Abstract
Objective
To quantify the combined effect of maternal pre-pregnancy obesity and maternal gestational weight gain (GWG) on the shape of infant growth throughout the first year of life.
Methods
A retrospective cohort of maternal-child dyads with children born between January 2007 and May 2012 was identified in a linked electronic medical record.
Data were abstracted to define the primary exposures of maternal pre-pregnancy BMI and GWG, and the primary outcome of infant growth trajectory.
Results
We included 499 maternal-infant dyads. The average maternal age was 28.2 years; 55% of mothers were overweight or obese prior to pregnancy and 42% of mothers had excess GWG, as defined by the Institute of Medicine. Maternal pre-pregnancy BMI (p<0.001), and the interaction between pre-pregnancy BMI and maternal GWG (p=0.02) showed significant association with infant growth trajectory through the first year of life after controlling for breastfeeding and other covariates, while GWG alone did not reach statistical significance (p=0.38). Among infants of mothers with excess GWG, a pre-pregnancy BMI of 40 kg/m2 versus 25 kg/m2 resulted in a 13.6% (95% CI 5.8%, 21.5%; p<0.001) increase in 3-month infant weight/length percentile that persisted at 12 months (8.4%, 95% CI 0.2%, 16.5%; p=0.04).
Conclusions
The combined effect of excess maternal GWG and pre-pregnancy obesity resulted in higher infant birth weight, rapid weight gain in the first 3 months of life, with a sustained elevation throughout the first year of life. These findings highlight the importance of the preconception and prenatal periods for pediatric obesity prevention.
Keywords: Obesity, Pediatric; Pregnancy; Weight Gain; Mothers; Infants
Introduction
Despite a recent leveling of childhood obesity rates, the absolute prevalence of childhood overweight and obesity remains at 31.8%.1 The lasting public health implications of this epidemic stem from the repeatedly demonstrated predisposition for obese children to become obese adults with increased risks of associated morbidity, such as the metabolic syndrome, type 2 diabetes, and coronary heart disease.2-7
Recent work has provided evidence that infancy is a critical period of child development where rapid weight gain and altered adiposity can shift a child’s growth trajectory towards a more obese phenotype in childhood and into adulthood.4,8 Previous work has shown that rapid weight gain in the first 3 to 6 months of life has been associated with both a predicted prevalence of childhood obesity of 40% at age 3,8 and the development of obesity and its metabolic consequences in early adulthood.4,6 Furthermore, children who are born small for gestational age and have rapid growth in the several years of life have increased risk of coronary artery disease as adults.7 However, little has been done to characterize the determinants or predictive value of the shape of the infant growth trajectory throughout the first year of life.
In 2009 the Institute of Medicine (IOM) highlighted both pre-pregnancy BMI and excess gestational weight gain (GWG) as significant contributors to infant development.9-11 Noting the well established association between maternal pre-pregnancy BMI with both elevated birth weight and later obesity, the IOM particularly focused on the link between excess gestational weight gain (GWG) and excess infant adiposity as a modifiable risk factor to improve both pediatric and maternal health outcomes.9,12-16 These studies focused on evaluating maternal pre-pregnancy BMI or excess GWG as independent correlates to the later development of childhood obesity. Recently, the interaction between pre-pregnancy BMI and maternal gestational weight gain has been shown to influence rapid infant weight gain, but the combined effect on the shape of infant growth has not been described.17
Our objective was to measure the interaction of maternal pre-pregnancy BMI and maternal gestational weight gain on infant growth trajectory in the first year of life. We hypothesized that infants of mothers who were obese prior to pregnancy and had excessive GWG, as defined by the IOM guidelines, would have a more rapid pattern of growth in the first 3-6 months of life that would persist throughout infancy compared to infants of mothers who were either obese prior to pregnancy or had excessive GWG alone. Elucidating the shape of the infant growth curve throughout the first year is an important next step in characterizing the phenotype for infants who are at risk for developing later obesity.
By understanding how the combined effect of maternal pre-pregnancy BMI and excess gestational weight gain during pregnancy influences growth during a child’s first year of life, we can support meaningful preventive recommendations to parents and healthcare providers to help turn the tide of this pervasive childhood obesity epidemic.
