Abstract
Purpose: Based on the hypothesis that oral nutraceuticals do not adequately reach all ocular tissues in the anterior segment, we evaluated the ability of a 3% concentration of the ingredients in a topical nutraceutical antioxidant formulation called Optixcare Eye Health (Optixcare EH) to ameliorate oxidative stress in rat models of age-related ocular diseases.
Methods: Diabetes was induced by tail-vein injection of streptozotocin, and the development of cataracts was monitored by slit lamp. Young rats were exposed to ultraviolet (UV) light, and the reduction in lens glutathione (GSH) levels and increase in 4-hydroxynonenol (4-HNE) were measured. Oxidative stress in the neural retina was generated by exposure of dark-adapted rats to 1,000 lx of light, and oxidative stress markers were measured. Dry eye was induced in rats by twice daily (b.i.d.) subcutaneous scopolamine injections. Topical Optixcare EH was administered b.i.d. and compared in select experiments to the multifunctional antioxidant JHX-4, the topical aldose reductase inhibitor (ARI) Kinostat™, oral Ocu-GLO™, and the topical ocular comfort agents Optixcare Eye Lube, Optixcare Eye Lube + Hyaluron, and Idrop Vet Plus hyaluronic acid.
Results: In diabetic rats, topical ARI treatment prevented cataract formation while the nutraceuticals delayed their development with Optixcare EH>Ocu-GLO. In UV-exposed rats, the reduction of GSH and increase in 4-HNE in the lens were normalized in order JHX-4>Optixcare EH>Ocu-GLO. In the retina, oxidative stress markers were reduced better by oral JHX-4 compared with topical Optixcare EH. In the scopolamine-induced dry-eye rats, tear flow was maintained by Optixcare EH treatment, while none of the comfort agents examined altered tear flow.
Conclusions: Topical administration of a 3% concentration of the ingredients in Optixcare EH reduces experimentally induced reactive oxygen species in rats exposed to several sources of ocular oxidative stress. In addition, Optixcare EH maintains tear volume in scopolamine-induced dry eye. This suggests that in the anterior segment, the ingredients in Optixcare EH may have clinical potential against ocular oxidative stress.
Introduction
Population aging is rapidly occurring in many parts of the industrialized world. In the United States, the population of those aged 65 years or older is expected to increase from 40.2 million in 2010 to 88.5 million in 2050.1 Accompanying this increase in population aging is the prevalence of age-related ocular diseases that include cataracts, macular degeneration (AMD), and keratoconjunctivitis sicca, also known as dry eye disease (DED).2 Cataracts account for the majority of blindness in the world today, while AMD is the leading cause of visual impairment and irreversible blindness in industrialized countries. DED, which affects the majority of people over the age of 65 years, results in significant ocular discomfort and visual dysfunction. While DED generally does not result in vision loss, superficial keratitis, if left untreated, can lead to corneal ulcers or scars, and result in pain.
At present, there is no established pharmaceutical treatment for cataracts, and vision in patients with cataracts can only be restored by surgery. Cataract surgery has become one of the most frequently performed procedures in ophthalmology, accounting for $6.8 billion of the $16.2 billion spent in direct medical costs in 2006 on visual disorders among US adults aged 40 years or older.3 Delaying the onset and progression of cataracts through pharmaceutical intervention by 10 years has been estimated to reduce the need for cataract surgery by 50%.4 The need for the development of a nonsurgical treatment for delaying cataract is supported by the recent finding that cataract surgery increases the incidence of late AMD.5 Similarly, at present, there is no medical treatment for preventing the onset or reducing the enlargement of geographic atrophy (dry AMD).
The pathogeneses of cataracts and AMD have not been elucidated. Both are multifactorial diseases where increased oxidative damage, accompanied by increased levels of iron, influences their development and progression.6–8 Risk factors for both cataracts and AMD include smoking, which is associated with both the generation of reactive oxygen species (ROS) and increased tissue iron levels.9–12 Cataracts are also associated with increased iron levels and iron disorders such as hyperferritinemia.13–16 ROS generated in the presence of iron results in hydroxyl radical formation through the Fenton reaction and mitochondrial dysfunction.17 Dysregulation of iron metabolism and iron handling proteins are also linked to the pathogenesis of AMD18 with increased chelatable iron observed in the retinal pigmented epithelial cells (RPE) and Bruchs membrane in retinas from AMD patients.19 Iron overload is associated with a maculopathy that clinically resembles AMD, including the development and progression of multiple subretinal yellowish-white lesions and RPE cell atrophy in patients with aceruloplasminemia.20 Smoking also increases the risk of dry-eye syndrome and exacerbates existing dry-eye conditions.21,22 Dry eye is a multifactorial disease involving oxidative stress whose pathogenesis has also not been clearly defined.23
Targeting oxidative pathways associated with these age-related diseases suggests therapeutic potential. For example, the clinical administration of a combined formulation of antioxidants and omega-3 essential fatty acids significantly improves the subjective symptoms of dry eye,24 while topical administration of the antioxidant and chelator epigallocatechin gallate reduces the clinical signs and inflammatory changes of DED in murine dry eye.25 Similarly, systemic administration of the iron chelator deferiprone protects against light-induced retinal degeneration and diminishes oxidative stress in the mouse retina.26 Melatonin, a neurohormone with free radical scavenger activity, protects cultured human RPE against hydrogen peroxide-induced cell death.27 The AREDS antioxidant formulation reduces the risk of progression to advanced AMD by 25%.28–30 Several large clinical studies have also examined whether antioxidants can clinically reduce cataract progression. However, these studies suggest that any effect of antioxidants on cataract development is likely to be very small and probably is of no clinical or public-health significance.31,32
ROS is also reduced by lowering tissue levels of iron, which can generate reactive hydroxyl radicals through the Fenton reaction. Studies with the deferoxamine suggest that the use of chelating agents to reduce lenticular ROS may be beneficial because patients with β-thalassemia receiving more chelation therapy had a lower incidence of cataracts.33 Similarly, in rats, cataracts from tobacco smoke exposure were decreased by deferoxamine treatment.10 Kador has synthesized novel multifunctional antioxidants with the ability to scavenge free radicals and chelate iron independently, and demonstrated that by oral administration, they both delay cataract formation and protect the retina against light damage, a model for AMD.34–36
Based on the hypothesis that oral administration of antioxidants generally fail to achieve adequate levels of antioxidants to the lens, a topical nutraceutical suspension called Optixcare Eye Health (Optixcare EH) is being developed. Composed of 4 unique antioxidants that are designed to reduce oxidative stress through free radical scavenging and chelating activity, these nutraceuticals are topically delivered in a viscous proprietary carbomer vehicle with established topical adhesion properties that have been shown to enhance the ocular uptake of select drugs. Using rat models associated with cataract formation, light-induced retinal degeneration, and scopolamine-induced dry eye, we demonstrate that the topical administration of a 3% suspension of the nutraceutical components in this formulation has therapeutic potential.
