Summary
The function of EspI, a 70-kDa protein in Mycobacterium tuberculosis, has remained unclear. Although EspI is encoded by a gene within the esx-1 locus, in this study we clarify previous conflicting results and show that EspI is not essential for ESX-1-mediated secretion or virulence in M. tuberculosis. We also provide evidence that reduction of cellular ATP levels in wild-type M. tuberculosis using the drug bedaquiline completely blocks ESX-1-mediated secretion. Remarkably, M. tuberculosis lacking EspI fails to exhibit this phenotype. Furthermore, mutagenesis of a highly conserved ATP-binding motif in EspI renders M. tuberculosis incapable of shutting down ESX-1-mediated secretion during ATP depletion. Collectively these results show that M. tuberculosis EspI negatively regulates the ESX-1 secretion system in response to low cellular ATP levels and this function requires the ATP-binding motif. In light of our results the potential significance of EspI in ESX-1 function during latent tuberculosis infection and reactivation is also discussed.
Keywords: Tuberculosis, ESX-1, secretion, EspI, ATP-binding motif
Introduction
The type-7 ESX-1 protein secretion system of Mycobacterium tuberculosis is encoded by 16 genes in the esx-1 locus and the unlinked espACD locus (Bitter et al., 2009). The majority of these genes are highly conserved in members of the M. tuberculosis complex as well as in other pathogenic mycobacteria such as Mycobacterium leprae, the agent of leprosy, and Mycobacterium marinum, a pathogen of ectothermic vertebrates (Bentley et al., 2012, Cole et al., 1998, Cole et al., 2001, Garnier et al., 2003, Stinear et al., 2008). This system is essential for the secretion of EsxA (ESAT-6), EsxB (CFP-10), EspA, EspB and EspC proteins, and plays a decisive role in the virulence of M. tuberculosis (Bitter et al., 2009, Simeone et al., 2009). Deletion of the entire esx-1 locus or of genes contained therein abrogates ESX-1-mediated secretion and strongly attenuates M. tuberculosis (Brodin et al., 2006, Guinn et al., 2004, Hsu et al., 2003, Lewis et al., 2003, Pym et al., 2002, Stanley et al., 2003).
EspI is a 70-kDa alanine- and proline-rich protein of unknown function and it is encoded by the espI gene (rv3876) located in the esx-1 locus. Bioinformatic analysis of the EspI amino acid sequence revealed an FlhG domain (Das et al., 2011). The FlhG protein is needed to negatively regulate the number of polar flagella in Vibrio cholerae, as such it was proposed that EspI might play a role in regulating the ESX-1 system (Das et al., 2011). An espI transposon insertion mutant of M. tuberculosis H37Rv was found to be defective in ESX-1 mediated secretion and attenuated (Guinn et al., 2004). Complementation of this mutant with espI alone reportedly restored the wild-type phenotype (Guinn et al., 2004). However, in a second study, an in-frame deletion mutant of espI was found to be as secretion-competent and virulent as the wild-type control strain suggesting that EspI is not an essential component of the ESX-1 secretion apparatus (Brodin et al., 2006). In light of these contradictory findings, the role of EspI in ESX-1-mediated secretion and virulence has remained puzzling and unclear.
In this study, we document a novel role of EspI in ESX-1 function. While not critical for ESX-1-mediated secretion or cytotoxicity, we provide evidence that EspI is involved in repressing ESX-1 activity under conditions of lowered cellular ATP levels in M. tuberculosis. We have also defined a conserved ATP-binding motif in EspI to be pivotal for this role.
Results
M. tuberculosis EspI is not essential for ESX-1-mediated secretion or cytotoxicity
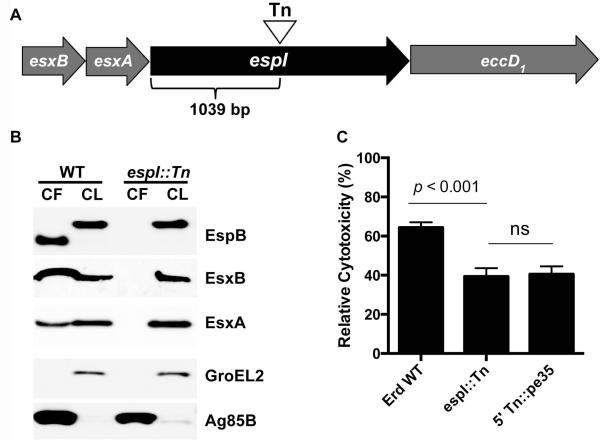
The espI (rv3876) gene is 2001 bp in length and located immediately upstream of eccD1 (rv3877) in the esx-1 locus (Fig. 1A). In this study, an M. tuberculosis Erdman mutant with a transposon insertion at nucleotide position 1039 of the espI gene was used for detailed analysis (Fig. 1A). The in vitro growth rate of the espI::Tn mutant strain was found to be indistinguishable from that of wild-type M. tuberculosis (data not shown). Unlike wild-type M. tuberculosis however, the secretion of EsxA, EsxB and the mature form of EspB was deficient in the espI::Tn mutant strain (Fig. 1B). By infecting and measuring its capacity to kill THP-1 macrophage cells, the espI::Tn strain was also found to be less cytotoxic than wild-type M. tuberculosis but as attenuated as the 5′ Tn::pe35 strain (Fig. 1C), shown previously to be deficient in ESX-1-mediated secretion and virulence (Chen et al., 2013). These results indicate that the espI::Tn mutant strain has a non-functional ESX-1 secretion system and is attenuated in macrophage cytotoxicity.
