Abstract
Few studies have evaluated habitual nutrient intake among HIV-infected youth in the United States, even though diet may influence disease progression and risk of comorbidities. This study determined habitual micronutrient and macronutrient intake in HIV-infected youth. HIV-infected subjects and healthy controls 1–25 years old were prospectively enrolled. Nutrient intake was assessed via 24-h dietary recalls performed every 3 months for 1 year and compared to recommended intake from the U.S. Dietary Reference Intakes (DRIs) and Acceptable Macronutrient Distribution Ranges (AMDRs). Subjects with two or more food recalls were analyzed (175 HIV+ and 43 healthy controls). Groups were similar in age, race, sex, body mass index, and kilocalorie intake. In both groups, intake of several micronutrients was below the DRI. In addition, HIV+ subjects had a lower percentage DRI than controls for vitamins A, D, E, pantothenic acid, magnesium, calcium, folate, and potassium. HIV+ subjects' percentage caloric intake from fat was above the AMDR and was higher than controls. Caloric intake was negatively correlated with current and nadir CD4 count. Zinc, riboflavin, and magnesium percentage DRI were positively associated with current CD4 count. In HIV+ subjects not on antiretroviral therapy, HIV-1 RNA levels were negatively correlated with protein intake. HIV+ youth have an inadequate dietary intake of several essential nutrients and poorer dietary intake compared to controls. Intake of some nutrients was associated with important HIV-related factors. Further investigation is warranted to determine the impact of dietary intake of specific nutrients on HIV progression and chronic complication risk in this population.
Introduction
According to the Centers for Disease Control and Prevention (CDC), there were 47,500 new diagnoses of HIV infection in the United States in 2010, with 20% between the ages of 0 and 24 years.1 Nutritional deficiencies are common among people with HIV, including HIV-infected youth.2–8 In HIV-infected adults, nutritional deficiencies have been shown to affect immune status, disease progression, and mortality.7–15
With the advent of highly-active antiretroviral therapy (HAART), concerns over nutritional deficiencies in the HIV-infected population have shifted from AIDS wasting syndrome, growth stunting, and chronic diarrhea to newly described long-term complications associated with chronic HIV infection secondary to increased inflammation, oxidative stress, and immune activation.6,7,16–23 For example, HIV-infected individuals have an increased risk of cardiovascular disease (CVD) that has been shown to be associated with nutritional deficiencies in other populations as well as within the HIV population.24–26 In addition, HIV-infected individuals are at an increased risk for lipid abnormalities and metabolic syndrome, which have been shown to improve with dietary intervention in the general population.18,22,24,26–28
Like HIV-infected adults, increased risk for CVD and metabolic abnormalities occur among HIV-infected youth.18,22,29–32 Combined with a rising prevalence of obesity in this population and higher nutritional risk due to growth and development demands, nutritional deficiencies in this population are particularly alarming.29,33 Moreover, chronic immune activation and increased oxidative stress in HIV-infected youth may result in increased nutrient needs beyond the recommended intakes,6,20,34 while those individuals with hyperlipidemia or metabolic dysfunction may need to decrease their total fat, trans fat, saturated fat, and cholesterol intake.30,35,36 To date, however, few studies have investigated nutritional intake in relation to population recommendations among HIV-infected youth in developed countries, despite the serious implications for HIV disease progression and complication development in this population.2,6,17,29,36–38 And, notably, the few studies that have investigated this important topic used less stringent methods than what is recommended for nutrient intake assessment and/or only explored a few specific micronutrients.
Thus, the primary objective of this study was to comprehensively evaluate the habitual micronutrient (vitamins, trace elements, minerals) and macronutrient (calories, fat, protein, carbohydrate) intake in HIV-infected youth seen at an HIV clinic in Atlanta, Georgia. Secondary objectives were to (1) compare specific nutrient intake in HIV-infected youth to that of current intake recommendations for the general population, (2) compare the nutrient intake in HIV-infected youth to that of healthy controls, (3) assess the associations between dietary intake and plasma lipid levels, and (4) determine whether nutrient intake of specific micronutrients or macronutrients is associated with HIV-related variables.
Materials and Methods
Study population
All patients between the ages of 1 and 25 years old with documented HIV-1 infection enrolled at the Ponce Youth HIV Clinic of the Grady Health System in Atlanta, Georgia were eligible for this study. Participants were recruited over a 10-month period of time while they were at the clinic for their regular HIV monitoring visits. Over 95% of approached patients consented to study participation.
Controls were recruited with advertisement flyers hung in the HIV clinic and by word of mouth, and selected so that the overall group matched the HIV-infected subjects in age, sex, and race, and shared similar socioeconomic demographics. Healthy controls included relatives of HIV-infected patients and HIV-negative patients seen at the clinic. Controls were eligible if they self-reported to be free of chronic disease and had no recent or active infection. Potential subjects ≥13 years of age were screened for HIV infection before enrollment with the OraQuick Advance Rapid HIV Test (OraSure Technologies, Inc., Bethlehem, PA). Controls <13 years of age were assumed to be HIV uninfected unless they were considered at high risk for having or contracting HIV.
All parents or legal guardians of subjects <18 years of age and subjects ≥18 years of age provided written informed consent to participate in the study, and those subjects 17 years of age signed the written consent along with their parent or legal guardian. Subjects 6–10 years old gave verbal assent and those 11–16 years gave written assent. The Institutional Review Boards of Emory University and Grady Health Systems approved the study, and all ethical principles of the institutions were followed throughout the study.