Methods
All reagents employed were of reagent grade. Optixcare Eye Lube (Optixcare) and Optixcare Eye Lube + Hyaluron (Optixcare + Hyaluron) were obtained from Aventix (Burlington, ON, Canada). The topical nutraceutical referred to here as Optixcare EH was prepared by blending 3% w/w of each of the 4 unique chemical grade antioxidants in Optixcare EH with Optixcare Eye Lube. Idrop Vet Plus was purchased from imed Pharma (Dollard-des-Ormeaux, QC, Canada), and the oral antioxidant formulation Ocu-GLO™ was purchased from Animal Health Quest (Bellingham, WA). The aldose reductase inhibitor Kinostat™ was obtained from Therapeutic Vision, Inc. (Omaha, NE). The multifunctional antioxidant JHX-4 (4-(5-hydroxypyrimidin-2-yl)-N,N-dimethyl-3,5-dioxopiperazine-1-sulfonamide (99% pure by HPLC)) was synthesized as previously described.36 Topical JHX-4 was prepared by suspending 3% (w/w) of compound in Optixcare Eye Lube. RIPA buffer was purchased from Cell Signaling Technology (Danvers, MA), and Halt Protease and Phosphatase Inhibitor Cocktail was obtained from VWR International (West Chester, PA). Antibodies against thioredoxin (TRx) were purchased from Cell Signaling Technology, and antibodies against thioredoxin reductase (TRxR) were purchased from Abcam, Inc. (Cambridge, MA). Chemiluminescent reagent and peroxide, biotinylated protein ladder, and prestained protein markers were obtained from Cell Signaling Technology. Polyacrylamide Ready Gels (4%–15% Tris-HCl) and Trans-BlotÒ nitrocellulose membrane were purchased from Bio-Rad Laboratories (Hercules, CA). OxiSelect™ HNE-His Adduct enzyme-linked immunosorbent assay (ELISA) kit, OxiSelect™ Nitrotyrosine ELISA kit, and the OxiSelect™ HNE Adduct Competitive ELISA Assay were purchased from Cell Biolabs, Inc. (San Diego, CA). Zone-Quick™ Phenol Red Threads were purchased from FCI Ophthalmics (Pembroke, MA).
Animals
All procedures involving live animals were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Sprague Dawley rats were purchased from Sasco (Wilmington, MA); Wistar rats and Lewis rats were obtained from Charles River Laboratories (Wilmington, MA).
Drug administration
The topicals Optixcare Eye Lube, Optixcare EyeLube + Hyaluron, Optixcare EH, Idrop Vet Plus, Kinostat™, and topical JHX-4 were administered twice daily (b.i.d) to each eye at approximately 8 am and 5 pm. The contents in the capsules of Ocu-GLO were dissolved in an appropriate amount of Tween 80 so that administration of 1 mL of the mixture delivered the manufacturer's suggested amount of nutraceutical formulation per weight of the animal. This 1 mL amount was administered daily by gavage at approximately 9 am. Oral JHX-4 was administered as 0.05% (w/w) in standard rat chow as previously described.34,35
Diabetic cataracts
Diabetes was induced in 24 young (100 g) Sprague Dawley rats by tail-vein injection of 75 mg/kg of streptozotocin as previously described.34 All rats with blood glucose levels >300 mg/dL were then equally divided into 4 groups of 6 rats each. The first group served as untreated controls. while the second group received 1 drop of the topical aldose reductase inhibitor Kinostat to each eye b.i.d., the third group topically received 1 drop of the nutraceutical Optixcare EH to each eye b.i.d., and the fourth group received 1 mL of Tween 80 Ocu-GLO solution once daily orally as described above. Lens changes were monitored weekly by hand-held slit lamp following dilation of each eye with 1% tropicamide ophthalmic solution, USP (Falcon Pharmaceuticals Ltd., Fort Worth, TX). Lens changes were evaluated on a scale of 0 to 3, with 0 corresponding to no lens changes, 0.5 to suture accentuation, 1 to vacuole formation, 2 to cortical opacity, and 3 to mature cataract. The study was terminated 8 weeks after induction of diabetes, and the lenses were carefully removed from each enucleated eye using a posterior approach and photo-documented. Blood glucose levels at the onset of the study were determined using a commercial glucometer (Freestyle by TheraSense, Alameda, CA). HbA1C levels were determined at the end of the study using the Bayer Metrika A1cNOW Plus System test kit (San Diego, CA).
UV irradiation of one eye only
Twenty-five female Sprague Dawley rats (100 g) were divided into 5 equal groups. One group served as the nonirradiated control, while the second group served as the untreated irradiated control. The third group received topical JHX-4, the fourth received topical Optixcare EH, and the fifth group received 1 mL of the oral Ocu-GLO as described above. Treatments began 3 days prior to irradiation and continued until each experiment was terminated. Prior to irradiation, each rat was anaesthetized by intraperitoneal injection of a mixture of 60 mg/kg of ketamine and 10 mg/kg of xylazine, and the right eye was dilated with 1% tropicamide ophthalmic solution. The contralateral eye was covered with an eye patch that was held in place with black tape. The dilated eye was then exposed for 15 min at a distance of 20 cm to 1,600 μW/cm2 of UV-B as above. Two days after irradiation, the experiment was terminated. Each rat was euthanized with carbon dioxide asphyxiation. Both eyes were enucleated, and the lenses were carefully removed by a posterior approach.
Oxidative stress analysis
Following morphological examination of each lens under retroillumination with a dissecting microscope, each lens was homogenized in a ground glass homogenizer with ice-cold lysis buffer (pH 7.5), and the insoluble proteins were removed by centrifugation at 4°C. Protein levels in an aliquot from each supernatant were measured according to Bradford Assay.37 Aliquots of the supernatant were then deproteinized with equal volumes of 20% TCA, and the glutathione (GSH) levels in the deproteinized supernatants were measured at 412 nm according to the DTNB method.38 4-Hydroxynonenal (4-HNE) levels were then determined by competitive ELISA in the remaining homogenate supernatants by using the OxiSelect HNE Adduct Competitive ELISA Assay Kit (Cell Biolabs, Inc.) according to the manufacturer's instructions. All assays were done in triplicate.
Light-induced retinal damage
This study was conducted as previously described in detail.35 Briefly, groups containing twelve 4–5-week-old male Wistar rats receiving either normal rat chow (untreated), normal rat chow and topically treated with Optixcare EH b.i.d., or an oral diet containing 0.05% of the multifunctional antioxidant JHX-4 as described above were dark adapted for 2 weeks. Rats from each group were then individually placed into acrylic, flat-bottom rodent restraints (Plas-Labs, Inc., Lansing, MI) in a light-box apparatus and exposed for 3 h to 1,000 lx of cool white fluorescent light (Lights of America, Los Angeles, CA; light-damaged rats, LD). An additional group of nontreated rats was also placed into the light-box apparatus for 3 h, but not exposed to light (nonlight-damaged rats, NLD). Immediately following light exposure, 8 LD rats from each group and 8 NLD rats were immediately euthanized, and the neural retinas were carefully dissected from the enucleated eye and frozen on dry ice. The remaining rats in each group were returned to the dark environment for 7 additional days. Each rat was then euthanized by carbon dioxide asphyxiation, and the dorsal ventral orientation of each eye was marked with a cautery tool. The eyes were then enucleated and fixed in a 3:1 (v/v) methanol:acetic acid solution overnight. The fixed samples were then processed by the UNMC Tissue Sciences Facility (www.unmc.edu/tissuesciences/) for subsequent morphological analyses.