Fig. 1. Characterization of an espI::Tn mutant.
(A) Schematic representation of the position of the transposon insertion in the espI::Tn mutant. (B) Immunoblots of cell lysates (CL) at 5 μg/well and culture filtrates (CF) at 10 μg/well of wild-type M. tuberculosis Erdman (WT) and espI::Tn grown in Sauton’s medium without Tween-80 for 5 days. Antibodies used are indicated. (C) Cytotoxicity assay in THP-1 cells infected with wild-type M. tuberculosis Erdman (Erd WT), espI::Tn mutant and 5′ Tn::pe35 at MOI of 5. Data represent the means and standard deviation of at least 4 independent experiments. The y-axis indicates cytotoxicity values relative to the uninfected control. Significance in difference was calculated using Student’s t-test.
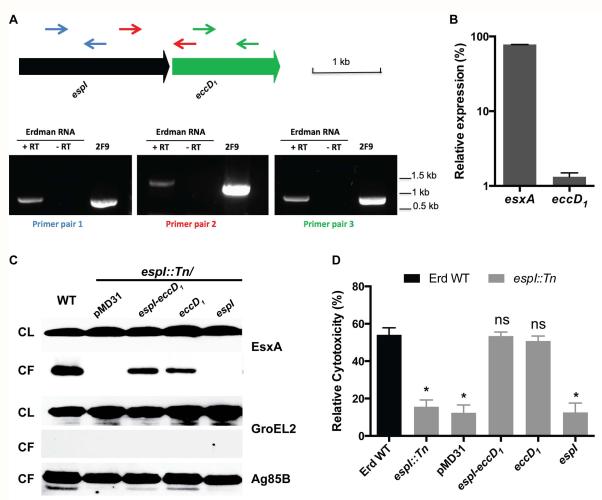
As espI is located directly upstream of eccD1, we examined if the transposon insertion in espI might be causing a polar effect on the transcription of eccD1, which is essential for ESX-1 secretion (Brodin et al., 2006, Pym et al., 2002). To this end, reverse transcriptase (RT)-PCR analysis of total RNA isolated from wild-type M. tuberculosis was performed. An RTPCR product spanning the intergenic region of espI and eccD1 was detected suggesting that they are co-transcribed (Fig. 2A). To further corroborate these results, eccD1 transcript levels in wild-type and espI::Tn strains were measured by quantitative real time-PCR. The mutant strain produced almost 100-fold less eccD1 mRNA compared to the wild-type, while esxA mRNA levels remained similar (Fig. 2B). These results show transposon insertion in the espI gene affects the transcription of eccD1 and likely accounts for the lack of ESX-1-mediated secretion and THP-1 cytotoxicity observed for the espI::Tn mutant strain.
Fig. 2. Complementation of the espI::Tn mutant.
(A) RT-PCR analysis of espI and eccD1 cotranscription. Upper panel shows genetic arrangement of espI and eccD1, and primer annealing sites. Blue arrows represent the internal primers for espI, green arrows the internal primers for eccD1 and red arrows the primers spanning the two genes. Lower panel shows RT-PCR analysis of total RNA from wild-type M. tuberculosis Erdman. 2F9 cosmid was used as a positive PCR control. - RT, no reverse transcriptase. (B) Quantitative RT-PCR analysis of mRNA levels of esxA and eccD1. Relative expression levels were calculated using the ΔΔCt method, normalizing transcript levels to sigA signals. Data are shown as percentage relative to wild-type M. tuberculosis. (C) Immunoblots of cell lysates (CL) at 5 μg/well and culture filtrates (CF) at 10 μg/well of wild-type M. tuberculosis Erdman (WT) and espI::Tn transformed with indicated plasmids grown in Sauton’s medium without Tween-80 for 5 days. Antibodies used are indicated. (D) Cytotoxicity assay in THP-1 cells infected with wild-type M. tuberculosis Erdman (Erd WT) (black bar) and espI::Tn (grey bars) transformed with indicated plasmids at MOI of 5. Data represent the means and standard deviation of at least 4 independent experiments. The y-axis indicates cytotoxicity values relative to the uninfected control. Significance in difference compared to wild-type M. tuberculosis was calculated using Student’s t-test. *, p < 0.0001; ns, no significant difference.
To verify that the lack of ESX-1 function in the espI::Tn mutant strain resulted from polar effects on eccD1 transcription, three different plasmids bearing espI or eccD1 only or both genes - pMDespI, pMDeccD1 and pMDespI-eccD1, all under the control of the espI promoter, were constructed. After transformation into the espI::Tn strain, levels of espI and eccD1 transcripts were measured using quantitative real time-PCR. All three transformants were found to produce espI and/or eccD1 mRNA effectively according to the plasmid constructs they harboured (Fig. S1). ESX-1-mediated secretion of these strains was then analyzed. Equivalent amounts of EsxA protein were detected in the culture filtrates of wild-type M. tuberculosis, espI::Tn strains transformed with pMDespI-eccD1 and pMDeccD1, but not pMDespI or empty pMD31 vector (Fig. 2C). The cytotoxicity of these strains was also measured using the THP-1 macrophage infection system described above. In contrast to espI::Tn strains with and without pMD31, espI::Tn transformed with pMDespI-eccD1 and pMDeccD1 were as cytotoxic to THP-1 macrophages as wild-type M. tuberculosis (Fig. 2D). However, the espI::Tn strain transformed with pMDespI remained attenuated (Fig. 2D). These results confirm espI and eccD1 are co-transcribed and indicate that provision of espI and eccD1 or eccD1 but not espI alone to the espI::Tn mutant strain restores ESX-1-mediated secretion and THP-1 cytotoxicity.