Study design
Each subject underwent anthropometric, clinical, laboratory, and nutritional intake assessments at enrollment. Subjects were then followed prospectively and one additional nutritional intake assessment was obtained per subject for each season of the year during routine clinic visits or by telephone interview. Only subjects with two or more nutritional assessments obtained during different seasons were included in the study. All nutritional intake assessments and data entry were performed by registered dietitian-trained investigators and were overseen by registered dietitians.
Nutritional assessments
Nutritional assessments were obtained for each season of the year by conventional 24-h diet recall in order to capture seasonal changes in dietary intake as well as day-to-day variations in diet. Each subject and/or guardian reported subject food intake over the past 24 h to a trained investigator, supervised by a registered dietitian of the Bionutrition Unit of the Atlanta Clinical and Translational Science Institute. Guardians assisted with food intake data for younger subjects, using the same methodology as the older participants. Data were then analyzed using the dietary analysis software, Nutrition Data System for Research (NDSR) versions 2010 and 2011, developed by the Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, Minnesota. Final calculations were completed using NDSR version 2011. NDSR is a dietary analysis program designed for the collection and analysis of 24-h dietary recalls and provides a detailed analysis of macronutrient and micronutrient intake in mean units (e.g., grams, milligrams, etc.) per day. Pre hoc criteria mandated that subjects were required to have completed two or more nonconsecutive 24-h food recalls available to be included in the analysis, as per the Institute of Medicine (IOM) recommendations for dietary intake analysis,39 and each recall had to be from a different season.
Nutrient intake data were assessed using dietary reference intakes (DRIs), which is an overall system of nutrient intake recommendations from the IOM of the U.S. National Academy of Sciences based on current scientific knowledge.40,41 The DRIs include recommended dietary allowances (RDA), the daily dietary intake level of nutrients considered sufficient by the IOM Food and Nutrition Board to meet the requirements of 97.5% of healthy individuals in each life-stage and sex group, and the adequate intake (AI) estimate, where no daily RDA has been established for the specific nutrient but the amount established is somewhat less firmly believed to be adequate for everyone in the demographic group. In the current study, micronutrient intake was compared to the RDA or the AI if no RDA existed for the nutrient. Individual recalls were assigned to an IOM life-stage group, based on the subject's gender and age on the day of the assessment. Due to collection of recalls taking place over time, subjects could have multiple life-stage groups and multiple RDAs (or AIs) for one micronutrient; therefore, the percentage of the RDA or AI was calculated for each recall for each nutrient and subsequently averaged for each subject. A total of 22 micronutrients were analyzed.
Mean macronutrient intake was compared to the Acceptable Macronutrient Distribution Ranges (AMDR).26 Total fat, saturated fat, trans fat, total cholesterol, and fiber intake were compared to the recommendations of the American Heart Association (AHA) for the general population (total fat intake: 25–35% of total calories; saturated fat: <7% of total calories; trans fats: <1% of total calories; total cholesterol: <300 mg per day; fiber: 14 g per 1,000 calories consumed per day). Normal lipoprotein profile levels were considered ≤200 mg/dl, ≤130 mg/dl, ≥40 mg/dl, and ≤140 mg/dl for total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG), respectively.2,28
Clinical assessments
Clinical measurements included weight, height, and waist and hip circumferences (with standardized measurements based on procedure recommendations from the Metabolic Study Group of the AIDS Clinical Trials Group). All HIV-infected subjects and controls (or guardians) completed questionnaires in order to obtain relevant demographic and medical information (including vitamins and supplements). An extensive chart review was conducted for the HIV-infected subjects, including detailed information on time of HIV diagnosis, past and current medical diagnoses, antiretroviral therapy (ART) use, and CD4 cell count nadir.
Laboratory assessments
Lipoprotein profiles were obtained after at least an 8-h fast. Current CD4 cell counts and HIV-1 RNA levels were obtained from the HIV-infected subjects.
Statistical analysis
Demographics, clinical characteristics, and laboratory parameters are described by HIV status. Continuous measures are described by means/standard deviations (SD), and nominal variables are described with frequencies/percentages. Variables that were not normally distributed were log-transformed and parametric tests were performed. If the variables were not normally distributed after log transformations, nonparametric tests were used for analysis.
Statistical tests used to make group comparisons and to compare variables to standards included Chi-squared for categorical variables, one-sample and independent t-tests for normally distributed means, and Mann–Whitney U test for nonnormally distributed means.
One-way analysis of variance (ANOVA) and Bonferroni post-hoc comparisons were used to determine differences in nutrient intakes between races. Pearson's correlation coefficient or Kendall's Tau were used to investigate associations between nutrient intake and variables of interest. Subanalyses were also completed based on race and sex.
The evaluation of nutrient intake in HIV-infected youth in this study was considered exploratory; therefore, no power analyses were determined. A p-value <0.05 was considered significant. All analyses were carried out using SPSS 18.0.
Results
Study population
Two hundred HIV-infected subjects (representing more than half of the patients in the clinic) and 50 healthy controls were enrolled. One hundred and seventy-five HIV-infected subjects and 43 controls completed two or more nutritional assessments and were included in the analysis. Subject characteristics are summarized in Table 1. Groups were matched for age, race, sex, body mass index (BMI), and waist-to-hip ratio. The prevalence of dyslipidemia was higher in the HIV-infected group compared to controls (TC=12% vs. 3%; LDL-C=8% vs. 3%; HDL-C=44% vs. 5%; and TG=9% vs. 1%). Mean HDL-C was significantly lower in the HIV-infected group and mean TG was significantly higher in the HIV-infected group. There were no significant differences between groups for TC and LDL-C.