Oxidative stress analysis
Oxidative stress markers were analyzed as previously described.35 Briefly, thawed retinas were homogenized in lysis buffer for ELISA determinations of 4-HNE-His Adduct and Nitrotyrosine using ELISA kits. Additional thawed retinas were homogenized in RIPA buffer containing Halt Protease and Phosphatase Inhibitor Cocktail for Western blot analysis of thioredoxin (TRx) and thioredoxin reductase (TRxR) expression levels. Protein samples were separated by SDS-PAGE and transferred onto nitrocellulose membranes. After blocking the membrane with 5% dry milk in 0.1% Tris-buffered saline with Tween (TBST), the membranes were then incubated overnight at 4°C with the primary antibodies anti-TRx (1:1,000 dilution) or anti-TRxR (1:2,000 dilution). Subsequently, after washing with TBST, the membranes were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h. The protein bands were then visualized by chemiluminescence and exposure to X-ray film. Finally, the membranes were reprobed with antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control. All visualized protein levels were quantified using the NIH ImageJ program (http://rsbweb.nih.gov/ij/).
Morphometric analysis
Digital images of the hematoxylin and eosin (H&E)-stained sections were obtained with a Zeiss AxioPlan microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) using Axiovision software. The outer nuclear layer (ONL) thickness was measured at 400-μm intervals, starting at the optic nerve head and extending toward the superior and inferior ora serrata using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD).
Dry-eye studies
Thirty 180–200-g female Lewis rats were divided into 5 groups: one served as an untreated control, while each of the other groups received 1 drop of Optixcare, Optixcare H, Optixcare EH, or I-Drop Vet Plus Ophthalmic Solution in each eye b.i.d. Dry eye was induced in all rats by subcutaneous injection of a 0.1 mL aqueous solution b.i.d. containing 24 mg of scopolamine (48 mg total dose per day).39 Tear flow was measured weekly prior to dosing using Zone-Quick™ Phenol Red Threads (FCI Ophthalmics, Pembroke, MA) by anesthetizing each rat with isofluorane. The lower eyelid was pulled down and, using forceps, the folded 3-mm end of each thread was placed into the palpebral conjunctiva at a point approximately one third of the distance from the lateral canthus. The string was left in the open eye for 15 s and then removed by gently pulling the lower eyelid down and removing the thread with an upward motion. The length of red-stained thread was then immediately measured. At each time point, the values determined for each rat are the average of the value determined from each eye.
Prior to termination of the study at 6 weeks, the cornea of each eye was clinically evaluated using Fluramene™, a proprietary Fluorescein Sodium and Lissamine Green dye combination (Noble Vision Group, Lisle, IL). Excess stain was removed by washing with 0.9% sodium chloride solution, and the corneas were examined by slit lamp under blue light.
Statistical analysis
Statistical comparisons between groups were performed with analysis of variance (ANOVA) test (Origin 8.1; OriginLab Corp., Northampton, MA). Differences of P<0.05 were considered significant.
Results
Cataract studies
The ability of topical Optixcare EH to alter lens changes associated with ROS was evaluated in 2 cataract rat models where lens changes were induced by diabetes-induced hyperglycemia and overexposure to UV irradiation. In these rats, the topical was compared with Ocu-GLO, an oral antioxidant formulation that is currently being marketed for the prevention/treatment of cataracts, and the multifunctional antioxidant JHX-4, which we have previously shown delays cataracts induced by diabetes and whole head gamma irradiation in rats.34
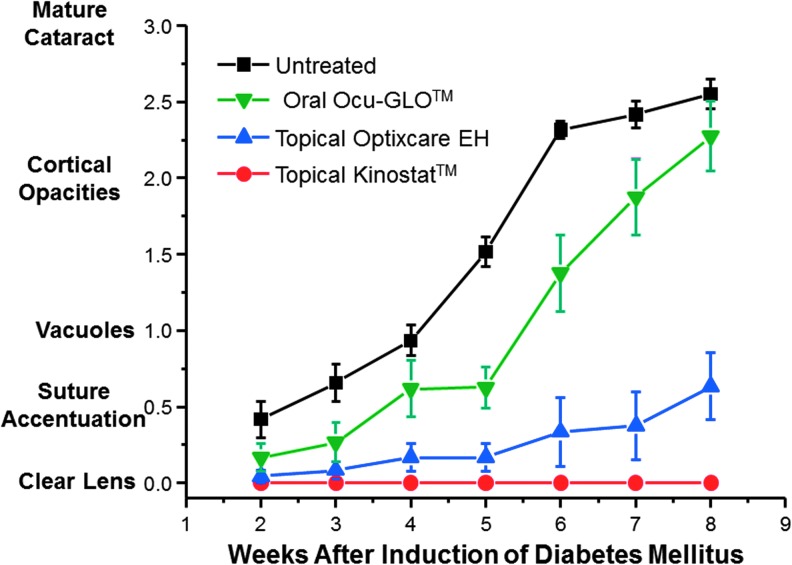
It is established that young diabetic rats rapidly develop “sugar cataracts” through osmotic stress that is linked to the intracellular accumulation of sorbitol whose formation is catalyzed by the enzyme aldose reductase.40,41 Osmotic stress induces endoplasmic reticulum (ER) stress that initiates an unfolded protein response that leads to the generation of ROS.42,43 Following the induction of diabetes with streptozotocin, lens changes were monitored weekly by hand-held slit lamp and graded on a scale of 0 (no lens change) to 3 (mature cataract). At the end of the study, all eyes were enucleated, and the grading of each lens was verified by examination of the subsequently dissected lens under a dissecting microscope. As summarized in Fig. 1, all young diabetic rats rapidly developed cataracts over an 8-week period, and this formation was inhibited by the topical administration of the aldose reductase inhibitor (ARI) Kinostat, which is currently undergoing a Food and Drug Administration (FDA)-approved clinical trial for the prevention of cataracts in diabetic dogs.44,45 The onset and progression of cataracts in these diabetic rats was delayed by the administration of nutraceuticals with the topical administration of Optixcare EH demonstrating a greater delay than the oral administration of the Ocu-GLO.
FIG. 1.
Effect of the topical aldose reductase inhibitor (ARI) Kinostat™ and nutraceutical antioxidants Ocu-GLO™ and Optixcare EH administration on the progression of sugar cataract formation in young (100 g) streptozotocin-induced diabetic rats. Administration of topical ARI from the onset of diabetes prevented sugar cataract formation, while similar administration of nutraceutical antioxidants delayed the onset and progression of sugar cataracts, with the topical nutraceutical Optixcare EH demonstrating a greater delay. Values represent mean±standard error of the mean (SEM); n=5.
These slit-lamp observations were confirmed at the end of the study by microscopic examination of the dissected lenses (Fig. 2). While no lens opacities were evident in lenses from the ARI-treated rats, both the untreated and orally treated Ocu-GLO rats developed lens changes ranging from cortical to mature cataracts (Fig. 2). In contrast, primarily suture accentuation, which is the initial stage of sugar cataract development, was present in the Optixcare EH-treated diabetic rats. These differences in sugar cataract formation were not linked to differences in the severity of diabetes because all groups were similarly hyperglycemic over the course of this study, as indicated by their glycosylated hemoglobin levels (HbA1c, mean±SEM untreated control 10.7±0.7; Kinostat 11.48±0.2; Optixcare EH 10.6±0.7; Ocu-GLO 10.4±0.76).