ESX-1-mediated secretion is blocked when cellular ATP levels in M. tuberculosis is reduced
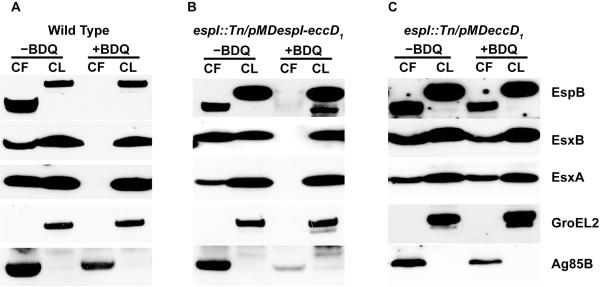
Three ESX-1 core component proteins, namely EccA1, EccCa1 and EccCb1, contain putative ATPase domains and have been proposed to power the multimerization and translocation of ESX-1 substrates (Stoop et al., 2012, Das et al., 2011). We sought to address whether perturbations in ATP synthesis and lowered cellular ATP levels might impact ESX-1 function. For this purpose, we assessed the effect of treatment with bedaquiline (BDQ), a drug that specifically targets the c subunit of mycobacterial ATP synthase and inhibits ATP synthesis, on ESX-1-mediated secretion by wild-type M. tuberculosis (Andries et al., 2005, Koul et al., 2007). BDQ treatment for 8 hours at 0.015, 0.03 and 0.06 μg/ml decreased cellular ATP levels in M. tuberculosis by 0%, 43% and 71%, respectively, relative to the untreated control (Fig. S2). Treatment of M. tuberculosis with BDQ at 0.03 μg/ml, which is half the MIC (Andries et al., 2005), completely blocked the secretion of EsxA, EsxB and EspB, while Ag85B secretion was partially affected compared to that of untreated cells (Fig. 3A). The effect of two cell-wall targeting antimycobacterial compounds, ethambutol (EMB) and isoniazid (INH) at 5 and 0.25 μg/ml respectively, on ESX-1-mediated secretion were also tested. EMB and INH at these concentrations had no effect on EsxA secretion despite being able to inhibit growth of wild-type M. tuberculosis to the same extent as BDQ at 0.03 μg/ml in vitro (Fig. S3). This suggests that blockage of ESX-1-mediated secretion by BDQ at 0.03 μg/ml is not due to the impaired growth of bacterial cells. Thus ESX-1 secretion is shut down when the ATP level in M. tuberculosis is reduced.
Fig. 3. Effect of BDQ treatment on ESX-1 secretion.
Immunoblots of cell lysates (CL) at 5 μg/well and culture filtrates (CF) at 10 μg/well of (A) wild-type M. tuberculosis Erdman strain, (B) espI::Tn transformed with pMDespI-eccD1, and (C) espI::Tn transformed with pMDeccD1 grown in Sauton’s medium without Tween-80 and with or without addition of BDQ (0.03 μg/ml) for 5 days. Antibodies used are indicated.
EspI is involved in blocking ESX-1 secretion during lowered cellular ATP levels in M. tuberculosis
Since EspI was predicted to negatively regulate ESX-1 function (Das et al., 2011), we reasoned that it might play a role in the blockage of ESX-1-mediated secretion under conditions of low cellular ATP. To test this, the impact of BDQ on ESX-1-mediated secretion in espI::Tn strains harbouring pMDespI-eccD1 and pMDeccD1 was assessed. When treated with BDQ at 0.03 μg/ml, the espI::Tn mutant strain transformed with pMDespI-eccD1 stopped secreting EsxA, EsxB and EspB much like wild-type M. tuberculosis (Fig. 3B). In stark contrast, the espI::Tn strain transformed with pMDeccD1 continued to secrete EsxA, EsxB and EspB, even in the presence of BDQ (Fig. 3C). These results indicate that EspI is involved in the repression of ESX-1-mediated secretion under conditions of low cellular ATP in M. tuberculosis.
