Abstract
Introduction
Rapid thrombelastography (rTEG) has been advocated as a point-of-care test to manage trauma-induced coagulopathy. rTEG activated clotting time (T-ACT) results become available much sooner than other rTEG values, thus offering an attractive tool to guide blood component transfusion in a hemorrhagic shock. We hypothesize that patients with a prolonged T-ACT require replacement of platelets (Plts) and cryoprecipitate (Cryo) in addition to plasma to correct trauma-induced coagulopathy.
Methods
A prospective trauma registry was reviewed for patients with an r-TEG available within 3 hours of injury. Blood was collected via a standardized protocol for rTEG. Patients were stratified into quartiles: low (T-ACT <113 seconds), mild (T-ACT 113–120 seconds), moderate (T-ACT 121–140 seconds), and severe (T-ACT >140 seconds). Transfusion requirements were evaluated during the first 6 hours after injury.
Results
A total of 114 patients were included. Median age was 39 years, injury severity score 20, base-deficit 10, and mortality rate 13%. T-ACT cohorts had similar age (P = .11), injury severity score (P = .55), and base deficit (P = .38). An T-ACT >140 seconds predicted a lower angle (median 57 vs 70, P <.000) and maximum amplitude (46 vs 60, P = .002), and patients received more Cryo (0.5 vs 0, P ≤ .000) and Plts (1 vs 0, P = .006).
Conclusion
Injured patients requiring resuscitation with blood transfusion that have a T-ACT > 140 seconds are polycoagulopathic and may benefit from early Cryo and Plts.
The bloody vicious cycle of death from trauma-induced coagulopathy (TIC) was described more than 30 years ago and inspired the concept of pre-emptive fresh-frozen plasma in civilian trauma centers.1,2 Combat experience in Iraq3 refocused interest in early plasma for patients at risk for TIC. Subsequently, fibrinogen deficiency4 and platelet (Plts) dysfunction5 have been implicated in TIC, suggesting that pre-emptive fibrinogen (cryoprecipitate) and Plts transfusions may be important in addition to plasma.
Thrombelastography (TEG) is superior to conventional laboratory measures for the assessment of TIC.6,7 Developed in the 1940s, this device allows for identification of the key phases of coagulation starting from clot initiation through clot propagation and strengthening and ending with clot degradation. The activated clotting time (T-ACT) in rapid TEG (r-TEG) represents this initial phase of clot formation. Prolongation of T-ACT has been associated with increased likelihood of requiring a massive transfusion (MT).8 This is an appealing point-of-care test because results are available within minutes.
As we have matured our understanding of TIC, phenotypes of bleeding disorders after major injury are becoming apparent.9 This observation caters to improving patient outcomes by personalizing trauma care. The success of personalized medicine is dependent on a reliable and clinically relevant product.10 We therefore wanted to determine the clinical value of T-ACT to guide early cryoprecipitate (Cryo) and Plts transfusions in patients at risk of TIC. Our hypothesis is prolongation of T-ACT represents a global coagulation disorder that would identify patients who will benefit from early product administration beyond plasma to correct deficient fibrinogen and dysfunctional Plts.
METHODS
Study population
A prospective trauma registry was reviewed for patients with an r-TEG available within 3 hours of injury before the administration of any blood products. Patients were excluded if they were younger than 18 years of age, had evidence of liver failure, were taking an anticoagulant, or died within 6 hours of injury. Patient demographics, emergency department vital signs, and initial laboratory values were obtained from this prospective registry, which is validated by the hospital trauma data bank. Blood product administration was prospectively recorded in the same registry. Total blood product administration was determined during the first 6 hours after injury.
Thrombelastography
Blood was collected from patients in 2.7-mL buffered sodium citrate (3.2%) sample tubes (Vacutainer; Becton-Dickinson, Franklin Lakes, NJ). Samples were run within 2 hours of collection. R-TEG assays were recalcified and run according to the manufacturer’s instructions on a TEG 5000 Thrombelastography Hemostasis Analyzer (Haemonetics Corp., Braintree, MA). The following parameters were recorded from the tracings of the r-TEG: T-ACT (seconds), angle (α, degrees), maximum amplitude (MA, mm), and lysis 30 minutes after MA (LY30, %). The significance of each aforementioned parameter has been described previously in the literature.11
Data analysis
TEG parameters were analyzed on all patients, and the Spearman Rho test was used to determine correlation between TEG parameters. Patients were stratified into quartiles on the basis of T-ACT using SPSS software visual binning (SPSS Institute, Cary, NC) with equal percentile cut points. Total blood product transfusions at 6 hours were compared between groups. The first 6-hour time interval was selected as previous literature supports that the majority of transfusions occur during this time,12 and increased plasma/red blood cell (RBC) ratio during this time may reduce the classic definition of massive transfusion of 10 units RBC in 24 hours.13 A massive transfusion in our study was considered 10 units or more of RBC within the first 6 hours.
