Dear Editor
In recent years the interest in melanoma therapies have shifted focus from the more conventional forms (e.g. – radiation and chemotherapy) to more molecular based strategies, highlighting the need to understand the underlying molecular mechanisms that contribute to melanoma progression. Along these lines, studies examining the aberrant expression of proteins in melanoma have identified Pax3 as an important marker (Medic and Ziman). Used in tumor staging, Pax3 expression is deregulated in a significant proportion of melanomas, particularly in malignant tumors (Scholl et al., 2001), and the abrogation of Pax3 expression in melanoma cells inhibits proliferation and induces apoptosis (He et al., 2010; He et al., 2005). However, the molecular contributions that Pax3 makes to melanoma phenotypes have yet to be determined.
Pax3 is phosphorylated at only three sites in myoblasts (Ser201, Ser205 and Ser209) (Dietz et al., 2011a; Miller et al., 2008); phosphorylation is dynamic and changes throughout myogenesis (Dietz et al., 2011b); and phosphorylation contributes to regulating Pax3 activity (Amstutz et al., 2008). Despite this knowledge, little is known about phosphorylation status of Pax3 in melanoma cells and how these events contribute to known melanoma phenotypes. A recent report demonstrated that GSK3β, the kinase that phosphorylates Pax3 at Ser201 (Dietz et al., 2011a), promotes melanoma cell survival by regulating Pax3 protein levels (Kubic et al., 2012). However, the phosphorylation of Ser201, Ser205, or Ser209 on endogenous Pax3 in melanoma cells and the role that phosphorylation plays to contribute to melanoma phenotypes are not known.
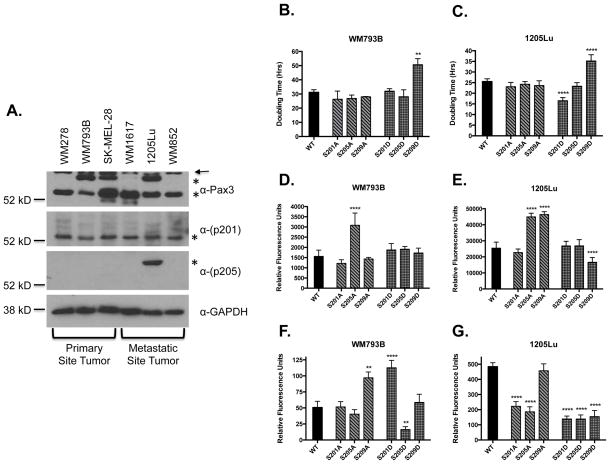
We used total cell extracts from six different melanoma cell lines (Appendix S1) to determine Pax3 expression and the presence of phosphorylation at Ser201 and Ser205. We observed the primary line WM278 expressed only the 56kD form while the remaining two primary lines (WM793B and SK-MEL-28) expressed both the 56kD and the 66kD forms (Appendix S1). We also observed two of the metastatic lines (WM1617 and WM852) express only the 56kD form while 1205Lu expresses both forms (Figure 1A). We observed phosphorylation of the 56kD form at Ser201 in all cell lines. In contrast, we found no phosphorylation at Ser205 in primary tumor melanoma cell lines that contain the 66kD species (WM793B and SK-MEL-28). However, we did detect phosphorylation at Ser205 of the 66kD species of Pax3 in the metastatic 1205Lu (Figure 1A). Since 1205Lu cells are derived from WM793B cells, this result suggests metastatic cells gain phosphorylation at Ser205 relative to the primary cells. The presence of phosphorylation at Ser209 was not detectable in either line (data not shown).
FIGURE 1. Pigment Cell & Melanoma Research Proof.