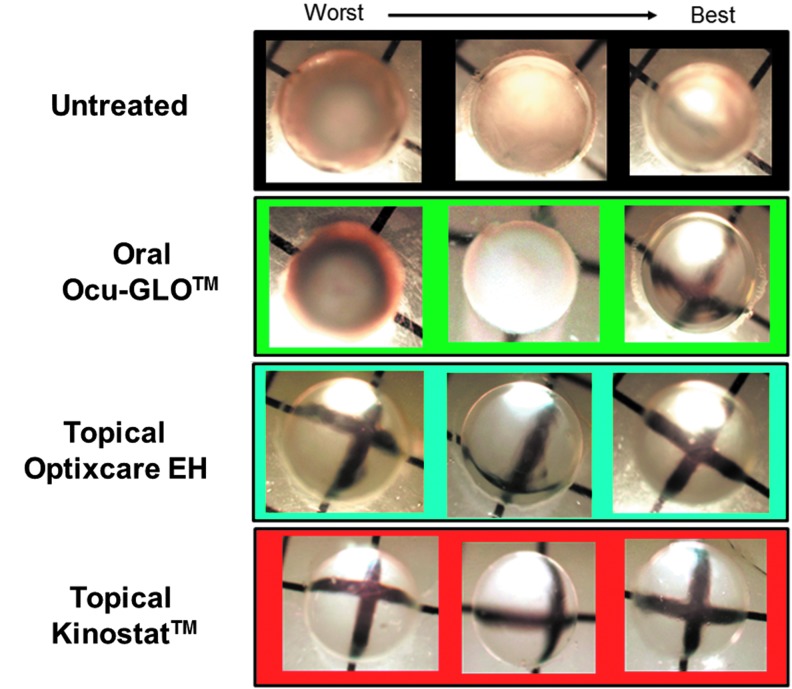
FIG. 2.
General appearance of diabetic lens changes at the termination of the study. Eyes were enucleated, and lenses were carefully removed by a posterior approach and photographed under a dissecting microscope. Lens changes in both the untreated and oral Ocu-GLO-treated rats ranged from mature cataracts (worst case) to cortical opacities (best case). In contrast, lens in the topical Optixcare EH-treated rats demonstrated primarily suture accentuation, while no lens changes were present in the Kinostat-treated rats.
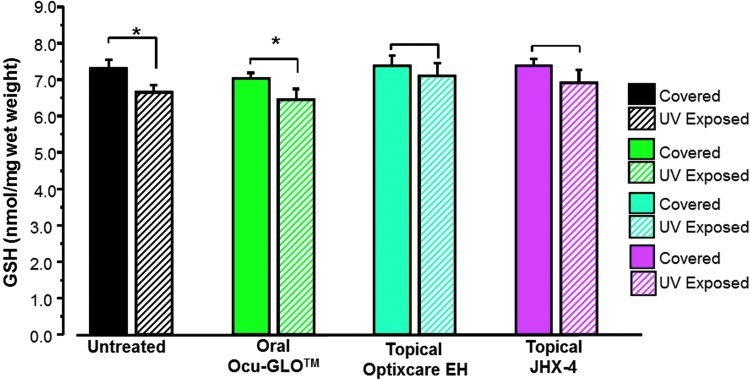
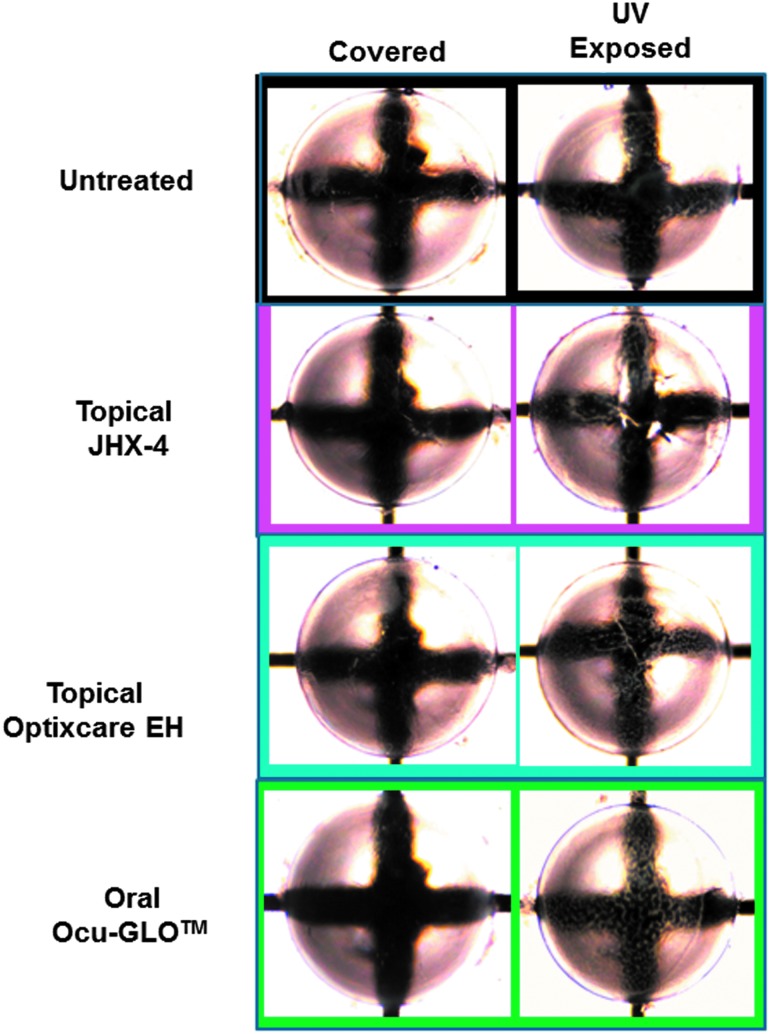
Another major risk factor of cataractogenesis is UV irradiation, which induces lens damage through the generation of ROS that leads to decreased GSH levels and subsequent lens opacification. Experimental exposure of young male albino rats to UV irradiation results within 2–3 days led to a rapid decrease in lens GSH levels and the appearance of anterior lens vacuoles. For these studies, each rat was anesthetized and then placed on its side with only one eye exposed for 15 min to 1,600 μW/cm2 of UV light (280–360 nm, UVmax=306 nm), while the covered contralateral eye was down and away from UV exposure. In rats exposed to UV radiation by this method, lens GSH levels were significantly lower (P<0.05) in the exposed eyes compared with GSH levels in their unexposed contralateral lenses (Fig. 3). Similar irradiation of rats pretreated for 3 days with topical 3% JHX-4 b.i.d., Optixcare EH b.i.d., or oral Ocu-GLO administered daily by gavage resulted in GSH levels being significantly reduced only in the Ocu-GLO-treated rats. GSH levels in irradiated lenses from rats topically treated with either Optixcare EH or 3% JHX-4 were not significantly lower (Fig. 3). Nevertheless, all exposed eyes developed anterior vacuoles independent of treatment, and vacuoles were only absent in nonexposed eyes (Fig. 4).
FIG. 3.
Comparison of GSH levels in young (100 g) female rats where one eye was exposed to ultraviolet (UV) irradiation, while the contralateral eye was covered and served as the nonirradiated control. Rats were pretreated for 3 days with topical multifunctional antioxidant, topical nutraceutical Optixcare EH, or the oral nutraceutical Ocu-GLO administered daily. Lens GSH levels measured 2 days after exposure were significantly lower in the exposed eyes from either untreated or orally Ocu-GLO-treated rats compared with GSH levels in their contralateral unexposed lenses. Mean±SEM; n=5–6 rats; *P<0.05. GSH, glutathione.