SPSS software was used for group comparisons, with two-tailed alpha set to 0.05 for comparisons of statistical significance. Intergroup comparisons were made with independent median test for continuous variables. Kruskal-Wallis and Mann Whitney U were used for intergroup comparisons for ordinal data. Follow-ups test for multiple group comparisons were completed with a Bonferroni adjustment. The χ2 test was used for nominal group comparisons.
RESULTS
Patient demographics
A total of 114 patients met inclusion criteria. Median age was 39 years, 75% were male, and 37% had penetrating injuries. Median injury severity score was 20 (interquartile range 21), base deficit of 10 (interquartile range 7), and mortality rate was 13%. T-ACT stratification by quartile did not have a difference in age (P = .11), injury severity score (P = .55), or base deficit (P = .38). The T-ACT had a high correlation with angle and MA, but no correlation with LY30 (Table I). Quartile distributions of T-ACT showed differences in blood product transfusion within the first 6 hours (Table II). Pairwise comparisons of quartile groups of follow-up analysis identified the upper quartile (T-ACT > 140) to have the largest differences in blood product transfusions between groups.
Table I.
T-ACT correlation with other TEG parameters
| Spearman’s rho | T-ACT | Angle | MA | Ly30 |
|---|---|---|---|---|
| T-ACT | ||||
| Correlation Coefficient | 1.000 | −0.655* | −0.504* | 0.025 |
| Sig. (2-tailed) | — | 0.000 | 0.000 | 0.794 |
| N | 114 | 114 | 114 | 114 |
| Angle | ||||
| Correlation Coefficient | −0.655* | 1.000 | 0.687* | 0.002 |
| Sig. (2-tailed) | 0.000 | — | 0.000 | 0.982 |
| N | 114 | 114 | 114 | 114 |
| MA | ||||
| Correlation Coefficient | −0.504* | 0.687* | 1.000 | 0.014 |
| Sig. (2-tailed) | 0.000 | 0.000 | — | 0.880 |
| N | 114 | 114 | 114 | 114 |
| Ly30 | ||||
| Correlation Coefficient | 0.025 | 0.002 | 0.014 | 1.000 |
| Sig. (2-tailed) | 0.794 | 0.982 | 0.880 | — |
| N | 114 | 114 | 114 | 114 |
Statistically significant.
Bivariate analysis with Spearman’s Rho demonstrates T-ACT has inverse correlation with angle and MA.
Ly30, Lysis at 30 minutes after reaching maximal amplitude; MA, maximal amplitude; T-ACT, activated clotting time in thrombelastography.
Table II.
Blood product transfusion stratified by TACT quartile
| ACT quartile | Blood product | Median | IQR | Median Test, P value | Versus quartile 4, P value |
|---|---|---|---|---|---|
| 1 | RBCs | 2 | 8 | .245 | NA |
| 2 | RBCs | 1.5 | 6 | NA | |
| 3 | RBCs | 3 | 10 | NA | |
| 4 | RBCs | 7 | 17 | NA | |
| 1 | Plasma | 0.5 | 3 | .016* | .578 |
| 2 | Plasma | 0 | 3 | .016* | |
| 3 | Plasma | 0 | 4 | .02* | |
| 4 | Plasma | 4 | 10 | NA | |
| 1 | Cryo | 0 | 0 | .001* | .008* |
| 2 | Cryo | 0 | 0 | .038* | |
| 3 | Cryo | 0 | 0 | .003* | |
| 4 | Cryo | 0.5 | 2 | NA | |
| 1 | Platelets | 0 | 1 | .027* | .2 |
| 2 | Platelets | 0 | 0 | .032* | |
| 3 | Platelets | 0 | 1 | .061 | |
| 4 | Platelets | 1 | 2 | NA |
Statistical significance P < .05.