The ATP-binding motif of EspI is required for blocking ESX-1 secretion during lowered cellular ATP levels in M. tuberculosis
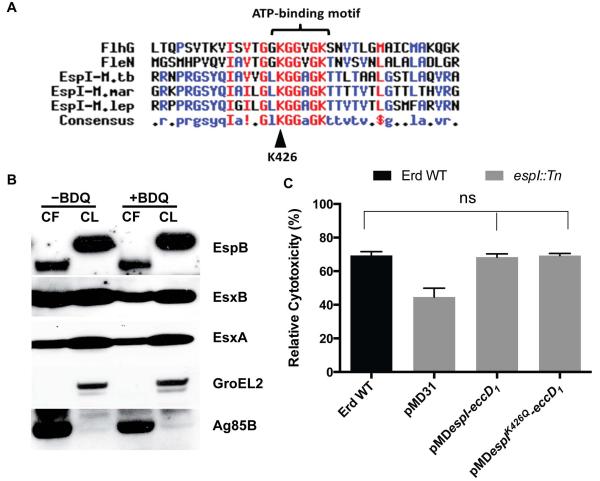
EspI was shown to contain an FlhG domain (Das et al., 2011). FlhG and FleN (FlhG-homolog) proteins in Vibrio cholerae and Pseudomonas aeruginosa respectively, regulate the number of flagella synthesized at the bacterial cell poles (Dasgupta & Ramphal, 2001, Correa et al., 2005). Furthermore, a lysine residue in the conserved ATP-binding motif of P. aeruginosa FleN was shown to be crucial for its function (Dasgupta & Ramphal, 2001). Amino acid sequence analysis revealed that like FlhG and FleN, mycobacterial EspI proteins including those of M. tuberculosis, M. leprae and M. marinum contain a highly conserved ATP-binding motif (Fig. 4A). To determine if the ATP-binding motif might be crucial for EspI involvement in blocking ESX-1-mediated secretion, a conserved lysine residue 426 (K426) in this motif was replaced with glutamine by site-directed mutagenesis of pMDespI-eccD1, thus generating pMDespIK426Q-eccD1 (Fig. 4A). An espI::Tn strain transformed with pMDespIK426Q-eccD1 was then treated with BDQ at 0.03 μg/ml. In the absence of the drug, the EspIK426Q-expressing strain (espI::Tn+ pMDespIK426Q-eccD1), like the fully complemented EspIWT-expressing strain (espI::Tn+pMDespI-eccD1), secreted ESX-1 substrates normally. In the presence of BDQ however, the EspIK426Q-expressing strain unlike the EspIWT-expressing strain did not block ESX-1-mediated secretion and abundant amounts of EsxA, EsxB and EspB were found in the culture filtrate (Fig. 4B). The cytotoxicity of the EspIK426Q-expressing strain was also assessed and it was found to kill THP-1 macrophages to the same extent as the wild-type and fully complemented strains (Fig. 4C). These results confirm that EspI is required to repress ESX-1-mediated secretion under low ATP conditions and show that its ATP-binding site is essential for this function.
Fig. 4. Impact of BDQ treatment on ESX-1-mediated secretion by the EspIK426Q-expressing strain.
(A) Multiple protein sequence alignment of amino acid sequences of V. cholerae O1 FlhG (residue 12 to 48), P. aeruginosa PAO1 FleN (residue 4 to 40), M. tuberculosis EspI (residue 410 to 446), M. marinum EspI (residue 623 to 659) and M. leprae EspI (residue 330 to 366). The lysine residue, K426, mutagenized and characterized in this study is indicated by a black arrow. (B) Immunoblots of cell lysates (CL) at 5 μg/well and culture filtrates (CF) at 10 μg/well of espI::Tn transformed with pMDespIK426Q-eccD1 grown in Sauton’s medium without Tween-80 and treated with BDQ (0.03 μg/ml) for 5 days. (C) Cytotoxicity assay in THP-1 cells infected with wild-type M. tuberculosis Erdman (Erd WT) (black bar), espI::Tn (grey bars) transformed with indicated plasmids at MOI of 5. Data represent the means and standard deviation of at least 4 independent experiments. The y-axis indicates cytotoxicity values relative to the uninfected control. Significance in difference was calculated using Student’s t-test. ns, no significant difference.
Discussion
Taking a genetic approach, we have confirmed in this study that EspI is not essential for the M. tuberculosis ESX-1 secretion system. However, we have discovered that EspI plays a novel role in negatively regulating ESX-1-mediated secretion under conditions where cellular ATP levels are depleted. Furthermore, we show that a conserved ATP-binding motif in the protein is required for this function.
Our data indicate that insertion of a transposon in espI causes a polar effect on the transcription of the downstream eccD1 gene; consequently, the espI::Tn mutant strain should be regarded as a double knock-out of espI and eccD1. The results of our complementation experiments confirm that EspI is not required for ESX-1-mediated secretion or THP-1 cytotoxicity and are consistent with the findings of Brodin and colleagues who had shown that an in-frame deletion of espI had no effect of EsxA secretion or on virulence (Brodin et al., 2006).
Little is known regarding the impact of ATP availability on ESX-1-mediated secretion and virulence. Given that EccA1, EccCa1 and EccCb1 contain putative ATPase domains and have been proposed to power ESX-1-mediated secretion (Stoop et al., 2012, Das et al., 2011) we surmised that an ATP deficit in M. tuberculosis might impact the secretion system. Indeed, treatment with sub-lethal concentrations of BDQ, a potent inhibitor of mycobacterial ATP synthesis, and the consequent reduction of cellular ATP levels in M. tuberculosis completely blocked ESX-1-mediated secretion in vitro.
EspI was suggested to negatively regulate ESX-1 function because it contains an FlhG domain (Das et al., 2011). This stems from the fact that FlhG and FleN (FlhG homolog) proteins are required by V. cholerae and P. aeruginosa, respectively, to regulate the number of flagella formed at the poles (Dasgupta & Ramphal, 2001, Correa et al., 2005). FleN-null and FlhG-null mutants of P. aeruginosa and V. cholerae respectively are hyper-flagellated. Conversely, cells overexpressing FleN and FlhG lack flagella (Dasgupta & Ramphal, 2001, Correa et al., 2005). Polar flagellar assembly in P. aeruginosa and V. cholerae is a tightly regulated, spatio-temporally coordinated process of multi-protein synthesis and translocation of proteins from the cytoplasm to outside the bacterial cell (Kazmierczak & Hendrixson, 2013). It has become evident that FlhG (and FleN) through a variety of different mechanisms play pivotal roles in this complex process (Kazmierczak & Hendrixson, 2013).