FIG. 4.
General appearance of contralateral lenses from rats 2 days after being exposed to UV radiation and treated with or without antioxidants. All UV-exposed lenses with or without antioxidant treatment demonstrated the presence of anterior lens vacuoles, which can easily be observed near the images of the black lines.
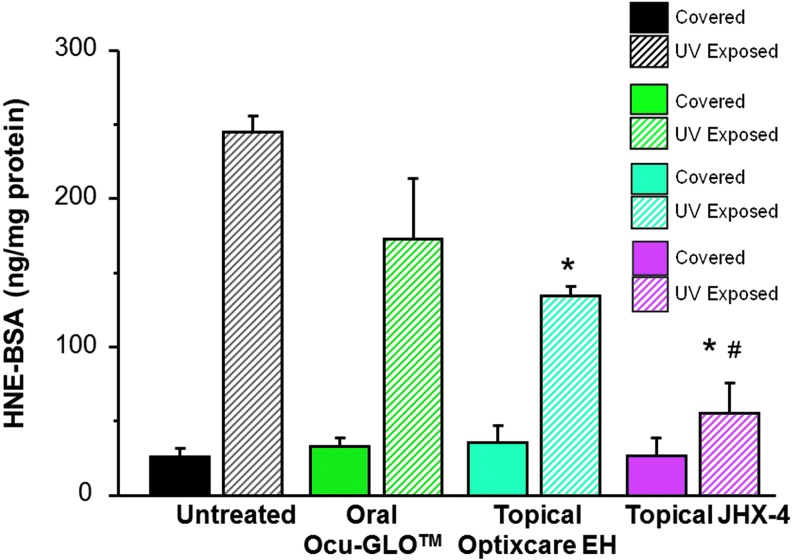
To confirm that the reduced GSH levels after UV exposure were linked to increased levels of ROS, the lenticular levels of the oxidized membrane lipid 4-hydroxynonenol (4-HNE) were also measured. As summarized in Fig. 5, UV exposure resulted in an increase in 4-HNE levels, which were reduced by antioxidant treatments in the order: topical JHX-4>topical Optixcare EH>oral Ocu-GLO. Compared with 4-HNE levels in lenses from untreated rats exposed to UV irradiation, 4-HNE levels were significantly lower in UV-exposed lenses from rats treated with JHX-4 (P<0.01) and Optixcare EH (P<0.05). 4-HNE levels in irradiated lenses from the Ocu-GLO-treated rats were not significantly different from levels in lenses from untreated rats exposed to UV radiation. 4-HNE lens levels from the UV exposed and unexposed contralateral eyes were statistically similar only in the JHX-4-treated rats.
FIG. 5.
Lenticular levels of 4-hydroxynonenol (4-HNE) in young female rats where one eye was exposed to UV light while the contralateral eye was covered and served as the nonirradiated control. Rats were pretreated for 3 days with topical multifunctional antioxidant, topical nutraceutical Optixcare EH, or the oral nutraceutical Ocu-GLO administered daily. Lens 4-HNE levels measured 2 days after exposure to UV irradiation were reduced by antioxidant treatments in the order: topical 3% JHX-4>topical Optixcare EH>oral Ocu-GLO. With the exception of topical JHX-4-treated rats, all rats had significantly higher (P<0.01) levels of 4-HNE in the UV-exposed lenses compared with the levels in the contralateral nonirradiated controls. Mean±SEM; n=5–6 rats; *P<0.01 compared with untreated irradiated lenses; #P<0.01 compared with irradiated Ocu-GLO-treated lenses.
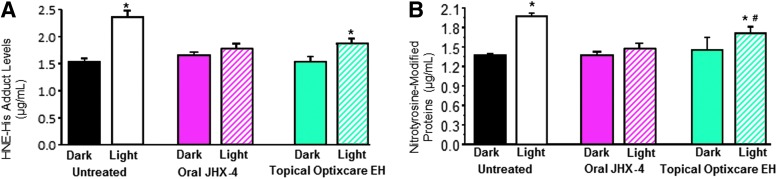
Exposure of dark-adapted rats to light also results in the generation of ROS, iron dysregulation, and the release of iron from ferritin in the neural retina.46,47 In this animal model of AMD, Wistar rats were either orally treated with a diet containing 0.05% of the multifunctional antioxidant JHX-4 or topically treated b.i.d. with Optixcare EH with treatment beginning at the onset of 2-week dark adaption. As previously reported by Randazzo et al.,35 exposure of neural retinas to 1,000 lx of light for 3 h resulted in an increase of oxidative stress markers that include 4-HNE adducts formed with histidine following the nonenzymatic oxidation of polyunsaturated fatty acids and nitrotyrosine-modified proteins resulting from tyrosine residues being oxidized by peroxynitrite formed by the reaction of nitric oxide with superoxide anions. After light exposure, both of these markers significantly increased, while neither oxidative marker significantly increased in similar light-exposed rats treated with JHX-4 (Fig. 6). Rats treated with Optixcare EH showed partial increases in both oxidative markers. However, these increases were significantly less than those in untreated rats.
FIG. 6.
Comparison of 4-HNE-histidine adduct levels (A) and nitrotyrosine adduct levels (B) in the neural retina of rats exposed to light after 2-week dark adaptation. HNE-histidine adduct levels, as determined by ELISA, in rats treated with or without the multifunctional antioxidant JHX-4 and Optixcare EH suggests that HNE levels were reduced by treatment. Similar conclusions were obtained with ELISA measurements of nitrotyrosine-modified proteins in rats treated with or without these antioxidants. The JHX-4 data have been previously published.35 Mean±standard deviation (SD); n=6. *P<0.05 compared with the untreated, nonlight-damaged (NLD) group. #P<0.05 compared with the untreated light damaged (LD) group. ELISA, enzyme-linked immunosorbent assay.
ROS and free radicals generated by retinal exposure to acute excessive light are also mediated by the thioredoxin (TRx)/thioredoxin reductase (TRxR) defense system.48 Therefore, the expression levels of both TRx and TRxR in the neural retinas of were also examined. Both TRx and TRxR expression increased in untreated rats exposed to light (Fig. 7). The expression of TRxR was significantly lowered (P<0.05) in the neural retinas of light-exposed rats treated with either JHX-4 or Optixcare EH. However, TRx expression was only reduced by JHX-4.
FIG. 7.
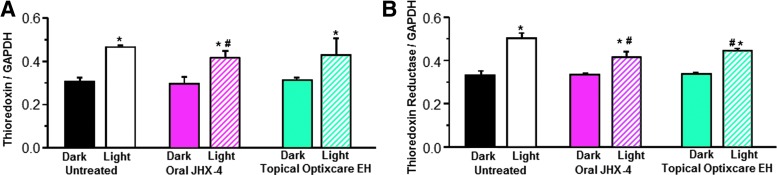
Comparison of expression of thioredoxin (TRx) (A) and thioredoxin reductase (TRxR) (B) in the neural retina of rats exposed to light after 2-week dark adaptation. Comparison of expression of TRx normalized to levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in rats treated with or without the multifunctional JHX-4 or topical Optixcare EH indicates that JHX-4 reduced the induction of TRx to a greater extent than Optixcare EH. Similarly, comparison of the expression of TRxR normalized to levels of GAPDH in rats treated with or without the multifunctional JHX-4 or topical Optixcare EH indicates that both JHX-4 and Optixcare reduced the induction of TRx compared with the levels observed in the untreated rats. The JHX-4 data have been previously published.35 Mean±SD; n=6. An asterisk (*) denotes a significant difference (P<0.05) when compared to the untreated NLD group while the hatched symbol (#) denotes a significant difference (P<0.05) compared to the untreated LD group.