Table 1.
Subject Characteristics
| Mean±SD or no. (%) | HIV+ (N=175) | Controls (N=43) | p-value |
|---|---|---|---|
| Age (years) | 17.44±4.79 | 17.26±6.14 | 0.688 |
| Race/ethnicity | |||
| Non-Hispanic, black | 166 (94.9%) | 39 (90.7%) | 0.183 |
| Non-Hispanic, white | 6 (3.4%) | 4 (9.3%) | |
| Hispanic, white | 3 (1.7%) | 0 (0%) | |
| Gender | |||
| Male | 94 (53.7%) | 24 (55.8%) | 0.804 |
| Female | 81 (46.3%) | 19 (44.2%) | |
| Body mass index (kg/m2) | 22.53±5.72 | 22.16±5.17 | 0.813 |
| Waist-to-hip ratio | 0.85±0.08 | 0.83±0.07 | 0.124 |
| Total cholesterol (mg/dl) | 155.29±38.929 | 150.67±31.471 | 0.473 |
| LDL-cholesterol (mg/dl) | 94.66±26.217 | 87.86±28.779 | 0.138 |
| HDL-cholesterol (mg/dl) | 44.1±15.923 | 51.16±13.009 | 0.001 |
| Triglycerides (mg/dl) | 84.35±53.8 | 58.56±23.757 | 0.001 |
| Currently on ART | 119 (68%) | — | |
| ART naive | 25 (14%) | — | |
| Perinatally infected | 113 (64%) | — | |
| Time from HIV diagnosis (years) | 10.9±10.4 | — | |
| CD4 cell count (cells/mm3) | 499±361 | — | |
| CD4 cell count % | 26.3±12.1 | — | |
| CD4 cell count nadir (cells/mm3) | 291±267 | — | |
| ΔCD4 (nadir-current) cell count (N=156) | 208±255 | — | |
| HIV-1 RNA <1,000 copies/ml | 101 (58%) | — | |
| Cumulative NRTI use (months) | 74.3±65.2 | — | |
| Cumulative PI use (months) | 53.3±54.5 | — |
LDL, low-density lipoprotein; HDL, high-density lipoprotein; ART, antiretroviral therapy; NRTI, nucleoside/nucleotide analogue reverse transcriptase inhibitor; PI, protease inhibitor.
Dietary intake, comparison to standards, and between-group differences
Recalls that were obvious outliers (<500 kcal or >5,000 kcal in one 24-h period) were excluded from the analysis. A total of 674 24-h recalls were analyzed. Five hundred and fifty-eight 24-h recalls were analyzed for the HIV-infected group (mean=3.2 recalls per subject): 75 subjects with four recalls (43%), 58 subjects with three recalls (33%), and 42 subjects with two recalls (24%). The control group had 113 recalls analyzed (mean=2.6 recalls per subject): 7 subjects had four recalls (16%), 13 subjects had three recalls (30%), and 23 subjects had two recalls (53%).
Mean (SD) intake was 1,954±611 and 1,990±541 kcal for the HIV-infected and control groups, respectively (p=0.723). For the HIV-infected group, the mean micronutrient intake was suboptimal for vitamin A, vitamin D, vitamin E, pantothenic acid, folate, calcium, magnesium, and potassium with a prevalence of suboptimal intake at 82%, 99%, 93%, 73%, 61%, 93%, 87%, and 100% of subjects, respectively (all p<0.001 compared to the RDA or AI) (Table 2). Although the controls also had suboptimal intake for six of these eight micronutrients, the HIV-infected subjects had a significantly lower intake compared to controls for all but vitamin D.
Table 2.
Micronutrients with Suboptimal Intake in the HIV-Infected Group
| HIV-infected (N=175) | Controls (N=43) | p-valuea | |
|---|---|---|---|
| Vitamin A | 78%±120 | 97%±75 | 0.003 |
| Vitamin D | 27%±21 | 36%±32 | 0.067 |
| Vitamin E | 54%±34 | 65%±28 | 0.009 |
| Pantothenic acid | 88%±38 | 106%±55 | 0.009 |
| Folate | 97%±48 | 114%±54 | 0.041 |
| Calcium | 58%±27 | 70%±31 | 0.013 |
| Magnesium | 64%±30 | 80%±40 | 0.003 |
| Potassium | 40%±14 | 45%±13 | 0.021 |
p-values represent differences between HIV-infected and control groups; bold type indicates statistically lower intake for the HIV-infected group compared to the controls.
Suboptimal intake is defined as daily intake below the recommended amount with p<0.001. Values for each micronutrient are expressed as the mean percentage (±standard deviation) of daily recommended intake. All micronutrients are compared with the Institute of Medicine (IOM) recommended dietary allowance (RDA). Pantothenic acid is compared to the IOM Adequate Intake (AI) guideline.
Both HIV-infected subjects and controls had 236% and 247%, respectively, greater than the recommended RDA for sodium (both p<0.001) with 99% of HIV-infected subjects consuming greater than the recommended amount.