T-ACT stratified by quartiles demonstrates differences in blood product transfusions between groups. Independent median test had statistical significance for differences in blood product transfusions between quartiles for plasma, cryo, and platelets. Follow-up adjusted paired analysis identified the 4th quartiles as different from the rest of the groups. None of the lower 3 quartiles had significant differences in product with adjusted pair wise comparisons.
Cryo, Cryoprecipitate; IQR, interquartile range; NA, not available; RBC, red blood cells; T-ACT, activated clotting time in thrombelastography.
Transfusion requirements
Overall 61% of patients required a transfusion, and 26% required a massive transfusion. Sixty eight percent of patients with a T-ACT >140 seconds required a transfusion which was similar to patients with a T-ACT <140 seconds (59%, P = .31). Massive transfusions were more frequent in the T-ACT >140 seconds cohort but did not reach statistical significance (37% vs 22%, P = .12). A T-ACT >140 seconds predicted a lower angle (median 57 vs 70, P < .000) and MA (46 vs 60, P = .002) compared with the lower three quartiles. This result correlated with the number of blood product transfusions over the first 6 hours. Patients with a T-ACT >140 seconds received more plasma (4 vs 0, P = .01) Cryo (0.5 vs 0, P ≤.000), and Plts (1 vs 0, P = .006). The correlation also was seen in the percentage of patients requiring blood product specific transfusions (Fig 1).
Fig 1.
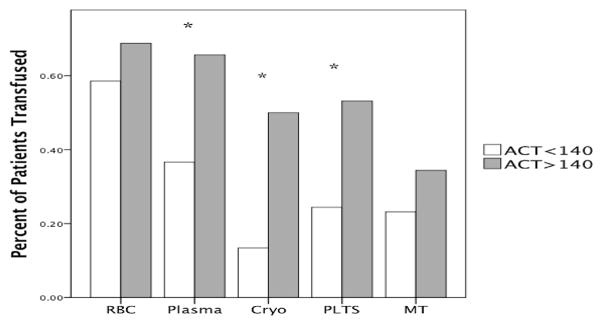
Percentage of patients receiving one or more blood product stratified by blood product and dichotomized by TACT. There was no difference in the percentage of patients receiving RBC transfusions or MT, but the remaining blood products were transfused more frequently in patients with a TACT >140 seconds. *P <.05. RBC, Red blood cells (>1 in first 6 hours); Cryo, cryoprecipitate; Plts, platelets; MT, massive transfusion (10 or more RBCs in 6 hours); T-ACT, TEG-activated clotting time.
Patient selectivity
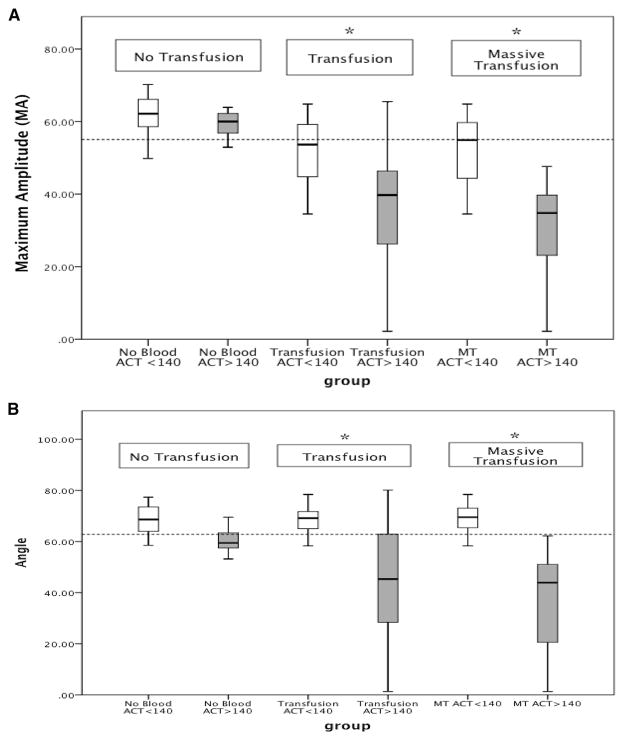
Additional stratification of group comparisons based on transfusion requirements (no transfusion, 1 or more transfusions, or massive transfusion) demonstrated that T-ACT gained a greater predictive value for identifying depressed angle and clot strength when applied to bleeding trauma patients. Patients that did not require a transfusion had no significant difference in angle (59.5 vs 68.6, P = .07) and MA (59.5 vs 62.2, P = .719) when T-ACT was greater than 140 vs less than. For patients requiring a transfusion angle (48 vs 70, P < .000) and MA (41 vs 57, P < .000) were both reduced in patients with a T-ACT >140 seconds compared with the lower quartiles. The same pattern was seen in massive transfusion (angle 44 vs 69.5, P < .000, MA 35 vs 55, P < .000). Perhaps even more importantly, based off our institutions transfusion thresholds for decreased MA and angle, an T-ACT >140 seconds in a patient undergoing a MT had 100% positive predictive value for indication of PLTS and CRYO transfusion based off of initial TEG (Fig 2, A and B).