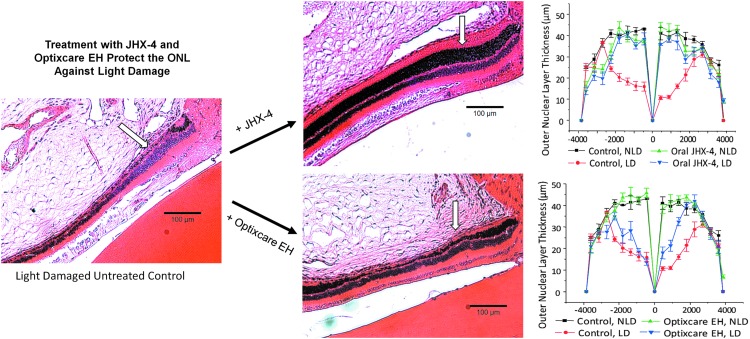
The reduction of oxidative stress by JHX-4 and Optixcare EH was confirmed by histological measurements of the photoreceptor layers, where intense light exposure resulted in photoreceptors thinning in the ONL, with damage in the superior hemisphere more pronounced than the inferior hemisphere. In rats treated with JHX-4 (Fig. 8A), the photoreceptor layer was only slightly decreased in both the inferior hemisphere and the superior hemisphere. In contrast, the photoreceptor layer was only partially protected in the Optixcare EH-treated rats (Fig. 8B). These results suggest that despite topical delivery, Optixcare EH can partially reduce oxidative stress in the retinas of light-damaged rats.
FIG. 8.
Light-induced destruction of the retinal outer nuclear layer (ONL) can be reduced by the presence of antioxidants. Photomicrographs illustrate changes observed in the ONL (arrow) of the photoreceptor layer progressing from the optic nerve head, which is located at the top right corner of each photomicrograph. Changes were quantified by measuring the ONL thickness of the inferior and superior hemispheres at each defined distance from the optic nerve. The NL thickness of NLD and LD retinas treated with or without the multifunctional JHX-4 or the nutraceutical antioxidant Optixcare EH are to the right of each respective photomicrograph. The JHX-4 data have been previously published35; n=3; mean±SEM.
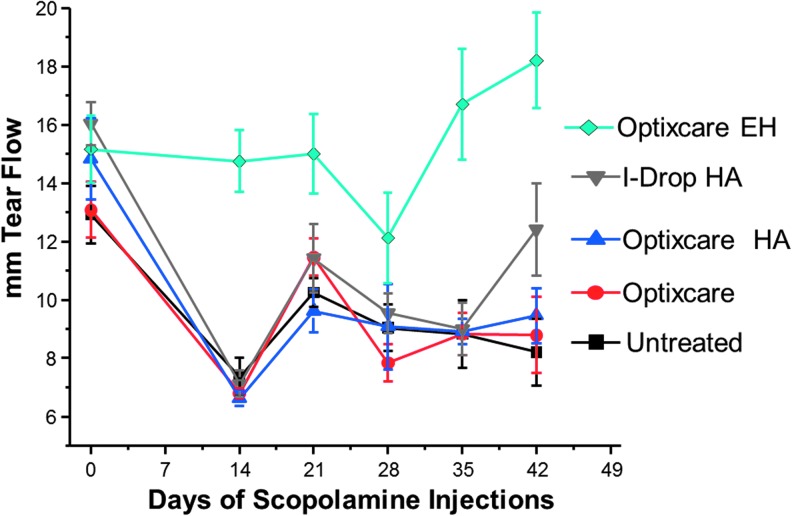
Optixcare is a carbomer-based eye lubricant that is used in the veterinary market to protect eyes from dehydration during anesthesia and as adjunctive support for dry eyes. Optixcare belongs to a class of ocular comfort agents that is composed of poly(acrylic acid) polymers that are designed to relieve ocular discomfort by augmenting the characteristics of the tear film so that the tear volume is stabilized and retained, while the ocular surface remains lubricated. Other such agents include hyaluronic acid (HA), which retains water. Based on this primary commercial use for Optixcare, we investigated whether the addition of nutraceutical antioxidants alter the ability of Optixcare to serve as an adjunctive treatment for dry eye. Dry eye was experimentally induced in adult Lewis rats by twice daily subcutaneous injections of scopolamine, and tear production was monitored with phenol red thread. As shown in Fig. 9, tear flow in each rat, determined as the average of both eyes, decreased over time in the untreated rats, and topical administration of either of the comfort agents Optixcare, Optixcare with HA, or i-Drop HA did not alter this decrease in tear flow. In contrast, tear flow was preserved in similar scopolamine-injected rats receiving the nutraceutical Optixcare EH. This suggests that this nutraceutical formulation may actually be beneficial in dry eye by maintaining tear flow. The corneal surface was also clinically evaluated by slit lamp with Fluramene™ stain prior to termination of the study. No sign of surface corneal or conjunctival erosion appeared to be present in eyes from either the nontreated or any of the treated groups, indicating that the scopolamine-induced dry eye was not clinically severe.
FIG. 9.
Reduction of tear flow induced by twice daily subcutaneous injections of scopolamine in adult Lewis rats topically treated with or without the comfort agents Optixcare, Optixcare with hyaluronic acid, i-Drop hyaluronic acid, and the nutraceutical Optixcare EH. Tear flow in each rat, determined with phenol red thread as the average of both eyes, decreased over time in the untreated rats, and topical administration of either of the comfort agents Optixcare, Optixcare with hyaluronic acid, or i-Drop hyaluronic acid did not alter this decrease. Concomitant treatment with nutraceutical Optixcare EH in similar scopolamine-injected rats appeared to preserve tear flow; n=6 rats.
Discussion
Worldwide, nutraceuticals are being sold with the premise that they are beneficial in ameliorating age-related diseases such as cataracts, AMD, and dry eye. “Support” for their use is primarily based on theoretical mechanisms or in vitro “test tube” results rather than experimental evidence from in vivo animal studies. When clinical trials with nutraceutical antioxidants and vitamin supplements have been conducted, the results obtained have often been discouraging, For example, the Roche European American Cataract Trial (REACT),49 the Linxian cataract study,50 the AREDS study,51 Centrum use in the AREDS study,52 Lutein/zeaxanthin for the treatment of age-related cataracts in the AREDS2 trial,53 the α-tocopherol β-Carotene Study (ATBC),54 and the Physicians Health Study,55 have all yielded minimal or no significant results in clinically reducing cataract development.56 A major problem with these studies has been the assumption that oral administration of antioxidants results in increased levels of these antioxidants in the lens. This has not been documented. In fact, patients given α-tocopherol, β-carotene, and vitamin C prior to lens extraction showed no increase in intralenticular antioxidants despite substantially raised blood levels of these antioxidants.57 In contrast, topical application can result in increased lens levels as demonstrated by Ohta et al.58 who reported that lenticular levels of α-tocopherol can be raised by topical instillation of α-tocopherol containing liposomes and that this delays sugar cataract formation in rats.