Vitamin K, C, thiamin, riboflavin, niacin, B6, B12, phosphorus, manganese, iron, zinc, copper, and selenium were consumed in sufficient amounts for both groups. Nonetheless, the HIV-infected subjects had a significantly lower mean percentage RDA compared to controls for vitamin K (–37.6%, p=0.038), vitamin C (–42.6%, p=0.003), thiamin (–25.45%, p=0.029), riboflavin (–42%, p=0.002), copper (–18.2%, p=0.048), phosphorus (–20%, p=0.033), and manganese (–18.1%, p=0.044).
While mean percentage of calories from carbohydrate and protein fell within the AMDR for both groups, total fat intake significantly exceeded the AMDR only for the HIV-infected group (p=0.018) (Fig. 1). Compared to AHA recommendations, both groups had significantly less fiber intake and a greater percentage of calories derived from saturated fat and trans fat, but normal total cholesterol intake. The HIV-infected group had a significantly higher percentage of kilocalories from total fat and trans fat and a significantly lower percentage kilocalories from carbohydrates (–2.9%, p=0.014) compared to the control group, but no difference in protein intake (p=0.177).
FIG. 1.
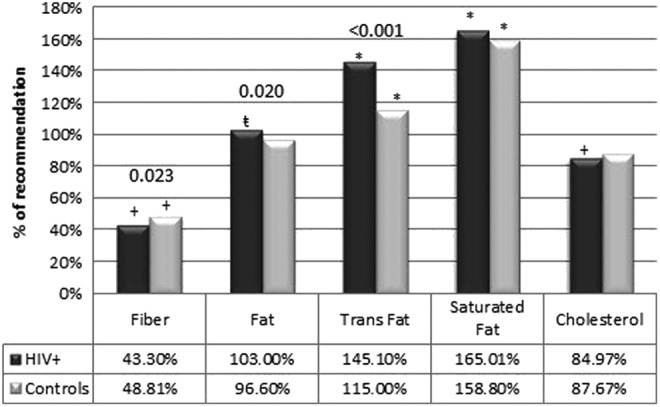
Intake compared to AHA recommendations. This figure shows the percentage intake of cholesterol, fat, and fiber compared to AHA recommendations (saturated fat <7% of kcal; trans fat <1% of kcal; cholesterol <300 mg/day; fiber >14 g/1,000 kcal). Both groups had significantly less fiber intake and a greater percentage of calories derived from saturated and trans fat, but only the HIV-infected group exceeded total fat intake recommendations. Both groups had optimal total cholesterol intake. p-values above the bars show significant differences between the HIV-infected and control groups. There was a significantly higher intake for total and trans fat and a lower intake of fiber in the HIV-infected group compared to controls. Symbols above the bar denote a significant difference from AHA recommendations (*statistically above p<0.001; t-statistically above p<0.05; +statistically below p<0.001). AHA, American Heart Association.
Within the HIV-infected group, females had significantly lower calorie intake (–387 kcal, p<0.001) and mean percent RDA or AI for folate (–0.184%, p=0.004), pantothenic acid (–0.182%, p=0.000), vitamin D (–0.09%, p=0.002), calcium (–0.17%, p<0.001), and potassium (–0.085%, p<0.001). HIV-infected African Americans had a lower percent RDA intake of vitamin D (–26.2%, p=0.027) and calcium intake (–31.2%, p=0.017) compared to HIV-infected whites. HIV-infected Hispanics had a significantly lower percentage of total fat intake from calories compared to HIV-infected African Americans (–10.8%, p=0.003) and HIV-infected whites (–10.1%, p=0.031).
Associations between nutrient intake and plasma lipid levels
Within the HIV-infected group, there was a positive correlation between trans fat intake and plasma LDL-C (r=0.158, p=0.040) as well as between fiber intake and plasma TG (r=0.157, p=0.041). There was a negative correlation between total fat intake and plasma TG (r=−0.170, p=0.027). In the control group, there was a positive correlation between TC intake and plasma TG levels (r=0.315, p=0.040).
Associations between nutrient intake and HIV-related factors
Within the HIV-infected group, calorie intake was negatively correlated with current CD4 cell count and nadir CD4 cell count (Table 3). The percentage RDA intake for vitamin A was positively associated with nadir CD4 cell count. The percentage of the RDA intake for zinc, magnesium, and riboflavin was positively associated with the current CD4 cell count.
Table 3.
Associations Between Nutrient Intake and HIV-Related Factors
| Current CD4 | CD4 nadir | HIV-1 RNA | ||||
|---|---|---|---|---|---|---|
| ra | p | r | p | r | p | |
| All HIV-infected subjects (N=175) | ||||||
| Calories | −0.116 | 0.024 | −0.105 | 0.041 | — | — |
| Vitamin A | — | — | 0.102 | 0.047 | — | — |
| Zinc | 0.128 | 0.013 | — | — | — | — |
| Riboflavin | 0.120 | 0.019 | — | — | — | — |
| Magnesium | 0.141 | 0.006 | — | — | — | — |
| HIV-infected subjects on ART (N=119) | ||||||
| Riboflavin | — | — | 0.129 | 0.033 | — | — |
| Vitamin B12 | — | — | 0.122 | 0.044a | — | — |
| Magnesium | 0.140 | 0.20 | — | — | — | — |
| HIV-infected subjects not on ART (N=56) | ||||||
| Protein | — | — | — | — | −0.296 | 0.046 |
r=Pearson's correlation coefficient.
No significant correlations were found between nutrients and those variables without numbers reported in the table or for those categories not listed in the table.