Although the flagellar assembly machinery and the ESX-1 secretion system may appear to share mechanistic similarities, closer analysis reveals that FlhG/FleN and EspI are quite different. For instance, V. cholerae FlhG is 291 amino acids in length while M. tuberculosis EspI comprises 666 amino acids. Furthermore, the two proteins only share 23.9% sequence identity and likely function differently. This is consistent with our observations that EspI deficiency does not result in the hyper-secretion of ESX-1 substrates (or affect ESX-1 secretion at all) when the tubercle bacillus is ATP-replete. Nevertheless, our intriguing demonstration that BDQ blocks ESX-1-mediated secretion prompted us to investigate whether EspI might be involved in this phenomenon and led us to serendipitously find that M. tuberculosis lacking EspI (espI::Tn+pMDeccD1) is rendered incapable of shutting down ESX-1-mediated secretion in the presence of BDQ. In addition to M. tuberculosis, EspI orthologues are also present in other pathogenic mycobacteria with ESX-1 systems such as M. bovis, M. africanum, M. canetti, M. leprae, M. kansasii and M. marinum. In M. smegmatis, a nonpathogenic species which appears to possess a functional esx-1 locus, the espI orthologue is 51% identical to the M. tuberculosis counterpart and is missing 577 bp encoding the N-terminal portion of M. tuberculosis EspI (Converse and Cox. JBac.2005vol.187p.1238). Furthermore, a conserved ATP-binding motif located in the amino-terminus of P. aeruginosa FleN appears to be crucial for its function although the precise mechanism remains unknown (Dasgupta & Ramphal, 2001). A similar ATP-binding motif was identified at the carboxy-terminus of EspI from M. tuberculosis and mycobacteria described above. The high degree of conservation of this motif among mycobacterial EspI proteins suggested it was of functional importance. Indeed, mutagenesis of a conserved K426 residue in the ATP-binding motif rendered M. tuberculosis incapable of repressing ESX-1 mediated secretion upon BDQ treatment and the consequent reduction in cellular ATP levels.
The precise molecular mechanism underlying EspI function and the role of the ATP-binding motif remain unknown and warrant further investigation. We hypothesize that a decline in ATP concentration within the tubercle bacillus may be sensed through the ATP-binding motif and confers a specific molecular conformation to EspI. This change in conformation could in turn trigger the transcriptional repression of core esx-1 genes required for ESX-1 secretion. Alternatively, reduced protein synthesis and/or destabilization of core ESX-1 components might be another pathway whereby EspI could exert its secretion-repressing effect. A change in EspI conformation could also enable the protein to physically obstruct ESX-1-mediated secretion at the M. tuberculosis cell membrane via protein-protein interactions. Indeed, three independent proteomic studies have found EspI to be particularly enriched in the cell membrane fraction of M. tuberculosis cells (Mawuenyega et al., 2005, Malen et al., 2010, de Souza et al., 2011).
Studies thus far have largely implicated transcriptional regulators, such as PhoP (Walters et al., 2006, Gonzalo-Asensio et al., 2008, Frigui et al., 2008), EspR (Blasco et al., 2012, Raghavan et al., 2008) and MprAB (Pang et al., 2013) in orchestrating ESX-1 gene expression. Here, EspI is shown for the first time to exert an additional layer of control which is dependent on cellular ATP levels in M. tuberculosis. Our data may therefore provide clues with respect to ESX-1 function during the chronic phase and even during latent M. tuberculosis infection. It is widely accepted that within granulomas and possibly during latent infection, ATP synthesis in non-replicating and dormant M. tuberculosis is significantly reduced (Gengenbacher & Kaufmann, 2012, Rittershaus et al., 2013). However, little is known about ESX-1 function during this phase of M. tuberculosis infection. Repression of ESX-1-mediated secretion involving EspI during this stage might be an important way not only to maintain appropriate ATP homeostasis but also to balance the virulence-promoting function of ESX-1 (Pym et al., 2002) and the T-cell stimulating properties of ESX-1 substrates (Pym et al., 2003). As such, the observation that EspI in M. tuberculosis is detected 90 days but not 30 days post-infection in guinea pigs raises the intriguing possibility that EspI may be more important during the chronic and later phases than in the acute phase of infection (Kruh et al., 2010). While we did not observe any impact of EspI deficiency in M. tuberculosis on THP-1 cytotoxicity, the short time-frame of our ex vivo experiments precludes us from ruling out possible effects on virulence during long-term chronic infection. For instance, decreased virulence during long-term in vivo infection might occur if EspI-deficient M. tuberculosis were unable to shut down ESX-1-mediated secretion, avoid presentation of the major immunogenic ESX-1 substrates and, as a consequence, elicit a more robust host immune response against the bacillus. In this context, manipulation of EspI in recombinant BCG strains complemented with the esx-1 locus could yield more efficacious TB vaccines.