Fig 2.
(A) Difference in maximum amplitude (MA) stratified by transfusion requirement. The dotted line at 55 represents our institution’s threshold for platelet transfusion. Patients who did not require a transfusion had no statistical difference in the change in MA with respect to T-ACT dichotomization. Comparison of patients who received a transfusion or MT showed decreased MA correlating to prolonged T-ACT. All 11 patients with a T-ACT >140 seconds and who required an MT had an MA indicative of platelet transfusion. No blood, no transfusion; Transfusion, patient received >1 unit of red blood cells; MT, massive transfusion (patients received 10 or more units of red blood cells in the first 6 hours); T-ACT, TEG-activated clotting time. *P <.01. (B) Difference in angle stratified by transfusion requirement. The dotted line represents our group’s transfusion threshold for cryoprecipitate. The same trend was seen with MA as angle. Patients who did not require a transfusion did not have a difference in angle, whereas patients requiring a transfusion or MT had an association with prolonged. Again, all 11 patients who required an MT had an angle indicative of a cryoprecipitate transfusion. No blood, no transfusion; Transfusion, patient received >1 unit of red blood cells; MT, massive transfusion (patients received >6 or more units of red blood cells in the first 6 hours); *P < .01.
DISCUSSION
The T-ACT from r-TEG is a rapidly available test with clinical relevance. Previous work from the Houston group6 and our own14 has shown TEG to have superiority to conventional labs and provide an early indication for the likelihood of requiring transfusion in trauma patients.7,8 An T-ACT >140 seconds identifies a patient population who were transfused a greater number of non–RBC products than patients with lower T-ACT values (Table II). The San Francisco General group principle component analysis based on soluble clotting factors has identified this patient phenotype of the polycoagulopathic patients.9 Our recent component analysis with TEG in massive transfusions has also identified this phenotype that likely would benefit from Cryo and Plts in addition to plasma during resuscitation. Waiting for the MA from r-TEG can take up to an hour to identify these patients. Therefore, T-ACT prediction of depression of both angle and MA is of clinical importance.
The pathophysiology of prolonged T-ACT in r-TEG is not clear. Although conventionally this portion of the TEG curve has been attributed to soluble coagulation factors,11 we are beginning to appreciate that Plts and fibrinogen are playing a role. For example, Johansson et al15 demonstrated that Plts impact the rate of thrombin generation. Plts stored for 7 days had a hypercoagable effect and decrease the R time (T-ACT equivalent in a nonrapid TEG) when added to whole blood. The addition of Plts after plasma dilution has the capacity to compensate for clotting factors, further supporting the role of Plts in clot initiation.16 After trauma, Plts lose their functionality and may have the opposite effect. Our group using a functional Plts assay found decreased platelet aggregation early in the post injury phase.5 Plts dysfunction is highly prevalent in trauma patients and associated with increased mortality independent of Plts count.17 Thus, there is a plausible biologic mechanism for prolonged clotting time and Plts dysfunction, supporting that patients with a severely prolonged T-ACT would benefit from a Plts transfusion.
Causality for prolonged T-ACT due to fibrinogen dysfunction is less clear. Fibrinogen replacement does not improve thrombin generation-like Plts.18 Interestingly, blood diluted with albumin and saline causing a prolonged clotting time is corrected by replacing fibrinogen levels; however, dilution using synthetic colloids does not have the same effect.19 The fibrin polymerization interaction with thrombin, fibrinogen, and factor XIII are impaired by artificial colloids.20 It is plausible that dysfunctional fibrinogen from trauma may have a similar reticence in initiating fibrin clot, but that is yet to be defined. However, our data support this mechanism as T-ACT has the greatest inverse correlation with angle compared with other R-TEG values (Table I).