Among HIV-infected subjects currently on ART, riboflavin and B12 intake was positively associated with CD4 cell count nadir. The current CD4 cell count was positively associated with magnesium intake. For subjects on ART for >6 months with an HIV-1 RNA <1,000 copies/ml, there were no significant associations between nutrient intake and change in CD4 cell count after starting ART (ΔCD4=current-nadir CD4).
There were no significant correlations with CD4 cell counts among those subjects currently not on ART. However, HIV-1 RNA was negatively correlated with mean daily protein intake (r=−0.296, p=0.046).
Discussion
This study showed that the nutrient intake in HIV-infected youth did not meet dietary intake guidelines for a number of critical micronutrients, including vitamins A, D, E, pantothenic acid, folate, calcium, magnesium, potassium, and sodium. Vitamin D, calcium, potassium, and vitamin E were consumed in the lowest amounts compared to the RDA or AI for these nutrients, while sodium intake was exceedingly excessive.
HIV-infected youth also had poorer micronutrient and macronutrient intake compared to healthy controls despite similarities in caloric intake. Proper nutrient intake is vital for optimal health in the HIV-infected population, as many micronutrients have been associated with diseases known to be increased in this population. For example, HIV-infected individuals are at an increased risk of CVD.2,22,30,31,42,43 Low serum 25-hydroxyvitamin D concentrations are associated with CVD risk factors in children and adults in both the HIV-infected and HIV-uninfected populations.42–45 Furthermore, consuming the recommended amounts of potassium and sodium can help regulate blood pressure, another important factor in reducing CVD risk.26
Importantly, in our cohort of HIV-infected youth, we did observe a greater prevalence of dyslipidemia and a significantly higher mean TG and significantly lower HDL-C than healthy controls. Moreover, the HIV-infected youth in this study had a percentage total fat, trans fat, and saturated fat intake from kilocalories that exceeded recommendations, and fiber intake was less than recommendations in all but one subject. Fat and fiber intake aberrations have been associated with CVD risk in the general adult population. For example, limiting the dietary intake of saturated and trans fat is associated with a lower risk of CVD, mostly due to its positive effects on LDL-C.26 Increased dietary fiber, both insoluble and soluble, has been linked to a lower CVD risk and decreased progression to CVD in high-risk adults.26
Adult HIV studies have also shown associations between trans fat intake and serum TG levels,24 as well as between percentage calories derived from dietary fat and serum TC and TG.46 Despite these findings previously found in adults, there were few meaningful associations between nutrient intake and lipid profiles in our cohort of HIV-infected youth. These associations have not been previously studied in HIV-infected youth and, thus, nutrient intake may not have the same relationship to serum or plasma lipid levels as found in adults. Alternatively, inadequate power and/or confounding factors may have played a role. These relationships should be analyzed more systematically in future larger studies, given the high prevalence of dyslipidemia and high fat intake in this population.
Studies indicate that micronutrient status may contribute to immune function and clinical outcomes in HIV-infected individuals.7,8,10–15,47–49 For instance, Baum and colleagues found that as serum vitamin A, B12, and zinc status improved, CD4 cell counts increased in HIV-infected adult males.12 This association between micronutrient sufficiency and HIV disease status has been repeated in other studies,5,12,14,15 despite some conflicting data that investigated micronutrient intake.38 Notably, Steenkamp et al. showed that ART-naive children with abnormally low serum levels of zinc and vitamin A had significantly lower CD4 cell counts and higher HIV-1 RNA levels.5 Decreased serum 25-hydroxyvitamin D concentrations have also been associated with increased mortality and HIV disease progression among several HIV-infected populations, including children born to HIV-infected woman with vitamin D deficiency.48,49 Additionally, research has demonstrated links between the serum status of selenium, an important antioxidant, and HIV-related outcomes such as the incidence of diarrhea and hospitalization, HIV-1 RNA level, and CD4 cell counts.50 Similarly, vitamin E is important for immune function and is a potent antioxidant, which may be particularly important in HIV-infected individuals who have increased oxidative stress.6,38,51
In our current study, we found some notable correlations between nutrient intake and variables used to assess HIV disease status. Vitamin A intake was positively correlated with nadir CD4 cell count, and zinc, riboflavin, and magnesium intake were positively associated with current CD4 cell count. In subjects not on ART and with uncontrolled viremia, HIV-1 RNA was also negatively correlated with dietary protein intake. Finally, total intake of kilocalories was negatively correlated with current and nadir CD4 cell counts, which may suggest that individuals with more advanced HIV disease (i.e., with a lower CD4 count and lower CD4 nadir) require a higher amount of calories compared to individuals with higher CD4 counts. Previous studies evaluating caloric intake did not find any associations between CD4 category and progression to AIDS15 or mortality.10 However, these previous adult studies used semiquantitative food frequency questionnaires instead of the IOM's recommendation to use at least two, 24-h recalls on nonconsecutive days to assess usual intake,39 and they were also done in the pre-HAART era. Thus, further investigation is warranted to determine total kilocalorie and protein needs at various clinical and virological stages of disease in HIV-infected youth. In HIV-infected adults, medical nutrition therapy has been shown to improve immune status, progression of HIV, and risk of mortality.21
There were several limitations to our study including a relatively small control group and a wide age range among our subject population. In addition, while the IOM recommends using 24-h recalls to determine nutrient intake,39 this technique has drawbacks. Due to their recall nature, there was likely overreporting or underreporting of food intake. We tried to account for this by omitting recalls that were clear outliers. Regardless, 24-h dietary recalls still remain the best method for adequately assessing diet and making comparisons to DRIs.39 Similarly, we are assuming that dietary intake is correlated with serum levels, and this may not be the case. However, in studies investigating HIV-infected adults, correlations were found between serum concentrations and dietary intake of B12,14 vitamin A, and zinc.12 Additional studies are needed to determine whether nutrient intake data correlate with serum nutrient concentrations in our current study population and whether the serum nutrient status has an impact on immune status and HIV progression in HIV-infected youth.