In conclusion, this study has increased our understanding of the regulation of ESX-1-dependent secretion. Better mechanistic insight into how EspI functions might reveal new modes of combating M. tuberculosis infection. For instance, small-molecules constitutively activating the ESX-1 inhibitory function of EspI, even under ATP-replete conditions, would make ideal anti-virulence drugs. In this respect, BDQ appears to act indirectly as an anti-virulence drug by lowering ATP-levels and thus ablating ESX-1 activity. Since the latter is required for reactivation of latent TB, it is conceivable that the highly pronounced bactericidal effect of BDQ on non-replicating M. tuberculosis seen in vivo may stem in part from inhibition of the ESX-1 system (Zhang et al., 2012)
Experimental Procedures
Enzymes and reagents
Restriction and DNA modification enzymes were purchased from New England Biolabs (Ipswich, MA, USA). High fidelity Pfu polymerase was purchased from Promega (Madison, WI, USA). Cosmid 2F9 (Pym et al., 2002) containing the M. tuberculosis H37Rv esx-1 genomic region was used as template for PCR cloning. All other chemicals and reagents used were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Bacterial strains and growth conditions
M. tuberculosis Erdman was grown in 7H9 broth (supplemented with 0.2% glycerol, 10% ADC and 0.05% Tween-80) or on 7H11 agar (supplemented with 0.5% glycerol, 10% OADC). M. tuberculosis Erdman Tn5370 transposon insertion mutants espI::Tn and 5′ Tn::pe35 were generated as described elsewhere (Dhar & McKinney, 2010) and grown in the presence of hygromycin (50 μg/ml). Escherichia coli TOP10TM (Invitrogen, Carlsbad, CA, USA) used for routine cloning was grown on Luria-Bertani agar or broth. When needed, kanamycin was used at a final concentration of 25 μg/ml for M. tuberculosis and at 50 μg/ml for E. coli.
Quantitative RT-PCR
Total mRNA was extracted from exponentially growing M. tuberculosis cells using TRIzol (Invitrogen, Carlsbad, CA). Purified mRNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI) according to the manufacturer’s instructions. Treated mRNA was checked for complete removal of contaminating genomic DNA by PCR before proceeding to the reverse transcription step. cDNA from mRNA was generated using the Superscript III first-strand synthesis system (Invitrogen, Carlsbad, CA) and random hexamers. Three different primer pairs were used to amplify internal fragments of espI and eccD1, and the portions encompassing the coding sequence and intergenic regions between the two genes from the cDNA generated as described above. To measure transcript levels of espI and eccD1, cDNA corresponding to 10 ng of input RNA was used in each RT-PCR reaction using 200 nM each of espI, eccD1, esxA and sigA specific primer pairs and SYBR-Green master mix (Applied Biosystems). Relative mRNA levels were calculated using the ΔΔCt method, normalizing transcripts levels to sigA signals. Data are shown as percentage relative to wild-type M. tuberculosis. All primer sequences are available upon request.
Vector construction and complementation
The espI-eccD1 gene cluster including 147 bp upstream of the espI start codon was PCR-amplified from M. tuberculosis H37Rv cosmid 2F9 (Pym et al., 2002) with primers that added 5′ and 3′ SbfI sites. The PCR product generated was digested, purified, and ligated to the mycobacterial-E. coli shuttle vector pMD31 (Donnelly-Wu et al., 1993) to obtain the episomal plasmid pMDespI-eccD1, which confers kanamycin resistance. Site-directed mutagenesis of espI in pMDespI-eccD1 for the expression of EspIK426Q mutant was performed using the Quickchange Site-Directed Mutagenesis Kit (Agilent Technologies) and corresponding oligonucleotides with their antisense strands (primer sequence available upon request). All constructs generated in this study were verified by DNA sequencing. M. tuberculosis espI::Tn mutant was grown to mid-logarithmic phase prior to electroporation of the above mentioned plasmids following standard procedures (Wards & Collins, 1996). M. tuberculosis espI::Tn transformants harbouring pMDespI-eccD1 or its derivatives were selected on 7H11 agar plates containing 25 μg/ml kanamycin.
THP-1 cell culture and infection
To evaluate the virulence of different M. tuberculosis mutants, infection of human THP-1 monocytic cells was utilized. Actively dividing THP-1 cells grown in complete RPMI medium (Gibco RPMI supplemented with glutamine and 10% FCS) were treated with 10 nM phorbol myristate acetate (PMA), seeded at the required densities in multi-well plates and allowed to differentiate into adherent phagocytic cells over 3 days. PMA-containing medium was replaced with fresh complete RPMI medium and the cells incubated overnight before infecting with M. tuberculosis at a multiplicity of infection of 5. Cytotoxicity induced by M. tuberculosis was determined by measuring the metabolism of surviving THP-1 cells after 3 days post-infection with PrestoBlue Cell Viability Reagent (Life Technologies). Relative cytotoxicity of mammalian cells infected with different M. tuberculosis strains was measured as a percentage of uninfected cells in each experiment.
Protein preparation for immunoblots
Culture filtrates and cell lysates were prepared and immunoblotted as described previously (Chen et al., 2012). M. tuberculosis grown to an OD 600nm of 0.5 - 0.6 in Sauton’s medium containing 0.05% Tween-80 was centrifuged, washed once with PBS, resuspended in Sauton’s medium without Tween-80 with addition of tested compounds if needed, and grown further at 37 °C with shaking for 4 or 5 days. Culture filtrate proteins were obtained after centrifugation of cultures, filtering of the supernatant through 0.2 micron filters and 100-fold concentration with 5-kDa-molecular-weight-cutoff membranes (Sartorius Stedim Biotech GmbH, Goettingen, Germany). Cell lysates were prepared from cell pellets lysed by bead beating in cold PBS containing Roche protease inhibitor cocktail. Total protein concentrations were determined by the BCA assay (Pierce) with bovine serum albumin as the standard. Indicated amounts of culture filtrate and cell lysate proteins were resolved in NuPAGE 4 to 12% Bis-Tris gels (Invitrogen, Carlsbad, CA, U.S.A.), transferred to nitrocellulose membranes and blocked with TBS-Milk (20 mM Tris-HCl, pH 7.5, 150 mM NaCl and 5% non-fat milk powder). Membranes were usually incubated overnight with the required primary antibody in TNT-BSA (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween-20 and 1% BSA fraction V) at 4°C and with the appropriate secondary antibody in TNT-BSA for 30 min at room temperature and developed using Lumi-Light Plus chemiluminescence reagent (Roche, Mannheim, Germany).