Analysis of Pax3 phosphorylation in melanoma cell lines. (A) Total extracts were made from primary tumor melanoma cells (WM278, WM793B, and SK-MEL-28) or metastatic tumor cells (WM1617, 1205Lu, and WM852). Endogenous Pax3 (top panels) or endogenous Pax3 phosphorylated at Ser201 or Ser205 (middle panels) was determined by Western blot analysis (Appendix S2). The asterisks indicate the migration of the 56kD and the 66kD forms of Pax3 and the arrow indicates the presence of a commonly seen non-specific band (top panel, data not shown). The presence of GAPDH demonstrates equal protein loading. Proliferation rates (B and C), invasive capacity (D and E), or anchorage independent growth (F and G) were determined on WM793B (B, D, and F) or 1205Lu (C, E, or G) cells stably transduced with FLAG-Pax3 (WT) or the indicated FLAG-Pax3 phospho-mutants as described in Appendix S2. Error bars represent the standard deviation from three independent determinations and P-values were computed using non-parametric one-way ANOVA analyses comparing each Pax3 mutant construct relative to cells expressing wild-type Pax3. (**P = 0.001, ****P < 0.0001)
To examine the effects of Pax3 phosphorylation on proliferation, we used WM793B and 1205Lu cells stably transduced with Pax3 or Pax3 phospho-mutants (Appendix S1) in proliferation assays (Appendix S2). We observed no significant changes in doubling times of WM793B or 1205Lu cells stably expressing the phospho-incompetent mutants Ser201A, Ser205A, or Ser209A or the phospho-mimetic Ser205D. In contrast, we observed significant increases in the doubling time of both lines stably expressing the phospho-mimetic Ser209D mutant (Figure 1B and C). Finally, the stable expression of the phospho-mimetic Ser201D mutant reduced the doubling time of 1205Lu cells from 25 hours to 16 hours, but had no effect on WM793B proliferation rates.
To investigate the effects of Pax3 phosphorylation on invasive potential, we used our stably transduced cells in standard invasion assays (Appendix S2). We observed no significant effect of any of the phospho-mimetic mutants or the phospho-incompetent Ser201A or Ser209A on WM793B invasive potential. However, the expression of the phospho-incompetent Ser205A enhanced WM793B invasion greater than two-fold (Figure 1D). Similarly, the expression of the phospho-mimetic Ser201D or S205D, or the phospho-incompetent Ser201A had no effect on the invasive capacity of the 1205Lu cells. In contrast, expression of the phospho-incompetent Ser205A or Ser209A increased the invasive capacity of 1205Lu cells greater than two-fold. Interestingly, expression of the phospho-mimetic Ser209D decreased the invasive capacity of the 1205Lu cells nearly two-fold (Figure 1E).
We next tested the ability of our stably transduced melanoma cells to exhibit anchorage-independent growth (Appendix S2), considered the benchmark for cellular oncogenic capacity. The phospho-incompetent Ser201A, Ser205A, or the phospho-mimetic Ser209D had no effect on WM793B soft agar formation. However, the expression of the phospho-incompetent Ser209A or the phospho-mimetic Ser201D increased WM793B colony formation greater than two-fold while the phospho-mimetic Ser205D resulted in a nearly 3-fold decrease in the transformation capacity of these same cells (Figure1F and S2A). In contrast, expression of wild-type Pax3 resulted in the vigorous colony formation for the 1205Lu cells. This ability was lost in the presence of the phospho-incompetent Ser201A and Ser205A or all phospho-mimetic mutants (Ser201D, Ser205D, or Ser209D). Only upon the expression of the phospho-incompetent Ser209A do these cells retain the enhanced anchorage-independent growth (Figure 1G and Figure S2B).
Our results demonstrate for the first time that alternative forms of Pax3 exist between melanoma cell lines and that these different forms exhibit changes in phosphorylation at Ser205 between primary and metastatic melanoma cells (Figure 1A). This result suggests that phosphorylation at Ser205 may either 1) contribute to tumor metastasis to promote the detachment of cells from the primary site, or 2) occurs as a consequence of metastasis, with phosphorylation being gained at the metastatic site. Regardless, these results suggest that the examination of the forms and phosphorylation status, minimally at Ser205, of Pax3 may potentially be used to further stratify melanoma tumors for diagnosis, prognosis, and potential treatment.
