western English Channel. Sci. Total Environ. 310, 245–256. ( 10.1016/S0048-9697(02)00645-9) [DOI] [PubMed] [Google Scholar]
- 42.Genner MJ, Halliday NC, Simpson SD, Southward AJ, Hawkins SJ, Sims DW. 2010. Temperature-driven phenological changes within a marine larval fish assemblage. J. Plankton Res. 32, 699–708. ( 10.1093/plankt/fbp082) [DOI] [Google Scholar]
- 43.Sims DW, Genner MJ, Southward AJ, Hawkins SJ. 2001. Timing of squid migration reflects North Atlantic climate variability. Proc. R. Soc. Lond. B 268, 2607–2611. ( 10.1098/rspb.2001.1847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sims DW, Wearmouth VJ, Genner MJ, Southward AJ, Hawkins SJ. 2004. Low-temperature-driven early spawning migration of a temperate marine fish. J. Anim. Ecol. 73, 333–341. ( 10.1111/j.0021-8790.2004.00810.x) [DOI] [Google Scholar]
- 45.Frid CLJ, Garwood PR, Robinson LA. 2009. Observing change in a North Sea benthic system: a 33 year time series. J. Mar. Syst. 77, 227–236. ( 10.1016/j.jmarsys.2008.01.011) [DOI] [Google Scholar]
- 46.Robinson LA, Frid CLJ. 2008. Historical marine ecology: examining the role of fisheries in changes in North Sea benthos. Ambio 37, 368–371. ( 10.1579/07-A-300.1) [DOI] [PubMed] [Google Scholar]
- 47.Henderson PA, Seaby RM, Soames JR. 2011. Community level response to climate change: the long-term study of the fish and crustacean community of the Bristol Channel. J. Exp. Mar. Biol. Ecol. 400, 78–89. ( 10.1016/j.jembe.2011.02.028) [DOI] [Google Scholar]
- 48.Mieszkowska N, Hawkins SJ, Burrows MT, Kendall MA. 2007. Long-term changes in the geographic distribution and population structures of Osilinus lineatus (Gastropoda: Trochidae) in Britain and Ireland. J. Mar. Biol. Assoc. UK 89, 537–545. ( 10.1017/S0025315407053799) [DOI] [Google Scholar]
- 49.Crisp DJ. 1964. The effects of the severe winter of 1962–63 on marine life in Britain. J. Anim. Ecol. 33, 165–210. ( 10.2307/2355) [DOI] [Google Scholar]
- 50.Frost MT, Leaper R, Mieszkowska N, Moschella P, Murua J, Smyth C, Hawkins SJ. 2004. Recovery of a biodiversity action plan species in northwest England: possible role of climate change, artificial habitat and water quality amelioration. Sabellaria alveolata. Peterborough, UK: English Nature. [Google Scholar]
- 51.Southward AJ. 1991. Forty years of changes in species composition and population density of barnacles on a rocky shore near Plymouth. J. Mar. Biol. Assoc. UK 71, 495–513. ( 10.1017/S002531540005311X) [DOI] [Google Scholar]
- 52.Mieszkowska N, Burrows M, Pannacciulli F, Hawkins SJ. 2013. Multidecadal signals within co-occurring intertidal barnacles Semibalanus balanoides and Chthamalus spp. linked to the Atlantic multidecadal oscillation. J. Mar. Sci. Technol. 133, 70–76. ( 10.1016/j.jmarsys.2012.11.008) [DOI] [Google Scholar]
- 53.Wilson JG. 2001. Long-term studies of bivalves in Dublin Bay Ireland. Porcupine Mar. Nat. Hist. Soc. Newsl. 7, 27–31 [Google Scholar]
- 54.Wilson JG. 2005. The intertidal system: sustainability and long-term indicators of system status. In The intertidal ecosystem (ed. Wilson JG.), pp. 171–189. Dublin, Ireland: Dublin Royal Irish Academy. [Google Scholar]
- 55.Southward AJ. 1963. The distribution of some plankton animals in the English Channel and approaches. III. Theories about long term biological changes, including fish. J. Mar. Biol. Assoc. UK 43, 1–29. ( 10.1017/S0025315400005208) [DOI] [Google Scholar]
- 56.Edwards M, Richardson AJ. 2004. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884. ( 10.1038/nature02808) [DOI] [PubMed] [Google Scholar]
- 57.Cushing DH. 1995. Population production and regulation in the sea: a fisheries perspective Cambridge, UK: Cambridge University Press [Google Scholar]
