Abstract
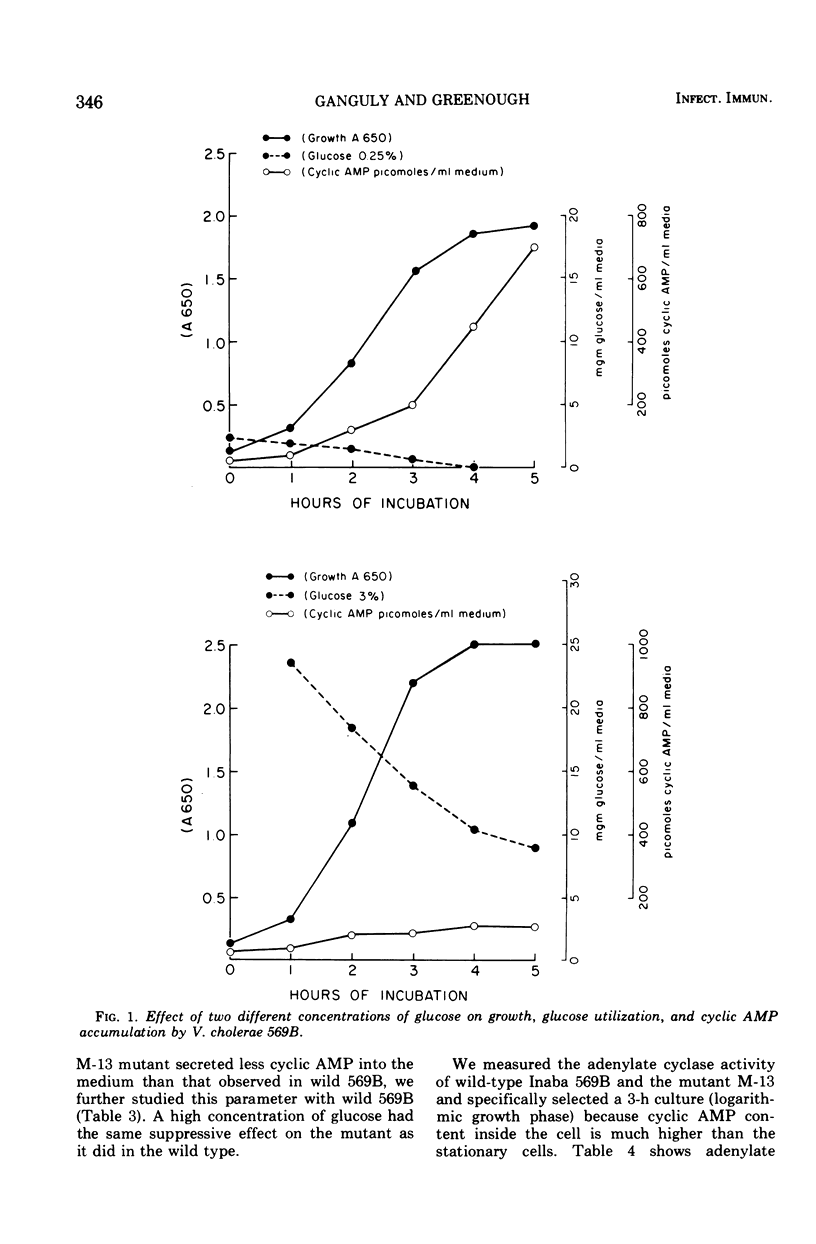
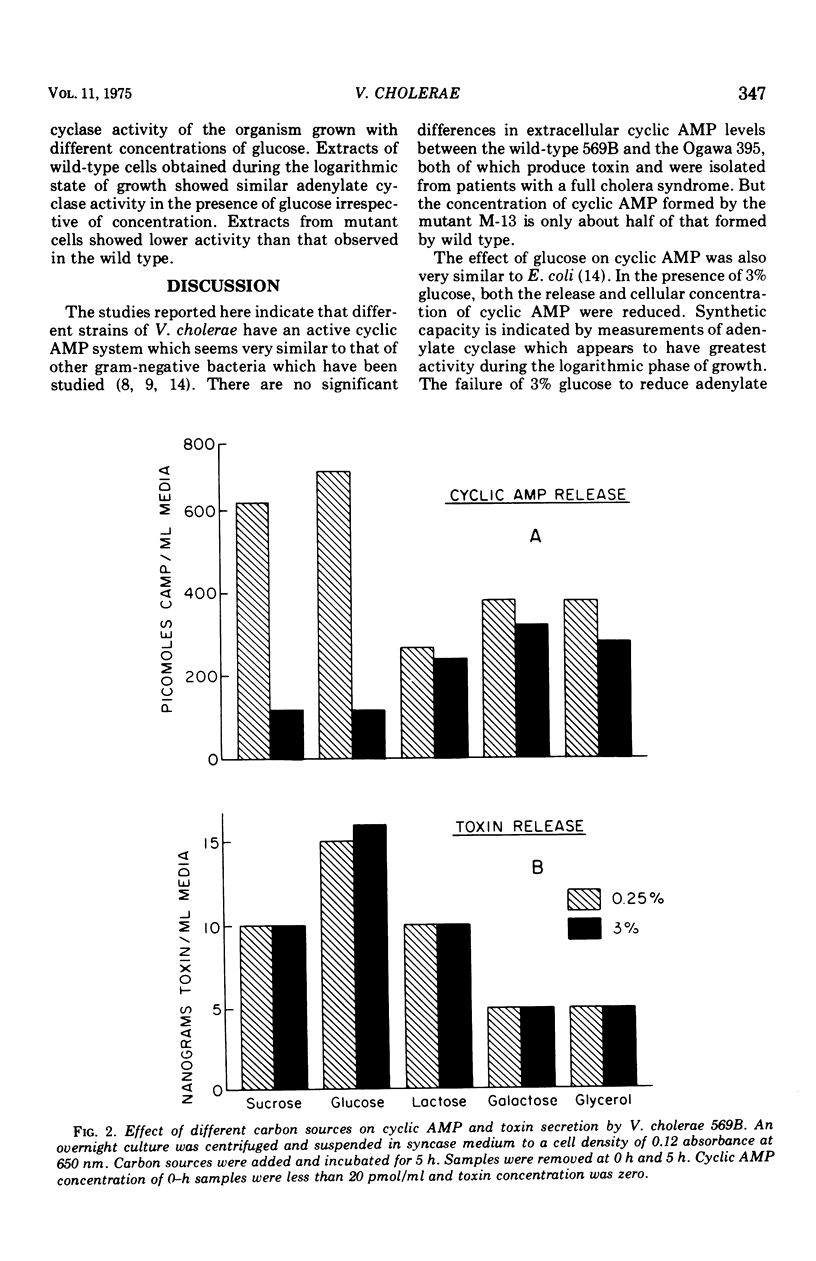
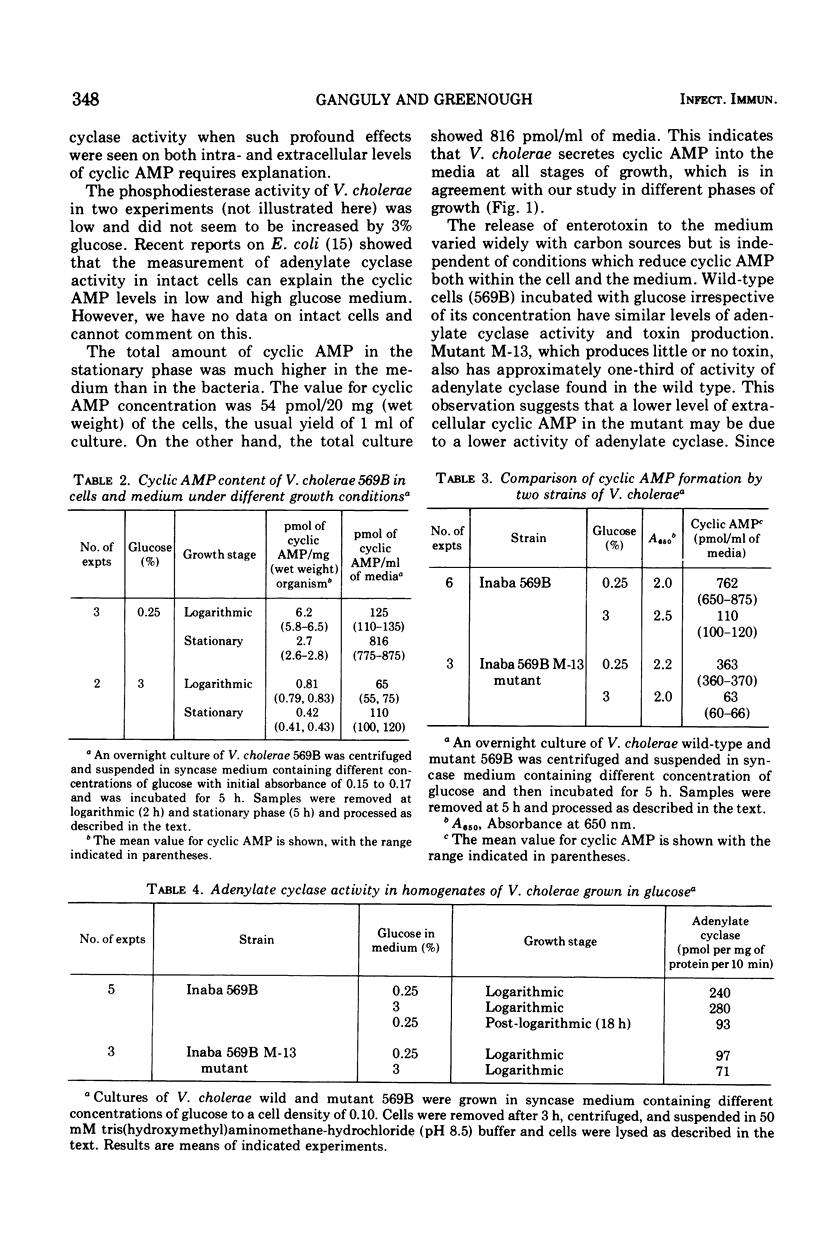
The extracellular concentration of cyclic adenosine 3',5'-monophosphate (AMP) of three different strains of Vibrio cholerae growing in syncase medium were measured. Cyclic AMP secreted by V. cholerae 569B varied widely, with different carbon sources. Mutant 13, which produced little or no toxin, released half the amount of cyclic AMP as the wild type. The release of less cyclic AMP into the medium by mutant 13 may be accounted for by the lower activity of adenylate cyclase observed. High glucose (3%) in the culture medium reduced the concentration of cyclic AMP both in wild type and mutant 13. Reduction of cyclic AMP levels at high concentrations of glucose (3%) occurred without change of adenylate cyclase activity. The release of enterotoxin to the medium varied with carbon sources but was independent of conditions which reduced the cyclic AMP both within the cell and the medium. Neither adenylate cyclase activity nor toxin production was reduced by an increase concentration of glucose in wild-type V. cholerae, whereas cyclic AMP levels