The present studies suggest that topical administration of Optixcare EH, which contains 4 nutraceuticals suspended in a viscous carbomer vehicle that also contains 1% cetrimide as both a preservative and corneal permeation enhancer, may be beneficial in delaying lens changes in 2 rat cataract models associated with ROS. As anticipated in the diabetic rat model, topical application delayed the onset and progression of sugar cataracts. This is because osmotic changes from intracellular sorbitol formation are the primary initiator of sugar cataract formation in diabetic rats, and ROS generated through sorbitol-linked osmotic stress is only a secondary contributor to lens changes. This premise has been confirmed by a number of prevention studies in rats and dogs where adequate ARI administration prevents sugar cataract formation, while antioxidants only delay the onset and progression of sugar cataracts.31,45,59,60 This observation has previously been reported by us by comparing the orally administered ARI AL1576 and orally administered multifunctional antioxidant JHX-4.34 The present study further confirms this premise by demonstrating that the topically administered ARI Kinostat, which is currently undergoing an FDA-approved clinical trial for the prevention of cataracts in diabetic dogs,44,45 also prevents cataract formation in rats, while the nutraceutical antioxidants Optixcare EH and Ocu-GLO delayed the onset and progression of sugar cataract development (Fig. 1). At the end of the study, little difference in cataract severity was observed between the untreated and orally Ocu-GLO-treated diabetic rats, while the topical Optixcare EH-treated rats primarily developed suture accentuation, which represents the initial stage of sugar cataract formation (Fig. 2). Many nutraceutical formulations also contain flavonoids that reduce AR activity. However, the ingredients in Optixcare EH do not directly inhibit AR. This suggests that the differences seen between Optixcare EH and Ocu-GLO are due to the strength of the antioxidant activities in the lens.
Exposure to UV light is a risk factor for cataracts. Experimental exposure to UV light also generates oxidative stress, which results in a decrease in reduced lens GSH levels and the subsequent development of lens opacification. In the present studies, rats with dilated eyes were then exposed to 1,600 μW/cm2 of UV-B radiation at a distance of 20 cm for 15 min, while the contralateral eyes were covered and not exposed. This resulted in a significant decrease in the lenticular antioxidant levels of GSH and a significant increase in lens 4-HNE levels, a marker of lens lipid peroxidation products, in only the exposed eyes. Similar UV exposure of rats treated either orally or topically with the multifunctional antioxidant JHX-4 prevented these significant changes in GSH and 4-HNE. JHX-4 is a small molecule containing a unique 2-amino-5-hydroxypyrimidine free radical scavenging ring system that readily reaches the lens and has been previously reported to delay the onset and progression of gamma-irradiation induced cataracts in rats. Topical administration of the ingredients in Optixcare EH to similar UV-exposed rats resulted in a similar, nonsignificant reduction of GSH levels compared with unexposed contralateral eyes and increases of 4-HNE levels that were significantly lower than those of similar exposed eyes from untreated rats. In UV-exposed rats treated with Ocu-GLO, little antioxidant protection was observed. The decreases in GSH levels compared with their unexposed contralateral eyes were significant, while the 4-HNE levels compared with similarly UV-exposed eyes from untreated rats were not significantly lower. This suggests that the lenticular antioxidant levels were inadequately lower in the orally administered Ocu-GLO rats. Interestingly, none of the antioxidants afforded protection against the development of UV-induced anterior lens vacuoles.
Topical ocular administration of drugs generally does not result in adequate retinal levels of drug being obtained. Therefore, topical administration of Optixcare EH to light-exposed, dark-adapted rats was not anticipated to result in any significant protection of retinal oxidative stress compared with the oral administration of the multifunctional antioxidant JHX-4. Surprisingly, beneficial retinal effects of topically administered Optixcare EH were observed with 4-HNE histidine adduct levels and nitrotyrosine-modified protein levels being significantly reduced compared with untreated rats (Fig. 8) and some preservation of the ONL photoreceptor layer histologically observed (Fig. 9). However, compared with that of JHX-4, this protection was less, and Optixcare EH gave no protection against ERG changes (data not shown). This observation suggests that better retinal antioxidant protection by Optixcare EH may be achieved if the nutraceutical ingredients in Optixcare EH are also concomitantly administered orally.
Another age-related problem affecting the anterior segment is dry eye. This disease, which is also linked to oxidative stress and inflammation, is not only treated with immune suppressants but also with nutraceuticals such as combined antioxidants and omega-3 essential fatty acids formulations24 or topical epigallocatechin gallate25 and comfort agents such as viscous water-retaining carbomer gels and HA. Since Optixcare carbomer gel and Optixcare containing HA are used in the veterinary market for alleviating the clinical symptoms of dry eye, and the nutraceutical ingredients in Optixcare EH have been reported to demonstrate not only antioxidant but also anti-inflammatory activity, studies comparing the effects of these carbomer gel ingredients on rats with scopolamine-induced dry eye were investigated. Surprisingly, the nutraceutical Optixcare EH maintained tear flow in the dry eye–induced rats, while tear flow was not affected by either the administration of carbomer gel with HA or HA alone. Further studies are planned to determine the potential clinical effects of Optixcare EH on dry eye.
In summary, Optixcare EH is a carbomer gel that contains 4 nutraceuticals possessing antioxidant, anti-inflammatory, and chelating activity along with the preservative cetrimide, which enhances corneal permeability. In the limited prevention studies in rats reported here, topical application of this formulation protects against oxidative stress not only in the lens but to a lesser extent in the retina. Moreover, the formulation appears to maintain tear flow in an experimental dry-eye rat model. These results suggest that further clinical studies of Optixcare EH are warranted.
Author Disclosure Statement
Research funds were obtained from Therapeutic Vision Inc., Omaha, NE, and CLC Medica LLC, Waterdown, Ontario, Canada. Dr. Kador is president of Therapeutic Vision, Inc. and inventor of a pending patent application.