Our study was composed of 95% African Americans, which limits our ability to generalize the results. However, to our knowledge, our data represent the largest such assessment of nutrient intake in HIV-infected African American youth. Similarly, the HIV-infected subjects comprised a relatively heterogeneous group in terms of ARV status and a wide range of ages. Due to the large number of variables that were tested in univariate fashion, there was a risk of Type I errors. Similarly, some differences observed between the HIV-infected group and controls for various nutrients may have been statistically significant, but may not be clinically relevant. Finally, the study did not investigate the potential causes of the observed differences in nutrient intake between the HIV-infected group and the controls. However, these exploratory data provide a substrate from which to design future trials.
This study offers insight into the diet of this vulnerable HIV-infected population and possible associations with HIV-related variables and chronic complication risk. These results are novel as few studies have evaluated nutrient intake in HIV-infected youth, and none has used 24-h food intake recall data compared to the current micronutrient and macronutrient RDAs, which are the gold standards according to the World Health Organization and IOM.9,39 Diet is a modifiable factor, and providing nutrition counseling early on in the disease process is likely to optimize health and improve long-term outcomes. Longitudinal, randomized, placebo-controlled trials are also needed not only to determine the impact of nutrition counseling on the nutrient status of HIV-infected youth, but to better define their actual intake requirements necessary to attenuate the risk of chronic complications, such as CVD, and minimize disease progression.
Acknowledgments
The study was supported by research grants to Dr. Eckard from GlaxoSmithKline, Emory-Egleston Children's Research Center, Emory's Center for AIDS Research (P30AI050409), to Dr. Eckard from NICHD (K23HD069199), to Dr. Ziegler from NIDDK (K24DK096574), to Dr. McComsey from NICHD (R01HD070490), and the Atlanta Clinical and Translational Science Institute (UL1RR025008). The funding agencies have no access to the raw data and no role in the analysis or writing of this article.
Data in part were presented previously at the 14th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV, Washington, DC, July 2012, and published as an abstract as Stricker LA, Ndurangu M, Nucci A, Ziegler TR, Tangpricha V, McComsey GA, Frediani JK, Millson EC, Seaton L, and Ross (Eckard) AC: Habitual nutrient intake in HIV-infected youth and associations with HIV-related factors. Antiviral Ther 2012;17(Suppl 2):A44.
Author Disclosure Statement
A.R.E. has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline, and has served as an advisor for Gilead. G.A.M. serves as a consultant and speaker and has received research funding from Bristol-Myers Squibb, GlaxoSmithKline, Gilead, and Tibotec. G.A.M. currently chairs a DSMB for a Pfizer-funded study.
References
- 1.Centers for Disease Control and Prevention: Estimated HIV incidence in the United States, 2007–2010. HIV Surveill Suppl Rep 2012;17(No. 4). www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental Published December2012. Accessed June20, 2014 [Google Scholar]
- 2.Tremeschin M, Sartorelli D, Cervi M, Negrini B, Salomao R, and Monteiro J: Nutritional assessment and lipid profile in HIV-infected children and adolescents treated with highly active antiretroviral therapy. Rev Soc Brasil Med Trop 2011;44(3):274–281 [DOI] [PubMed] [Google Scholar]
- 3.Chao D, Rutstein R, Steenhoff A, Shanbhag M, Andolaro K, and Zaoutis T: Two cases of hypocalcemia secondary to vitamin D deficiency in an urban HIV-positive pediatric population. AIDS 2003;17(16):2401–2403 [DOI] [PubMed] [Google Scholar]
- 4.Fawzi W: Micronutrients and HIV type 1 disease progression among adults and children. Clin Infect Dis 2003;37:112–116 [DOI] [PubMed] [Google Scholar]
- 5.Steenkamp L, Dannhauser A, Walsh D, Joubert G, Veldman FJ, Van der Walt E, Cox C, Hendricks MK, and Dippenaar H: Nutritional, immune, micronutrient and health status of HIV-infected children in care centres in Mangaung. S Afr J Clin Nutr 2009;22(3):131–136 [Google Scholar]
- 6.Stephensen C, Marquis G, Jacob R, Kruzich L, Douglas S, and Wilson C: Vitamin C and E in adolescents and young adults with HIV infection. Am J Clin Nutr 2006;83:870–879 [DOI] [PubMed] [Google Scholar]
- 7.Pee S. and Semba R: Role of nutrition in HIV infection: Review of evidence for more effective programming in resource-limited settings. Food Nutr Bull 2010;31(4):313–344 [PubMed] [Google Scholar]