Our results also demonstrate how phosphorylation of Pax3 affects melanoma phenotypes. We demonstrate that the absence of phosphorylation at Ser205 promotes the invasive capacity of both primary and metastatic cells (Figure 1D and E). Further, we found that phosphorylation at Ser201 enhances the proliferative capacity of the metastatic cell line (Figure 1C). In addition, our data supports the idea that two independent forms of phosphorylated Pax3 contribute to anchorage independent growth: 1) phosphorylation of Pax3 at Ser201 and Ser205 are both present in 1205Lu cells (Figure 1A); 2) these phosphorylation events do not occur on the same molecule of Pax3 (phosphorylation at Ser205 occurs on the 66kD while phosphorylation at Ser201 occurs on the 56kD form); and 3) each of these events alone are incapable of enhancing the transforming ability of 1205Lu cells (Figure 1F and G). Further our results demonstrating efficient colony formation in cells expressing Pax3 capable of having both Ser201 and Ser205 individually phosphorylated (wild-type or Ser209A) support this idea. In contrast, our results suggest phosphorylation of Pax3 at Ser209 would inhibit tumor formation. We found that Pax3 “permanently” phosphorylated at Ser209 inhibits the proliferative capacity of both primary and metastatic melanoma cells (Figure 1B and C), which would impede proliferation and provide a significant growth disadvantage to melanoma cells. Taken together, our results support the idea that phosphorylation of Pax3 affects melanoma phenotypes and therefore may be a viable biological target for the development of therapies (Appendix S1).
Supplementary Material
Methods
Supplemental results and discussion
Figure S1 – Ectopic expression of Pax3 phosphomutants
Figure S2 – Phase contrast images of anchorage independent growth
Acknowledgments
Funding for this project is from the National Cancer Institute grant R01CA138656.
References
- Amstutz R, Wachtel M, Troxler H, Kleinert P, Ebauer M, Haneke T, Oehler-Janne C, Fabbro D, Niggli FK, Schafer BW. Phosphorylation regulates transcriptional activity of PAX3/FKHR and reveals novel therapeutic possibilities. Cancer Res. 2008;68:3767–76. doi: 10.1158/0008-5472.CAN-07-2447. [DOI] [PubMed] [Google Scholar]
- Dietz KN, Miller PJ, Iyengar AS, Loupe JM, Hollenbach AD. Identification of serines 201 and 209 as sites of Pax3 phosphorylation and the altered phosphorylation status of Pax3-FOXO1 during early myogenic differentiation. Int J Biochem Cell Biol. 2011a;43:936–45. doi: 10.1016/j.biocel.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KN, Miller PJ, Iyengar AS, Loupe JM, Hollenbach AD. Identification of serines 201 and 209 as sites of Pax3 phosphorylation and the altered phosphorylation status of Pax3-FOXO1 during early myogenic differentiation. Int J Biochem Cell Biol. 2011b doi: 10.1016/j.biocel.2011.03.010. (Revision submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Li CG, Slobbe L, Glover A, Marshall E, Baguley BC, Eccles MR. PAX3 knockdown in metastatic melanoma cell lines does not reduce MITF expression. Melanoma Res. 2010 doi: 10.1097/CMR.0b013e328341c7e0. [DOI] [PubMed] [Google Scholar]
- He SJ, Stevens G, Braithwaite AW, Eccles MR. Transfection of melanoma cells with antisense PAX3 oligonucleotides additively complements cisplatin-induced cytotoxicity. Mol Cancer Ther. 2005;4:996–1003. doi: 10.1158/1535-7163.MCT-04-0252. [DOI] [PubMed] [Google Scholar]
- Kubic JD, Mascarenhas JB, Iizuka T, Wolfgeher D, Lang D. GSK-3 promotes cell survival, growth, and PAX3 levels in human melanoma cells. Mol Cancer Res. 2012;10:1065–76. doi: 10.1158/1541-7786.MCR-11-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medic S, Ziman M. PAX3 expression in normal skin melanocytes and melanocytic lesions (naevi and melanomas) PLoS ONE. 5:e9977. doi: 10.1371/journal.pone.0009977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PJ, Dietz KN, Hollenbach AD. Identification of serine 205 as a site of phosphorylation on Pax3 in proliferating but not differentiating primary myoblasts. Protein Sci. 2008;17:1979–86. doi: 10.1110/ps.035956.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl FA, Kamarashev J, Murmann OV, Geertsen R, Dummer R, Schafer BW. PAX3 is expressed in human melanomas and contributes to tumor cell survival. Cancer Res. 2001;61:823–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods
Supplemental results and discussion
Figure S1 – Ectopic expression of Pax3 phosphomutants
Figure S2 – Phase contrast images of anchorage independent growth