Patients requiring a transfusion or MT with a prolongation of T-ACT predicted depressed Angle and MA (Fig 2, A and B). High fibrinogen to RBC ratio has been advocated to improve survival during massive transfusion,21 and this concept is more universally adopted in Europe.4 In the United States, Cryo is infrequently used and in the recent PROMMTT (prospective, observational, multicenter, major trauma transfusion) trial, there was not enough to data draw benefit or harm.22 In a large retrospective study, Holcomb et al23 found an increased ratio of Plts to RBC to correlate with improved survival; however, prospective data from PROMMTT indicated that few patients receive Plts early during resuscitation and delaying transfusions beyond 4 hours does not increase survival.24 The 1:1:1 ratio is not the solution for all massively bleeding trauma patients; survival after penetrating trauma is inferior used fixed ratios of blood products compared with point-of-care, TEG-directed resuscitation.25
Limitations
The retrospective nature of this study is reflective of clinician behavior and does not necessarily represent what the patients should have optimally received. The rTEG transfusion parameters of our institution were developed from algorithms used from cardiac and liver transplant surgery. Although our treatment algorithm has been in practice for more than half a decade, it is becoming increasingly apparent that TIC is distinct from other forms of coagulopathy. Therefore, these transfusion triggers determined by TEG parameters are subject to change over time. Patients with a T-ACT >140 seconds who did not receive Cryo had a greater ratio of plasma to RBC. These patients had roughly 3 extra units of plasma per Cryo transfusion compared with others in the T-ACT >140 seconds cohort. Interestingly, this ratio is similar to the expected ratio of fibrinogen between these two products and is encouraging that the current transfusion triggers are in an effective range. Although it would appear that greater volumes of plasma correct deficiencies, increasing the number of these transfusions is associated with multiple organ failure and may not have the most advantageous results.
Cotton et al8 identified T-ACT >127 seconds as a risk for requiring a MT. In our study we did not find this correlation, likely because of a different patient population as our study had more than 4 times the percentage of patients receiving a MT and half the number of patients. We observed patients who did not require a transfusion lacked the same predictive value of their T-ACT as patients receiving blood, which demonstrates the importance of selective patient use of TEG for guiding resuscitation, because bleeding patients TEG parameters correlate differently from nonbleeding patients (Fig 2, A and B).
In conclusion, Injured patients requiring resuscitation with blood transfusion that have a T-ACT >140 seconds are polycoagulopathic. These patients may benefit by the addition of early cryoprecipitate and Plts transfusions during their resuscitation with RBC and plasma. The identification of unique phenotypes of TIC linked to TEG parameters is a compelling research agenda. TEG enables rapid results during trauma resuscitation, catering to the first step in personalized medicine for severely injured patients.
Acknowledgments
Supported in part by National Institute of General Medical Sciences grants T32-GM008315, P50-GM049222 1, UM 1HL120877, and CCTSI supported in part by Colorado CTSA Grant UL1 TR001082 from National Center for Advancing Translational Sciences/National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences, National Heart, Lung, and Blood Institute, or National Institutes of Health.
Footnotes
Presented at the 9th Annual Academic Surgical Congress in San Diego, CA, February 4–6, 2014.