- 58.Mieszkowska N, Milligan G, Burrows MT, Freckleton R, Spencer M. 2013. Dynamic species distribution models from categorical survey data. J. Anim. Ecol. 82, 1215–1226. ( 10.1111/1365-2656.12100) [DOI] [PubMed] [Google Scholar]
- 59.Spencer M, et al. 2011. Temporal change in UK marine communities: trends or regime shifts?. Mar. Ecol. 32(Suppl. 1), 10 ( 10.1111/j.1439-0485.2010.00422.x) [DOI] [Google Scholar]
- 60.Poloczanska ES, Brown CJ, Sydeman WJ, Kiessling W, Schoeman DS, Moore PJ, Richardson AJ. 2013. Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925. ( 10.1038/nclimate1958) [DOI] [Google Scholar]
- 61.IPCC. 2007. Climate change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, p. 104. [Google Scholar]
- 62.Natural Environment Research Council, Nature Conservancy. 1969. Conservation Policy in shallow seas. (Joint action meeting with the committee for Wales Scottish Committee the Scientific Policy Committee for England). [Google Scholar]
- 63.Nature Conservancy Council, Natural Environment Research Council. 1979. Nature conservation in the marine environment. In Report of the NCC/NERC joint working party on marine wildlife conservation London, UK: London Nature Conservancy Council. [Google Scholar]
- 64.Hiscock K. 1996. Marine Nature Conservation Review: rationale and methods. Peterborough, UK: Joint Nature Conservation Committee. [Google Scholar]
- 65.Duarte CM, Cebrian J, Marba N. 1992. Uncertainty of detecting sea change. Nature 356, 190 ( 10.1038/356190a0) [DOI] [Google Scholar]
- 66.HM Government Northern Ireland. 2010. UK Marine Science Strategy: shaping supporting co-ordinating and enabling the delivery of world class marine science for the UK 2010–2025. HM Government Northern Ireland. [Google Scholar]
- 67.European Commission 2008 Directive 56 of The European Parliament and of The Council of 17th June 2008. Establishing a framework for community action in the field of environmental policy. Marine Strategy Framework Directive, pp. 19–40. [Google Scholar]
- 68.Reid PC, et al. 2010. Charting progress 2 healthy and biological diverse seas feeder report: section 3.3: plankton. In UKMMAS (2010) charting progress 2 healthy and biological diverse seas feeder report (eds Frost M, Hawkridge J.), p. 69 London, UK: Department for Environment Food and Rural Affairs on behalf of UKMMAS. [Google Scholar]
- 69.Probst WN, Kloppmann N, Kraus G. 2013. Indicator-based status assessment of commercial fish species in the North Sea according to the EU Marine strategy framework directive (MSFD). ICES J. Mar. Sci. 70, 694–706. ( 10.1093/icesjms/fst010) [DOI] [Google Scholar]
- 70.Burrows MT, Mieszkowska N, Hawkins SJ. 2014. Development of climate change condition and boulder turning indicators for intertidal rocky habitats. Peterborough, UK: JNCC Report. [Google Scholar]
- 71.Marine Climate Change Impacts Partnership. See http://www.mccip.org.uk/. [Google Scholar]
- 72.Benjamins SJ, Mieszkowska N. 2010. Charting progress 2: UK marine habitats. (ed. Defra ), 209. [Google Scholar]
- 73.Austen M, Malcon S, Frost M, Hattam C, Mangi S, Mieszkowska N, Stenford G. 2010. Marine habitats. In National ecosystem assessment, pp. 459–498. Cambridge, UK: UNEP-WCMC. [Google Scholar]
- 74.MCCIP. 2011. Marine Climate Change Impacts Annual Report Card 2010–2011, p. 12 Lowestoft, UK: MCCIP. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Many of the sustained observations datasets can be accessed via the websites of the responsible institutes or organizations, and many of the Plymouth timeseries from the Western Channel Observatory website (http://www.westernchannelobservatory.org.uk/). Several including the MarClim dataset are held on the National Biodiversity Network Marine Recorder Database and in the DASSH.