Methods
Study Design and Data Sources
We assembled a retrospective cohort of maternal-child dyads, who were identified in the electronic medical record (EMR). Index children were born between January 2007 and May 2012 and were patients at the pediatric primary care clinics at a large urban academic medical center in Nashville, TN. Patients served by these practices come from a broad range of socio-economic strata and include a large cross-section of diverse cultural backgrounds. A pre-existing link between the index child’s medical record and the mother’s medical record was used to identify maternal-child dyads. All dyads received medical care at the primary care clinics throughout pregnancy and the first year of the child’s life. These clinics consisted of faculty-only practices and resident continuity clinics. This study was conducted at a single institution and approved by the Vanderbilt University Medical Center Institutional Review Board.
Population
Once maternal-child dyads had been identified electronically, a strict set of inclusion and exclusion criteria were used to avoid the potential for confounding. Children were eligible if they: 1) were at least one year old as of June 1, 2013; 2) had measures of height and weight taken concurrently on at least 3 occasions in the first year of life; and 3) had at least one concurrent measurement of child height and weight prior to 6 months of life, and 4) one after 6 months of life and ≤ 15 months of life. Mothers were eligible if they: 1) were at least 18 years old at the time of the index child’s birth; 2) had height recorded in the EMR within 365 days of the estimated date of conception (defined as delivery date minus estimated gestational age); and 3) had maternal weight recorded in the EMR up to 365 days prior to the estimated date of conception. To preserve independence of observations, only a mother’s first eligible pregnancy was included in the cohort, although it may not have been her first pregnancy. Exclusion criteria included: 1) multiple gestation; 2) children with medical conditions known to affect infant growth as defined by ICD-9 search in the EMR (e.g. Trisomy 21, hypothyroidism, cancer, etc.); 3) children with a diagnosis of failure to thrive in the first year of life; 4) children who required a percutaneous gastrostomy tube in the first year of life, as defined by CPT code in the EMR; 5) maternal insulin use during pregnancy; and 6) enrollment in another ongoing clinical trial. Children born between May 1, 2007 and May 31, 2012 were included, with potential data collected up to one year before and 15 months after.
Chart Abstraction and Data Collection
Data were abstracted from the EMR using a combination of information electronic data abstraction and manual chart reviews. A trained team of research nurses conducted the manual chart review data collection. Research nurses were trained by the principal investigator, who reviewed the first 10 charts and then a random sampling of 10% of the charts thereafter for accuracy.
EMR data that were abstracted electronically were obtained using a computer algorithm, and the principal investigator verified accuracy by manually reviewing 10% of medical records. Once length and weight measurements were obtained, a blinded reviewer visually inspected all growth curves to remove points such as a loss of height or other implausible measurements. In three such instances, data were changed to missing. Subsequently, the difference in both raw length and weight was calculated for each child between each measured time point. The highest and lowest 10% of measurements were verified from the EMR. Study data were managed using the secure Research Electronic Data Capture tools (REDCap) hosted at Vanderbilt University.18
Pre-Pregnancy BMI and Maternal Gestational Weight Gain
We evaluated maternal pre-pregnancy BMI and maternal gestational weight gain obtained from EMR measurements obtained as a part of routine clinical care visits. Pre-pregnancy BMI was calculated from weight and height measurements recorded within one year of the estimated date of conception. Gestational weight gain was calculated by subtracting the pre-pregnancy weight from the last recorded weight prior to delivery. Excess gestational weight gain was defined based on the 2009 IOM recommendations (Supplemental Table 1), and represented a range of weight gain for each of four categories of maternal pre-pregnancy BMI (underweight, normal weight, overweight, obese).9 While maternal pre-pregnancy BMI was included in the statistical models as a continuous variable, in some places it is reported categorically for ease of interpretation. These categorizations are based on CDC recommendations: 1) underweight BMI <18.5 kg/m2; 2) normal weight BMI between 18.5 and 24.9 kg/m2; 3) overweight BMI between 25.0 and 29.9 kg/m2; and 4) obese BMI ≥ 30 kg/m2. Furthermore, to illustrate findings from our statistical model, we a priori selected representative values for maternal gestational weight gain that covered the full range of weight gain described in the IOM guidelines (5kg, 10kg, 15kg).