References
- 1.Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma---a unified approach. J Trauma. 1982;22:672–9. doi: 10.1097/00005373-198208000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Ledgerwood AM, Lucas CE. A review of studies on the effects of hemorrhagic shock and resuscitation on the coagulation profile. J Trauma. 2003;54(5 Suppl):S68–74. doi: 10.1097/01.TA.0000064513.59253.70. [DOI] [PubMed] [Google Scholar]
- 3.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 4.Schlimp CJ, Schochl H. The role of fibrinogen in trauma-induced coagulopathy. Hamostaseologie. 2014;34:29–39. doi: 10.5482/HAMO-13-07-0038. [DOI] [PubMed] [Google Scholar]
- 5.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, et al. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214:739–46. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256:476–86. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 7.Pezold M, Moore EE, Wohlauer M, Sauaia A, Gonzalez E, Banerjee A, et al. Viscoelastic clot strength predicts coagulation-related mortality within 15 minutes. Surgery. 2012;151:48–54. doi: 10.1016/j.surg.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, Wade C, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma. 2011;71:407–14. doi: 10.1097/TA.0b013e31821e1bf0. discussion 414–7. [DOI] [PubMed] [Google Scholar]
- 9.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74:1223–9. doi: 10.1097/TA.0b013e31828b7fa1. discussion 1229–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363:301–4. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 11.Salooja N, Perry DJ. Thrombelastography. Blood Coagul Fibrinolysis. 2001;12:327–37. doi: 10.1097/00001721-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–70. doi: 10.1097/TA.0b013e31817de3e1. discussion 270–1. [DOI] [PubMed] [Google Scholar]
- 13.Kautza BC, Cohen MJ, Cuschieri J, Minei JP, Brackenridge SC, Maier RV, et al. Changes in massive transfusion over time: an early shift in the right direction? J Trauma Acute Care Surg. 2012;72:106–11. doi: 10.1097/TA.0b013e3182410a3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashuk JL, Moore EE, Wohlauer M, Johnson JL, Pezold M, Lawrence J, et al. Initial experiences with point-of-care rapid thrombelastography for management of life-threatening post-injury coagulopathy. Transfusion. 2012;52:23–33. doi: 10.1111/j.1537-2995.2011.03264.x. [DOI] [PubMed] [Google Scholar]
- 15.Johansson PI, Svendsen MS, Salado J, Bochsen L, Kristensen AT. Investigation of the thrombin-generating capacity, evaluated by thrombogram, and clot formation evaluated by thrombelastography of platelets stored in the blood bank for up to 7 days. Vox Sang. 2008;94:113–8. doi: 10.1111/j.1423-0410.2007.01011.x. [DOI] [PubMed] [Google Scholar]
- 16.Schols SE, Feijge MA, Lance MD, Hamulyak K, ten Cate H, Heemskerk JW, et al. Effects of plasma dilution on tissue-factor-induced thrombin generation and thromboelastography: partly compensating role of platelets. Transfusion. 2008;48:2384–94. doi: 10.1111/j.1537-2995.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 17.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73:13–9. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ninivaggi M, Feijge MA, Baaten CC, Kuiper GJ, Marcus MA, Ten Cate H, et al. Additive roles of platelets and fibrinogen in whole-blood fibrin clot formation upon dilution as assessed by thromboelastometry. Thromb Haemost. 2013;111:447–57. doi: 10.1160/TH13-06-0493. [DOI] [PubMed] [Google Scholar]
- 19.Fries D, Innerhofer P, Reif C, Streif W, Klingler A, Schobersberger W, et al. The effect of fibrinogen substitution on reversal of dilutional coagulopathy: an in vitro model. Anesth Analg. 2006;102:347–51. doi: 10.1213/01.ane.0000194359.06286.d4. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen VG. Colloids decrease clot propagation and strength: role of factor XIII-fibrin polymer and thrombin-fibrinogen interactions. Acta Anaesthesiol Scand. 2005;49:1163–71. doi: 10.1111/j.1399-6576.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 21.Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64(2 Suppl):S79–85. doi: 10.1097/TA.0b013e318160a57b. discussion S85. [DOI] [PubMed] [Google Scholar]
- 22.Holcomb JB, Fox EE, Zhang X, White N, Wade CE, Cotton BA, et al. Cryoprecipitate use in the PROMMTT study. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S31–9. doi: 10.1097/TA.0b013e31828fa3ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holcomb JB, Zarzabal LA, Michalek JE, Kozar RA, Spinella PC, Perkins JG, et al. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318–28. doi: 10.1097/TA.0b013e318227edbb. [DOI] [PubMed] [Google Scholar]
- 24.del Junco DJ, Holcomb JB, Fox EE, Brasel KJ, Phelan HA, Bulger EM, et al. Resuscitate early with plasma and platelets or balance blood products gradually: findings from the PROMMTT study. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S24–30. doi: 10.1097/TA.0b013e31828fa3b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ, Jr, et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74:378–85. doi: 10.1097/TA.0b013e31827e20e0. discussion 385–6. [DOI] [PubMed] [Google Scholar]