- 8.Baum MK Shor-Posner G, Lai S, Zhang G, Lai H, Sauberlich H, and Page BJ: High risk of HIV-related mortality is associated with selenium deficiency. JAIDS 1997;15(5):370–374 [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization: Nutrient requirements for people living with HIV/AIDS. World Health Organization, Geneva, 2003 [Google Scholar]
- 10.Tang A, Graham N, and Saah A: Effects of micronutrient intake on survival in human immunodeficiency virus type 1 infection. Am J Epidemiol 1996;143(12):1244–1256 [DOI] [PubMed] [Google Scholar]
- 11.Baeten JM, McClelland RS, Wener MH, Bankson DD, Lavreys L, Mandaliya K, Bwayo JJ, and Kreiss JK: Relationship between markers of HIV-1 disease progression and serum beta-carotene concentrations in Kenyan women. Int J STD AIDS 2007;18:202–206 [DOI] [PubMed] [Google Scholar]
- 12.Baum MK, Shor-Posner G, Lu Y, Rosner B, Sauberlich HE, Fletcher MA, Szapocznik J, Eisdorfer C, Buring JE, and Hennekens C: Micronutrients and HIV-1 disease progression. AIDS 1995;9:1051–1056 [DOI] [PubMed] [Google Scholar]
- 13.Tang AM, Graham N, Semba RD, Saah AJ: Association between serum vitamin A and E levels and HIV-1 disease progression. AIDS 1997;11:613–620 [DOI] [PubMed] [Google Scholar]
- 14.Tang AM, Graham N, Chandra RK, and Saah AJ: Low serum vitamin B-12 concentrations are associated with faster human immunodeficiency virus type 1 (HIV-1) disease progression. J Nutr 1997;127:345–351 [DOI] [PubMed] [Google Scholar]
- 15.Tang AM, Graham N, Kirby AJ, McCall DL, Willett W, and Saah AJ: Dietary micronutrient intake and risk of progression to acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus type 1 (HIV-1)-infected homosexual men. Am J Epidemiol 1993;138(11):937–951 [DOI] [PubMed] [Google Scholar]
- 16.Johann-Liang R, O'Neill L, Cervia J, Haller I, Giunta Y, Licholai T, and Noel G: Energy balance, viral burden, insulin-like growth factor-1, interleukin-6 and growth impairment in children infected with human immunodeficiency virus. AIDS 2000;14:683–690 [DOI] [PubMed] [Google Scholar]
- 17.Henderson R, Talusan K, Hutton N, Yolken R, and Caballero B: Serum and plasma markers of nutritional status in children infected with the human immunodeficiency virus. J Am Diet Assoc 1997;97(12):1377–1381 [DOI] [PubMed] [Google Scholar]
- 18.Dube M, Stein J, Aberg J, Fichtenbaum C, Gerber J, Tashima K, Henry W, Currier J, Sprecher D, and Glesby M: Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: Recommendations of the HIV medicine association of the infectious disease society of America and the adult AIDS clinical trials group. Clin Infect Dis 2003;37:613–627 [DOI] [PubMed] [Google Scholar]
- 19.Rhoads M, Lanigan J, Smith C, and Lyall E: Effect of specific ART drugs on lipid changes and the need for lipid management in children with HIV. J Acquir Immune Defic Syndr 2011;57(5):404–412 [DOI] [PubMed] [Google Scholar]
- 20.Baum MK, Shor-Posner G, Bonvehi P, Cassetti I, Lu Y, Mantero-Atienza E, Beach RS, and Sauberlich HE: Influence of HIV-infection on vitamin status and requirements. Ann NY Acad Sci 1992;669:165–173 [DOI] [PubMed] [Google Scholar]
- 21.Suttajit M: Advances in nutrition support for quality of life in HIV +/AIDS. Asia Pac J Clin Nutr 2007;16:318–322 [PubMed] [Google Scholar]
- 22.Charakida M, Donald AE, Green H, Storry C, Clapson M, Caslake M, Dunn DT, Halcox JP, Gibb DM, Klein NJ, and Deanfield JE: Early structural and functional changes of the vasculature in HIV-infected children: Impact of disease and antiretroviral therapy. Circulation 2005;112(1):103–109 [DOI] [PubMed] [Google Scholar]
- 23.Banerjee T, Pensi T, Banerjee D, and Grover G: Impact of HAART on survival, weight gain, and resting energy expenditure in HIV-1-infected children in India. Ann Trop Paediatr 2010;30:27–37 [DOI] [PubMed] [Google Scholar]
- 24.Shah M, Tierney K, Adams-Huet B, et al. : The role of diet, exercise and smoking in dyslipidaemia in HIV-infected patients with lipodystrophy. HIV Med 2005;6:291–298 [DOI] [PubMed] [Google Scholar]
- 25.Triant VA, Lee H, Hadigan C, and Grinspoon S: Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92(7):2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtenstein A, Appel L, Brands M, et al. : Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association nutrition committee. J Am Heart Assoc 2006;114:82–96 [DOI] [PubMed] [Google Scholar]
- 27.Capili B. and Anastasi J: HIV and hyperlipidemia: Current recommendations and treatment. Medsurg Nurs 2006;15:14–20 [PubMed] [Google Scholar]
- 28.National Institute of Health: National Cholesterol Education Program. ATP III Guidelines At-A-Glance Quick Desk Reference. Bethesda, MD: National Heart, Lung, and Blood Institute, 2001 [Google Scholar]
- 29.Kruzich L, Marquis G, Wilson C, and Stephensen C: HIV-infected US youth are at high risk of obesity and poor diet quality: A challenge for improving short- and long-term health outcomes. J Am Diet Assoc 2004;104(10):1554–1560 [DOI] [PubMed] [Google Scholar]