GroEL2 was used as a lysis control for culture filtrates. EsxA, EsxB and EspB were detected using anti-EsxA mouse monoclonal antibodies (HYB 76-8), anti-EsxB rabbit serum, and anti-EspB rat serum, respectively.
Statistical analysis
The significance in differences between experimental groups was determined using Student’s t-test (two-tailed, unpaired with equal variances) in the GraphPad Prism version 6 software.
Supplementary Material
Acknowledgements
The authors thank Koen Andries for kindly providing BDQ and Ida Rosenkrands for anti-EspB rat serum. J.M.C. received post-doctoral fellowships from the Canadian Thoracic Society and the Canadian Institutes of Health Research. J.R. was supported by the German Federal Ministry of Research and Education (BMBF grant 01KI1017). This study received funding from the Swiss National Science Foundation (31003A-140778) and the European Community’s Seventh Framework Program (FP7/2007-2013) under grant agreements n°201762 and 260872. Antibodies against GroEL2 and Antigen-85 complex were received as part of the National Institutes of Health, National Institute of Allergy and Infectious Diseases contract (no. HHSN266200400091c) entitled “Tuberculosis Vaccine Testing and Research Materials”, awarded to Colorado State University. The authors have no conflicts of interest.
References
- Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- Bentley SD, Comas I, Bryant JM, Walker D, Smith NH, Harris SR, Thurston S, Gagneux S, Wood J, Antonio M, Quail MA, Gehre F, Adegbola RA, Parkhill J, de Jong BC. The genome of Mycobacterium africanum West African 2 reveals a lineage-specific locus and genome erosion common to the M. tuberculosis complex. PLoS neglected tropical diseases. 2012;6:e1552. doi: 10.1371/journal.pntd.0001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter W, Houben ENG, Bottai D, Brodin P, Brown EJ, Cox JS, Derbyshire K, Fortune SM, Gao L-Y, Liu J, Gey van Pittius NC, Pym AS, Rubin EJ, Sherman DR, Cole ST, Brosch R. Systematic Genetic Nomenclature for Type VII Secretion Systems. PLoS Pathog. 2009;5:e1000507. doi: 10.1371/journal.ppat.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco B, Chen JM, Hartkoorn R, Sala C, Uplekar S, Rougemont J, Pojer F, Cole ST. Virulence regulator EspR of Mycobacterium tuberculosis is a nucleoid-associated protein. PLoS Pathog. 2012;8:e1002621. doi: 10.1371/journal.ppat.1002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P, Majlessi L, Marsollier L, de Jonge MI, Bottai D, Demangel C, Hinds J, Neyrolles O, Butcher PD, Leclerc C, Cole ST, Brosch R. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect Immun. 2006;74:88–98. doi: 10.1128/IAI.74.1.88-98.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Boy-Röttger S, Dhar N, Sweeney N, Buxton RS, Pojer F, Rosenkrands I, Cole ST. EspD is critical for the virulence-mediating ESX-1 secretion system in Mycobacterium tuberculosis. J Bacteriol. 2012;194:884–893. doi: 10.1128/JB.06417-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Zhang M, Rybniker J, Boy-Rottger S, Dhar N, Pojer F, Cole ST. Mycobacterium tuberculosis EspB binds phospholipids and mediates EsxA-independent virulence. Mol Microbiol. 2013;89:1154–1166. doi: 10.1111/mmi.12336. [DOI] [PubMed] [Google Scholar]
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honore N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. Massive gene decay in the leprosy bacillus. Nature. 2001;409:1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- Correa NE, Peng F, Klose KE. Roles of the regulatory proteins FlhF and FlhG in the Vibrio cholerae flagellar transcription hierarchy. J Bacteriol. 2005;187:6324–6332. doi: 10.1128/JB.187.18.6324-6332.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das C, Ghosh TS, Mande SS. Computational analysis of the ESX-1 region of Mycobacterium tuberculosis: insights into the mechanism of type VII secretion system. PLoS One. 2011;6:e27980. doi: 10.1371/journal.pone.0027980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta N, Ramphal R. Interaction of the antiactivator FleN with the transcriptional activator FleQ regulates flagellar number in Pseudomonas aeruginosa. J Bacteriol. 2001;183:6636–6644. doi: 10.1128/JB.183.22.6636-6644.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza GA, Leversen NA, Malen H, Wiker HG. Bacterial proteins with cleaved or uncleaved signal peptides of the general secretory pathway. Journal of proteomics. 2011;75:502–510. doi: 10.1016/j.jprot.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Dhar N, McKinney JD. Mycobacterium tuberculosis persistence mutants identified by screening in isoniazid-treated mice. Proc Natl Acad Sci U S A. 2010;107:12275–12280. doi: 10.1073/pnas.1003219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly-Wu MK, Jacobs WR, Jr., Hatfull GF. Superinfection immunity of mycobacteriophage L5: applications for genetic transformation of mycobacteria. Mol Microbiol. 1993;7:407–417. doi: 10.1111/j.1365-2958.1993.tb01132.x. [DOI] [PubMed] [Google Scholar]
