Abstract
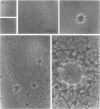
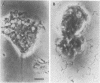
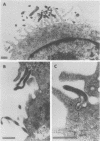
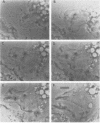
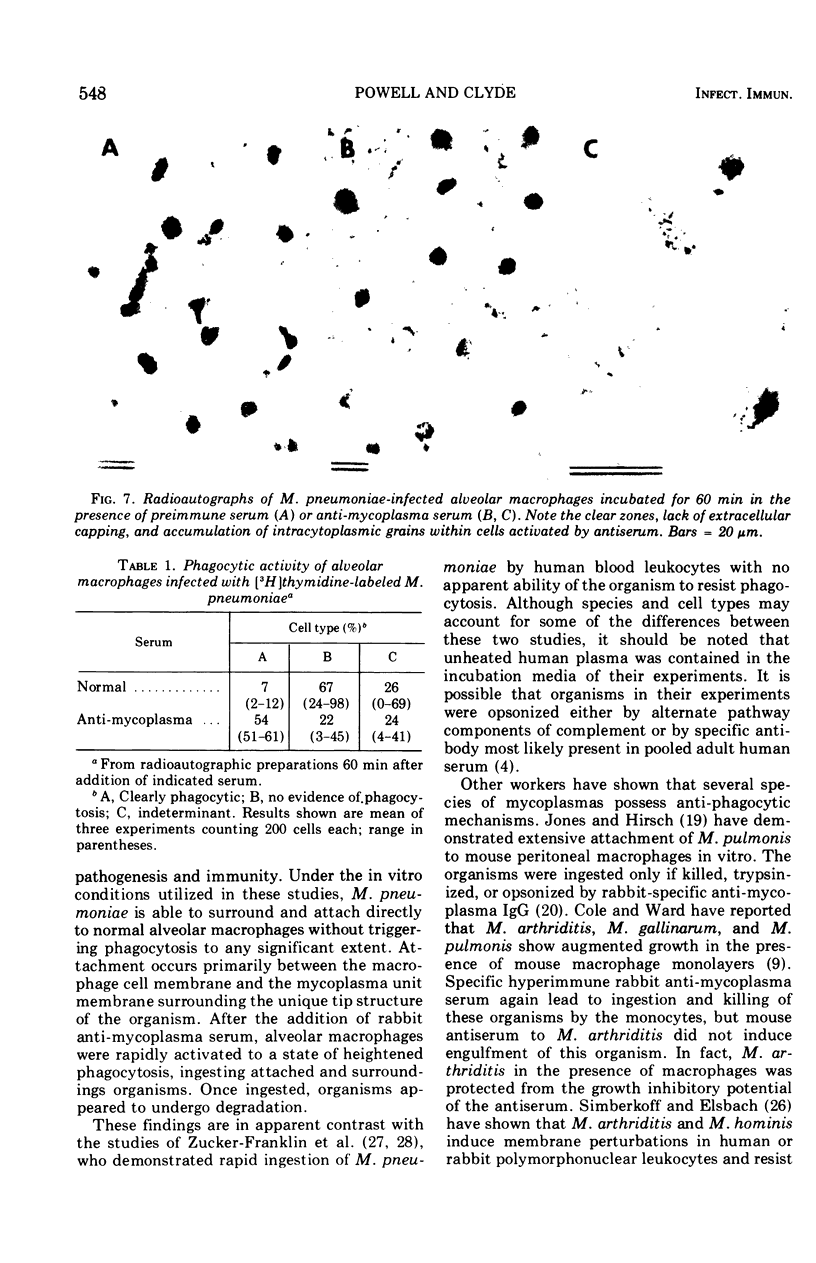
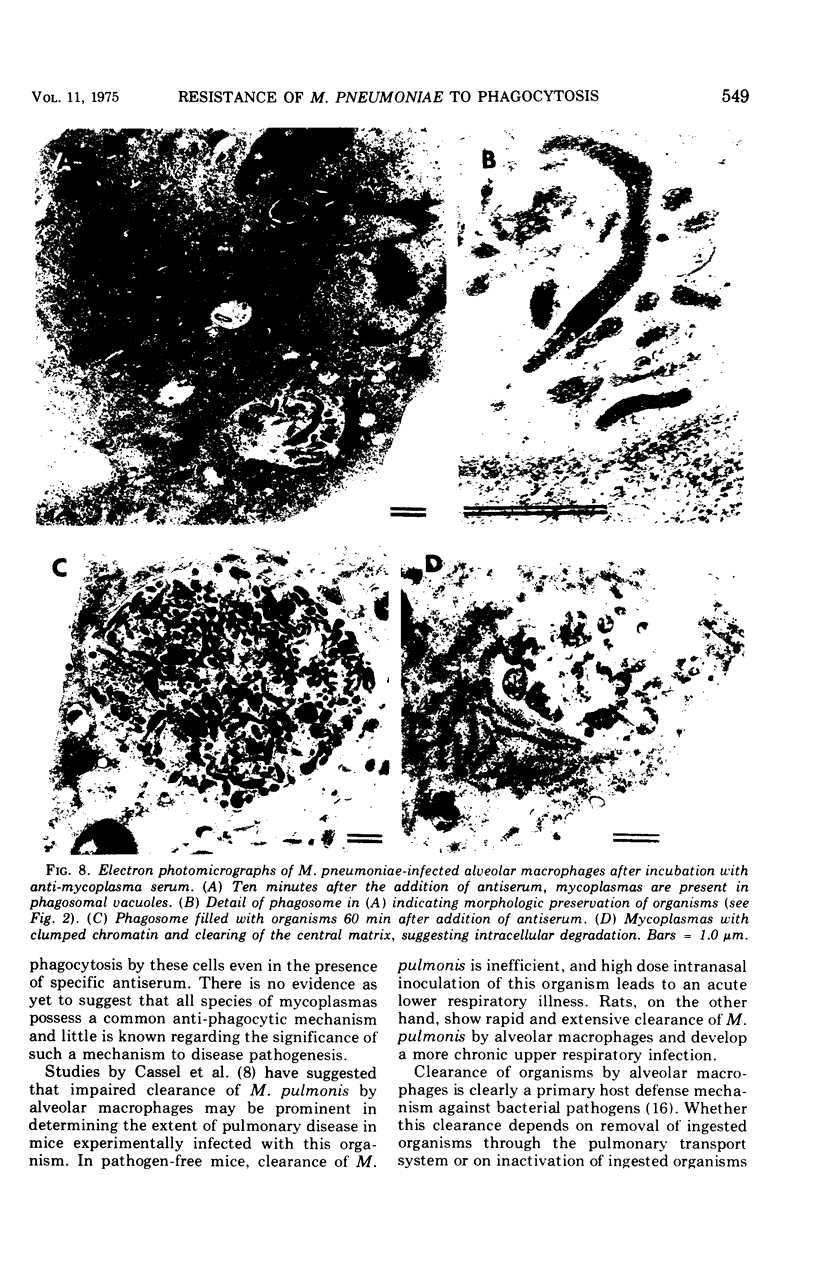
Several species of mycoplasmas are responsible for respiratory disease in animals and man. As yet, little is known about the interaction of these pathogens with alveolar macrophages, one of the primary components of pulmonary resistance to infections. The present study was undertaken to develop an in vitro model to examine this organism-cell interaction, using a human pathogen, mycoplasma pneumoniae, and normal guinea pig alveolar macrophages. During a 24-h incubation of M. pneumoniae with a monolayer of macrophages, mycoplasmas were found to attach directly to the surface of the cells without inducing significant phagocytosis. Ultrastructurally, the organisms appeared bound to the cell membrane by their characteristic attachment organelles. Only after the addition of specific anti-mycoplasma serum were cells able to engulf attached and surrounding organisms. These data suggest that the interaction of M. pneumoniae and alveolar macrophages is a potentially important aspect of disease pathogenesis, and immune factors which might alter this interaction merit further examination.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biberfeld G., Sterner G. Antibodies in bronchial secretions following natural infection with Mycoplasma pneumoniae. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(4):599–605. doi: 10.1111/j.1699-0463.1971.tb03818.x. [DOI] [PubMed] [Google Scholar]
- Brunner H., Greenberg H. B., James W. D., Horswood R. L., Couch R. B., Chanock R. M. Antibody to Mycoplasma pneumoniae in nasal secretions and sputa of experimentally infected human volunteers. Infect Immun. 1973 Oct;8(4):612–620. doi: 10.1128/iai.8.4.612-620.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner H., James W. D., Horswood R. L., Chanock R. M. Experimental Mycoplasma pneumoniae infection of young guinea pigs. J Infect Dis. 1973 Mar;127(3):315–318. doi: 10.1093/infdis/127.3.315. [DOI] [PubMed] [Google Scholar]