Outcome
The primary outcome was infant growth trajectory as measured by weight-for-length percentile defined by the 2006 World Health Organization growth curves.2,3,19 Each child had growth parameters abstracted from existing height and weight measurements in the EMR up to 6 times during the first year of life: birth, 2 months, 4 months, 6 months, 9 months, and 12 months. Weight-for-length percentile was calculated using an algorithm in STATA version 12.1,20 with 10% of calculated values being manually verified using the World Health Organization (WHO) published tables for accuracy. The median number of repeated weight-for-length measurements for children included in the study was 5 (Range 3-6, Inter-Quartile Range 5-6).
Covariates for adjustment
Covariates were obtained from the EMR, and were selected a priori based on clinical significance. Child characteristics included: estimated gestational age;21 and exclusive breast-feeding for the first 6 months of life.22,23 Maternal characteristics included: maternal age at the time of delivery; number of previous pregnancies;24,25 smoking during pregnancy;25,26 illicit drug use during pregnancy;27 alcohol use during pregnancy;27 hypertension treated during pregnancy;28 diagnosis of depression during pregnancy;29 levothyroxine use during pregnancy; and gestational diabetes.30 Maternal race and ethnicity were also evaluated as potential confounding factors as they were considered to be associated with both the exposure and the outcome (both maternal and childhood obesity prevalence are higher among racial minorities).1,31 Race and ethnicity were categorized into 5 mutually exclusive categories of: White, Non-Hispanic; Black, Non-Hispanic; Asian, Non-Hispanic; Other, Non-Hispanic; and Hispanic. Health insurance status was also included as a covariate as a proxy for socioeconomic status.
Statistical Analyses
Descriptive statistics were calculated as the frequency and proportion, mean with standard deviation (SD), or median with interquartile range according to the distribution of the variables. Maternal pre-pregnancy BMI and GWG were included as continuous variables. To evaluate the primary outcome of infant growth trajectory, the main effects of maternal pre-pregnancy BMI, maternal GWG and an interaction term were examined in a generalized least square model with adjustments for above covariates. Maternal pre-pregnancy BMI, GWG, and children’s age were included as non-linear effect using restricted cubic splines. The number and location of splines were determined by the distribution of each variable.32 For this model we included age with 5 knots, placed at 5%, 27.5%, 50%, 72.5% and 95% of the age variable. We included maternal age, maternal pre-pregnancy BMI, and maternal GWG with 3 knots each, located at 10%, 50%, and 90%. We report the overall effect of each variable of interest, which reflects the combined effects of the main effect of the variable and all interaction terms including the variable. This allowed us to examine whether the effect of the variable is significant at the level of an effect modifier. P-values were derived by Wald test. Specific comparisons were calculated via linear combination of regression coefficients (i.e. contrast, main effects, cross-products terms). Within patient correlation over time was accounted for using a first order autoregressive correlation structure as it produced the lowest Akaike information criterion.33 We tested the model assumptions by residual analyses. Statistical analysis was performed using R studio 3.0.1 (online at www.r-studio.com). All tests were 2-sided and a P value of less than 0.05 was considered significant.