- Frigui W, Bottai D, Majlessi L, Monot M, Josselin E, Brodin P, Garnier T, Gicquel B, Martin C, Leclerc C, Cole ST, Brosch R. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog. 2008;4:e33. doi: 10.1371/journal.ppat.0040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier T, Eiglmeier K, Camus JC, Medina N, Mansoor H, Pryor M, Duthoy S, Grondin S, Lacroix C, Monsempe C, Simon S, Harris B, Atkin R, Doggett J, Mayes R, Keating L, Wheeler PR, Parkhill J, Barrell BG, Cole ST, Gordon SV, Hewinson RG. The complete genome sequence of Mycobacterium bovis. Proc Natl Acad Sci U S A. 2003;100:7877–7882. doi: 10.1073/pnas.1130426100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengenbacher M, Kaufmann SH. Mycobacterium tuberculosis: success through dormancy. FEMS microbiology reviews. 2012;36:514–532. doi: 10.1111/j.1574-6976.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo-Asensio J, Mostowy S, Harders-Westerveen J, Huygen K, Hernandez-Pando R, Thole J, Behr M, Gicquel B, Martin C. PhoP: a missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS One. 2008;3:e3496. doi: 10.1371/journal.pone.0003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, Smith S, Sherman DR. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–370. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR., Jr. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci U S A. 2003;100:12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak BI, Hendrixson DR. Spatial and numerical regulation of flagellar biosynthesis in polarly flagellated bacteria. Mol Microbiol. 2013 doi: 10.1111/mmi.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul A, Dendouga N, Vergauwen K, Molenberghs B, Vranckx L, Willebrords R, Ristic Z, Lill H, Dorange I, Guillemont J, Bald D, Andries K. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nature chemical biology. 2007;3:323–324. doi: 10.1038/nchembio884. [DOI] [PubMed] [Google Scholar]
- Kruh NA, Troudt J, Izzo A, Prenni J, Dobos KM. Portrait of a pathogen: the Mycobacterium tuberculosis proteome in vivo. PLoS One. 2010;5:e13938. doi: 10.1371/journal.pone.0013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. J Infect Dis. 2003;187:117–123. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malen H, Pathak S, Softeland T, de Souza GA, Wiker HG. Definition of novel cell envelope associated proteins in Triton X-114 extracts of Mycobacterium tuberculosis H37Rv. BMC Microbiol. 2010;10:132. doi: 10.1186/1471-2180-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Forst CV, Dobos KM, Belisle JT, Chen J, Bradbury EM, Bradbury AR, Chen X. Mycobacterium tuberculosis functional network analysis by global subcellular protein profiling. Molecular biology of the cell. 2005;16:396–404. doi: 10.1091/mbc.E04-04-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X, Samten B, Cao G, Wang X, Tvinnereim AR, Chen XL, Howard ST. MprAB regulates the espA operon in Mycobacterium tuberculosis and modulates ESX-1 function and host cytokine response. J Bacteriol. 2013;195:66–75. doi: 10.1128/JB.01067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46:709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Griffiths KE, Marchal G, Leclerc C, Cole ST. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9:533–539. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Manzanillo P, Chan K, Dovey C, Cox JS. Secreted transcription factor controls Mycobacterium tuberculosis virulence. Nature. 2008;454:717–721. doi: 10.1038/nature07219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittershaus ES, Baek SH, Sassetti CM. The normalcy of dormancy: common themes in microbial quiescence. Cell Host Microbe. 2013;13:643–651. doi: 10.1016/j.chom.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone R, Bottai D, Brosch R. ESX/type VII secretion systems and their role in host-pathogen interaction. Curr Opin Microbiol. 2009;12:4–10. doi: 10.1016/j.mib.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Stanley SA, Raghavan S, Hwang WW, Cox JS. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci U S A. 2003;100:13001–13006. doi: 10.1073/pnas.2235593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear TP, Seemann T, Harrison PF, Jenkin GA, Davies JK, Johnson PD, Abdellah Z, Arrowsmith C, Chillingworth T, Churcher C, Clarke K, Cronin A, Davis P, Goodhead I, Holroyd N, Jagels K, Lord A, Moule S, Mungall K, Norbertczak H, Quail MA, Rabbinowitsch E, Walker D, White B, Whitehead S, Small PL, Brosch R, Ramakrishnan L, Fischbach MA, Parkhill J, Cole ST. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 2008;18:729–741. doi: 10.1101/gr.075069.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop EJM, Bitter W, van der Sar AM. Tubercle bacilli rely on a type VII army for pathogenicity. Trends Microbiol. 2012;20:477–484. doi: 10.1016/j.tim.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Walters SB, Dubnau E, Kolesnikova I, Laval F, Daffe M, Smith I. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol Microbiol. 2006;60:312–330. doi: 10.1111/j.1365-2958.2006.05102.x. [DOI] [PubMed] [Google Scholar]
- Wards BJ, Collins DM. Electroporation at elevated temperatures substantially improves transformation efficiency of slow-growing mycobacteria. FEMS microbiology letters. 1996;145:101–105. doi: 10.1111/j.1574-6968.1996.tb08563.x. [DOI] [PubMed] [Google Scholar]
- Zhang M, Sala C, Hartkoorn RC, Dhar N, Mendoza-Losana A, Cole ST. Streptomycin-Starved Mycobacterium tuberculosis 18b, a Drug Discovery Tool for Latent Tuberculosis. Antimicrob Agents Chemother. 2012;56:5782–5789. doi: 10.1128/AAC.01125-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.