References
- 1.Vincent G., and Velkoff V.The Next Four Decades, The Older Population in the United States: 2010 to 2050, Population Estimates and Projections. Washington, DC: US Census Bureau; 2010 [Google Scholar]
- 2.Akpek E.K., and Smith R.A.Overview of age-related ocular conditions. Am J Manag Care. 19:S67–75, 2013 [PubMed] [Google Scholar]
- 3.Rein D.B., et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 124:1754–1760, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Kupfer C.Bowman lecture. The conquest of cataract: a global challenge. Trans Ophthalmol Soc UK. 104:1–10, 1985 [PubMed] [Google Scholar]
- 5.Klein B.E., et al. The relationship of cataract and cataract extraction to age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 119:1628–1633, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukinova N., et al. Iron chelation protects the retinal pigment epithelial cell line ARPE-19 against cell death triggered by diverse stimuli. Invest Ophthalmol Vis Sci. 50:1440–1447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodge W.G., Whitcher J.P., and Satariano W.Risk factors for age-related cataracts. Epidemiol Rev. 17:336–346, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Castineiras S.Iron, the retina and the lens: a focused review. Exp Eye Res. 90:664–678, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suner I.J., et al. Nicotine increases size and severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 45:311–317, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Avunduk A.M., et al. Cataractous changes in rat lens following cigarette smoke exposure is prevented by parenteral deferoxamine therapy. Arch Ophthalmol. 117:1368–1372, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Avunduk A.M., et al. Prevention of lens damage associated with cigarette smoke exposure in rats by alpha-tocopherol (vitamin E) treatment. Invest Ophthalmol Vis Sci. 40:537–541, 1999 [PubMed] [Google Scholar]
- 12.Rhone M., and Basu A.Phytochemicals and age-related eye diseases. Nutr Rev. 66:465–472, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Aydin E., et al. Levels of iron, zinc, and copper in aqueous humor, lens, and serum in nondiabetic and diabetic patients: their relation to cataract. Biol Trace Elem Res. 108:33–41, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Bush A.I., and Goldstein L.E.Specific metal-catalysed protein oxidation reactions in chronic degenerative disorders of ageing: focus on Alzheimer's disease and age-related cataracts. Novartis Found Symp. 235:26–38; discussion 38–43, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Ozawa Y., et al. Neuroprotective effects of lutein in the retina. Curr Pharm Des. 18:51–56, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troutbeck R., et al. Therapeutic targeting of the complement system in age-related macular degeneration: a review. Clin Exper Ophthalmol. 40:18–26, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Thomas C., et al. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: implications for diseases associated with iron accumulation. Redox Rep. 14:102–108, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Wong R.W., et al. Iron toxicity as a potential factor in AMD. Retina. 27:997–1003, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Hahn P., Milam A.H. and Dunaief J.L.Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch's membrane. Arch Ophthalmol. 121:1099–1105, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Dunaief J.L., et al. Macular degeneration in a patient with aceruloplasminemia, a disease associated with retinal iron overload. Ophthalmology. 112:1062–1065, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Satici A., et al. The effects of chronic smoking on the ocular surface and tear characteristics: a clinical, histological and biochemical study. Acta Ophthalmol Scand. 81:583–587, 2003 [DOI] [PubMed] [Google Scholar]
- 22.El-Shazly A.A., et al. Passive smoking as a risk factor of dry eye in children. J Ophthalmol. 2012:130159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson W., Chauhan S.K., and Dana R.Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 130:90–100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinazo-Duran M.D., et al. Effects of a nutraceutical formulation based on the combination of antioxidants and omega-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clin Interv Aging. 8:139–148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H.S., et al. Therapeutic efficacy of topical epigallocatechin gallate in murine dry eye. Cornea. 30:1465–1472, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song D., et al. Systemic administration of the iron chelator deferiprone protects against light-induced photoreceptor degeneration in the mouse retina. Free Radic Biol Med. 53:64–71, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang F.Q., et al. Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Exp Eye Res. 78:1069–1075, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 119:1439–1452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Age-Related Eye Disease Study Research Group.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 119:1417–1436, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnadev N., Meleth A.D., and Chew E.Y.Nutritional supplements for age-related macular degeneration. Curr Opin Ophthalmol. 21:184–189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer C.H., and Sekundo W.Nutritional supplementation to prevent cataract formation. Dev Ophthalmol. 38:103–119, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Chiu C.J., and Taylor A.Nutritional antioxidants and age-related cataract and maculopathy. Exp Eye Res. 84:229–245, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Popescu C., et al. [The mechanism of cataract formation in persons with beta-thalassemia]. Oftalmologia. 45:10–13, 1998 [PubMed] [Google Scholar]
- 34.Randazzo J., et al. Orally active multi-functional antioxidants delay cataract formation in streptozotocin (type 1) diabetic and gamma-irradiated rats. PLoS One. 6:e18980, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randazzo J., et al. Orally active multi-functional antioxidants are neuroprotective in a rat model of light-induced retinal damage. PLoS One. 6:e21926, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin H., et al. Multifunctional antioxidants for the treatment of age-related diseases. J Med Chem. 53:1117–1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradford M.M.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 38.Lou M.F., and Dickerson J.E., Jr.Protein-thiol mixed disulfides in human lens. Exp Eye Res. 55:889–896, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Chen W., et al. Keratoconjunctivitis sicca modifies epithelial stem cell proliferation kinetics in conjunctiva. Cornea. 26:1101–1106, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Kinoshita J.H.Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 13:713–724, 1974 [PubMed] [Google Scholar]
- 41.Zhang P., et al. Osmotic stress, not aldose reductase activity, directly induces growth factors and MAPK signaling changes during sugar cataract formation. Exp Eye Res. 101:36–43, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulhern M.L., et al. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Invest Ophthalmol Vis Sci. 47:3951–3959, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Mulhern M.L., et al. Cellular osmolytes reduce lens epithelial cell death and alleviate cataract formation in galactosemic rats. Mol Vis. 13:1397–1405, 2007 [PubMed] [Google Scholar]
- 44.Kador P.F., et al. Topical aldose reductase inhibitor formulations for effective lens drug delivery in a rat model for sugar cataracts. J Ocul Pharmacol Ther. 23:116–123, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Kador P.F., et al. Topical KINOSTAT ameliorates the clinical development and progression of cataracts in dogs with diabetes mellitus. Vet Ophthalmol. 13:363–368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Organisciak D.T., and Vaughan D.K.Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 29:113–134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohishi K., et al. Iron release analyses from ferritin by visible light irradiation. Free Radic Res. 39:875–882, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Tanito M., Agbaga M.P., and Anderson R.E.Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med. 42:1838–1850, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Chylack L.T. Jr., et al. The Roche European American Cataract Trial (REACT): a randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthalmic Epidemiol. 9:49–80, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Sperduto R.D., et al. The Linxian cataract studies. Two nutrition intervention trials. Arch Ophthalmol. 111:1246–1253, 1993 [DOI] [PubMed] [Google Scholar]
- 51.Mitchell P., et al. Nutritional factors in the development of age-related eye disease. Asia Pac J Clin Nutr. 12:S5, 2003. 15023590 [Google Scholar]
- 52.Milton R.C., et al. Centrum use and progression of age-related cataract in the Age-Related Eye Disease Study: a propensity score approach. AREDS report No. 21. Ophthalmology. 113:1264–1270, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chew E.Y., et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 131:843–850, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teikari J.M., et al. Incidence of cataract operations in Finnish male smokers unaffected by alpha tocopherol or beta carotene supplements. J Epidemiol Community Health. 52:468–472, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christen W.G., Gaziano J.M., and Hennekens C.H.Design of Physicians' Health Study II—a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 10:125–134, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Mares J.A.High-dose antioxidant supplementation and cataract risk. Nutr Rev. 62:28–32, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Bates C.J., et al. Quantitation of vitamin E and a carotenoid pigment in cataractous human lenses, and the effect of a dietary supplement. Int J Vitam Nutr Res. 66:316–321, 1996 [PubMed] [Google Scholar]
- 58.Ohta Y., et al. Preventive effect of vitamin E-containing liposome instillation on cataract progression in 12-month-old rats fed a 25% galactose diet. J Ocul Pharmacol Ther. 16:323–335, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Osawa T., and Kato Y.Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann N Y Acad Sci. 1043:440–451, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Kador P.F.Ocular pathology of diabetes mellitus. In: Tasma W., Jaeger E.A., eds. Duane's Foundations of Clinical Ophthalmology. Vol. 3 Philadelphia: Lippincott Williams & Wilkins; 2008; p. 84 [Google Scholar]