Results
Patient characteristics
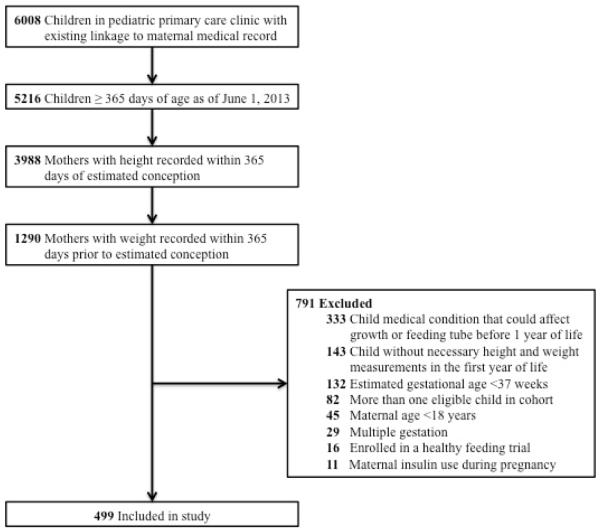
We identified 6008 maternal-infant dyads from the pediatric primary care panel and after application of the inclusion criteria and exclusion of data errors, 499 dyads were included in the analysis (Figure 1). Baseline characteristics of the cohort are presented in Table 1. The median estimated gestational age of the index children was 39.4 weeks (Interquartile range [IQR] 38.8, 40.3 weeks). About half (53%) of children were female, and the median birth weight was 3.4 kg (IQR 3.1, 3.7 kg). Based on information from well child clinic visits, 19% of children were exclusively breast fed for the first 6 months of life. The median maternal age at the time of delivery was 28.0 years (IQR 23.0, 32.5 years), and represented the first pregnancy for 35% of mothers. The distribution of maternal race/ethnicity included 54% White, Non-Hispanic, 32% Black, Non-Hispanic, 8% Asian, Non-Hispanic, and 6% Hispanic. Maternal co-morbidities included hypertension treated during pregnancy (3%), depression (10%), and gestational diabetes (9%). Prior to pregnancy the median maternal BMI was 25.6 kg/m2 (IQR 22.6, 31.1 kg/m2), with 1% of mothers classified as underweight (BMI ≤ 18.5), 44% classified as normal weight (BMI between 18.5 and 25), 26% classified as overweight (BMI between 25 and 30), and 29% classified as obese (BMI ≥ 30). Among the 142 mothers who were obese prior to pregnancy, 68 (48%) had class 1 obesity (BMI between 30 and 35), 41 (29%) had class 2 obesity (BMI between 35 and 40), and 33 (28%) had class 3 obesity (BMI≥ 40).
Figure 1.
Study Cohort of Maternal-Infant Dyads
Table 1.
Baseline Characteristics.
| Child Characteristics | N(%) or Median (IQR) |
|---|---|
| Child Gender: Female | 265 (53%) |
| Estimated Gestational Age (weeks) | 39.4 (38.8, 40.3) |
| Birth Weight (kg) | 3.4 (3.1, 3.7) |
| Exclusively Breast Fed for 6 months | 95 (19%) |
| Year of Child Birth | |
| 2007 | 38 (8%) |
| 2008 | 81 (16%) |
| 2009 | 90 (18%) |
| 2010 | 111 (22%) |
| 2011 | 131 (26%) |
| 2012 | 48 (10%) |
| Insurance Type | |
| Private Insurance | 257 (52%) |
| TN Medicaid | 242 (48%) |
| Maternal Characteristics | |
|
| |
| Age at Child’s Birth (years) | 28.0 (23.0, 32.5) |
| Number of Prior Live Births | 2 (1, 3) |
| Race/Ethnicity | |
| White, Non-Hispanic | 259 (54%) |
| Black, Non-Hispanic | 154 (32%) |
| Asian, Non-Hispanic | 38 (8%) |
| Other, Non-Hispanic | 2 (0%) |
| Hispanic | 28 (6%) |
| Maternal Pre-Pregnancy BMI | 25.6 (22.6, 31.1) |
| Maternal GWG (kg) | 12.3 (7.3, 16.8) |
| Maternal Medical Conditions | |
|
| |
| Diagnosed with Depression | 51 (10%) |
| Treated for HTN | 14 (3%) |
| Gestational Diabetes | 44 (9%) |
| Treated with Levothyroxine | 18 (4%) |
| Tobacco Use During Pregnancy | 46 (9%) |
| ETOH Use during pregnancy | 18 (4%) |
| Illicit Drug Use during pregnancy | 24 (5%) |
Data are presented as N (%) or median (interquartile range).
Association between maternal BMI, GWG, and infant growth trajectory
The median maternal gestational weight gain was 12.3 kg (IQR 7.3, 16.8 kg) and based on the recommendations from the IOM, 211 (42%) of mothers had excess gestational weight gain (Table 2). The percentage of mothers with excess GWG was highest for women who were overweight prior to pregnancy (55%). Mothers who were obese prior to pregnancy and had excess GWG gained an average of 15.8 kg (SD 5.8 kg), which was much higher than upper limit of 9.0 kg recommended by the IOM. Furthermore, of the 33 mothers who were morbidly obese prior to pregnancy (BMI ≥ 40 kg/m2), 33% experienced excess GWG. After adjustment for covariates in the generalized least square model, the main effect of older child age (p<0.001), higher maternal pre-pregnancy BMI (p<0.001), and the interaction term of maternal-pregnancy BMI and GWG (p=0.01) were significantly associated with infant growth patterns through the first year of life. The overall effect of GWG did not reach statistical significance (p=0.38).