- Brunner H., James W. D., Horswood R. L., Chanock R. M. Measurement of Mycoplasma pneumoniae mycoplasmacidal antibody in human serum. J Immunol. 1972 Jun;108(6):1491–1498. [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr Appearance of Mycoplasma pneumoniae in lungs of experimentally infected hamsters and sputum from patients with natural disease. Am Rev Respir Dis. 1974 Dec;110(6):765–773. doi: 10.1164/arrd.1974.110.6P1.765. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny F. W., Clyde W. A., Jr, Glezen W. P. Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control. J Infect Dis. 1971 Jan;123(1):74–92. doi: 10.1093/infdis/123.1.74. [DOI] [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A., Jr, Bienenstock J. Immunoglobulin-containing cells in lungs of hamsters infected with Mycoplasma pneumoniae. J Immunol. 1972 May;108(5):1400–1408. [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A., Jr, Denny F. W. Factors influencing growth inhibition of Mycoplasma pneumoniae by immune sera. Proc Soc Exp Biol Med. 1967 Oct;126(1):161–166. doi: 10.3181/00379727-126-32391. [DOI] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. Immune enhancement of pulmonary bactericidal activity in murine virus pneumonia. J Clin Invest. 1973 Nov;52(11):2878–2884. doi: 10.1172/JCI107484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction in vitro of Mycoplasma pulmonis with mouse peritoneal macrophages and L-cells. J Exp Med. 1971 Feb 1;133(2):231–259. doi: 10.1084/jem.133.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake E. S., Evans D. G., Myrvik Q. N. Ultrastructural patterns of bacterial breakdown in normal and granulomatous rabbit alveolar macrophages. J Reticuloendothel Soc. 1971 Feb;9(2):174–199. [PubMed] [Google Scholar]
- McCormick D. P., Wenzel R. P., Senterfit L. B., Beam W. E., Jr Relationship of pre-existing antibody to subsequent infection by Mycoplasma pneumoniae in adults. Infect Immun. 1974 Jan;9(1):53–59. doi: 10.1128/iai.9.1.53-59.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvik Q. N. Function of the alveolar macrophage in immunity. J Reticuloendothel Soc. 1972 May;11(5):459–468. [PubMed] [Google Scholar]
- Simberkoff M. S., Elsbach P. The interaction in vitro between polymorphonuclear leukocytes and mycoplasma. J Exp Med. 1971 Dec 1;134(6):1417–1430. doi: 10.1084/jem.134.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. I. HeLa cells, neutrophils, and eosinophils. J Exp Med. 1966 Sep 1;124(3):521–532. doi: 10.1084/jem.124.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]