were reduced by sixfold. A lower activity of the adenylate cyclase was observed in a mutant of V. cholerae which produced no detectable toxin. Thus, a correlation exists between toxin production and adenylate cyclase activity in V. cholerae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buettner M. J., Spitz E., Rickenberg H. V. Cyclic adenosine 3',5'-monophosphate in Escherichia coli. J Bacteriol. 1973 Jun;114(3):1068–1073. doi: 10.1128/jb.114.3.1068-1073.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan L. T., 3rd, Richardson S. H. Biochemistry of Vibrio cholerae virulence. 3. Nutritional requirements for toxin production and the effects of pH on toxin elaboration in chemically defined media. Infect Immun. 1973 Apr;7(4):567–572. doi: 10.1128/iai.7.4.567-572.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld O. Notes on food, beverages and fomites contaminated with Vibrio cholerae. Bull World Health Organ. 1965;33(5):725–734. [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P., Chulasamaya M., Charunmethee P. Pathogenesis of experimental cholera: biologic ativities of purified procholeragen A. J Immunol. 1966 Mar;96(3):440–449. [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough W. B., 3rd, Pierce N. F., Vaughan M. Titration of cholera enterotoxin and antitoxin in isolated fat cells. J Infect Dis. 1970 May;121(Suppl):111+–111+. doi: 10.1093/infdis/121.supplement.s111. [DOI] [PubMed] [Google Scholar]
- Khandelwal R. L., Hamilton I. R. Purification and properties of adenyl cyclase from Streptococcus salivarius. J Biol Chem. 1971 May 25;246(10):3297–3304. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Mosinger B., Vaughan M. Effects of electrolytes on epinephrine stimulated lipolysis in adipose tissue in vitro. Biochim Biophys Acta. 1967 Dec 5;144(3):556–568. doi: 10.1016/0005-2760(67)90045-8. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Glucose and the metabolism of adenosine 3':5'-cyclic monophosphate in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2794–2798. doi: 10.1073/pnas.68.11.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Measurements of rates of adenosine 3':5'-cyclic monophosphate synthesis in intact Escherichia coli B. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2149–2152. doi: 10.1073/pnas.70.7.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]