- 30.Miller T, Grant Y, Almeida D, Sharma T, and Lipshultz S: Cardiometabolic disease in human immunodeficiency virus-infected children. J Cardiometab Syndr 2008;3:98–105 [DOI] [PubMed] [Google Scholar]
- 31.Taylor P, Worrell C, Steinberg S, et al. : Natural history of lipid abnormalities and fat redistribution among human immunodeficiency virus-infected children receiving long-term, protease inhibitor-containing, highly active antiretroviral therapy regimens. Pediatrics 2004;114 (2):e235–242 [DOI] [PubMed] [Google Scholar]
- 32.Vigano A, Bedogni G, Zuccotti G, et al. : Both HIV-infection and long-term antiretroviral therapy are associated with increased common carotid intima-media thickness in HIV-infected adolescents and young adults. Curr HIV Res 2010;8(5):411–417 [DOI] [PubMed] [Google Scholar]
- 33.Position of the American Dietetic Association: Nutrition intervention and human immunodeficiency virus infection. J Am Diet Assoc 2010;110:1105–1119 [DOI] [PubMed] [Google Scholar]
- 34.Jahoor F, Abramson S, and Heird W: The protein metabolic response to HIV infection in young children. Am J Clin Nutr 2003;78:182–189 [DOI] [PubMed] [Google Scholar]
- 35.American Dietetic Association: HIV/AIDS macronutrient composition. Internet: www.adaevidencelibrary.com/template.cfm?template=guide_summary&key=2821
- 36.Sharma T, Kinnamon D, Duggan C, Weinberg G, Furuta L, Bechard L, Nicchitta J, Gorbach S, and Miller T: Changes in macronutrient intake among HIV-infected children between 1995 and 2004. Am J Clin Nutr 2008;88:384–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuin G, Comi D, Fontana M, Tornaghi R, Brugnani M, Fadini S, and Principi N: Energy and nutrient intakes in HIV-infected children. Pediat AIDS HIV Infect 1994;5(3):159–161 [Google Scholar]
- 38.Kruzich L, Marquis G, Carriquiry A, Wilson C, and Stephensen C: US youths in the early stages of HIV disease have low intakes of some micronutrients important for optimal immune function. J Am Diet Assoc 2004;104(7):1095–1101 [DOI] [PubMed] [Google Scholar]
- 39.Institute of Medicine: Dietary Reference Intakes: Applications in Dietary Assessment/Subcommittee on Interpretation and Uses of Dietary Reference Intakes and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes [e-book]. National Academy Press, Washington, DC, 2000 [Google Scholar]
- 40.Otten JJ, Hellwig JP, and Meyers LD: Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. The National Academies Press, Washington, DC, 2006 [Google Scholar]
- 41.Institute of Medicine: Dietary Reference Intakes: Recommended Intakes for Individuals: 2010. Internet: http://fnic.nal.usda.gov/dietary-guidance/dietary-reference-intakes/dri-tables Accessed October24, 2011
- 42.Ross AC, Judd S, Kumari M, Hileman C, Storer N, Labbato D, et al.: Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antivir Ther 2011;16:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi AI, Lo JC, Mulligan K, Schnell A, Kalapus SC, Li Y, et al.: Association of vitamin D insufficiency with carotid intima-media thickness in HIV-infected persons. Clin Infect Dis 2011;52:941–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganji V, Zhang X, Shaikh N, and Tangpricha V: Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001–2006. Am J Clin Nutr 2011;94:225–233 [DOI] [PubMed] [Google Scholar]
- 45.Ross AC, Judd S, Ziegler TR, Camacho-Gonzalez AF, Fitzpatrick AM, Hadley GR, Grossmann RF, et al. : Risk factors for vitamin D deficiency and relationship with cardiac biomarkers, inflammation, and immune restoration in HIV-infected youth. Antivir Ther 2012;17(6):1069–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrios A, Blanco F, Garcia-Benayas T, Gomez-Viera J, de la Cruz J, Soriano V, and Gonzalez J: Effect of dietary intervention on highly active antiretroviral therapy-related dyslipidemia. AIDS 2002;16(15):2079–2081 [DOI] [PubMed] [Google Scholar]
- 47.Ross AC. and McComsey GA: The role of vitamin D deficiency in the pathogenesis of osteoporosis and in the modulation of the immune system in HIV-infected patients. Clinic Rev Bone Miner Metab 2012;10(4):277–278 [Google Scholar]
- 48.Mehta S, Giovannucci E, Mugusi FM, Spiegelman D, Aboud S, Hertzmark E, Msamanga GI, Hunter D, and Fawzi WW: Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One 2010;5(1):e8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viard JP, Souberbielle JC, Kirk O, Reekie J, Knysz B, Losso M, Gatell J, Pedersen C, Bogner JR, Lundgren JD, et al. : Vitamin D and clinical disease progression in HIV infection: Results from the EuroSIDA study. AIDS 2011;25(10):1305–1315 [DOI] [PubMed] [Google Scholar]
- 50.Stone CA, Kawai K, Kupka R, and Fawzi WW: Role of selenium in HIV infection. Nutr Rev 2010;68(11):671–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McComsey GA. and Morrow JD: Lipid oxidative markers are significantly increased in lipoatrophy, but not in sustained asymptomatic hyperlactatemia. J Acquir Immune Defic Syndr 2003;34:45–49 [DOI] [PubMed] [Google Scholar]