Table 2.
Maternal Gestational Weight Gain by Pre-Pregnancy Body Mass Index and Institute of Medicine Recommendations
| Maternal Pre- Pregnancy BMI |
Number of Mothers |
IOM Recommended Weight Gain (kg) |
# Classified as Excess Gestational Weight Gain n(%) |
Mean (SD) Gestational Weight Gain for Mothers with Excess GWG (kg) |
|---|---|---|---|---|
| < 18.5 | 8 | 12.5-18.0 | 3 (37.5%) | 22.4 (3.0) |
| 18.5-24.9 | 218 | 11.5-16.0 | 74 (33.9%) | 20.7 (4.0) |
| 25.0-29.9 | 131 | 7.0-11.5 | 72 (55.0%) | 17.8 (4.9) |
| ≥30.0 | 142 | 5.0-9.0 | 62 (43.7%) | 15.8 (5.8) |
Data are presented as N (%) or median (interquartile range).
When stratified by maternal pre-pregnancy BMI alone, the overall shape of the infant growth curves was similar until reaching the highest category of maternal pre-pregnancy obesity (BMI 40 kg/m2) (Supplemental Figure 1). Infants of morbidly obese mothers were more likely to have a higher birth weight followed by a rapid increase in weight-for-length in the first 3 months of life. These infants subsequently developed a decrease in growth trajectory, resulting in a markedly different shape of the early growth curve when compared with children of mothers with normal pre-pregnancy BMI. For example, among infants of mothers with gestational weight gain in the recommended range (5kg), those born to morbidly obese mothers with a pre-pregnancy BMI (40 kg/m2) had an 8.3% higher weight/length percentile at 3 months (95% confidence interval [CI] 0.8%, 15.8%; p=0.03) compared with infants of mothers who were overweight prior to pregnancy (BMI= 25 kg/m2).
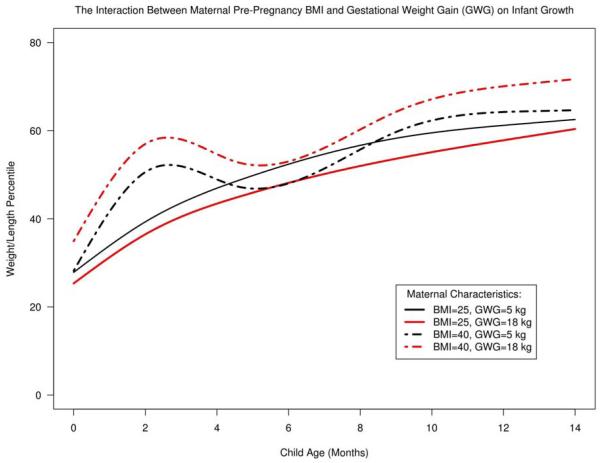
Maternal excess gestational weight gain amplified the effect of maternal pre-pregnancy BMI on infant growth. For infants of mothers with obese pre-pregnancy BMI, excess maternal GWG resulted in a statistically significant elevation of the growth curve at all points through the first year of life (Figure 2). For example, among infants of mothers with excess gestational weight gain (15kg), an obese pre-pregnancy BMI (40 kg/m2), resulted in a 13.6% increase in 3-month infant weight/length percentile, when compared with overweight pre-pregnancy BMI (25 kg/m2) (95% CI 5.8%, 21.5%; p<0.001). This effect was sustained at 12 months of age, where infants of mothers with a BMI of 40 kg/m2 had an 8.4% higher weight/length percentile compared to infants of mothers with a BMI of 25 kg/m2 (95% CI 0.2%, 16.46%; p=0.04). Infants of mothers with normal pre-pregnancy BMI and excess GWG normalized their growth by the first year.
Figure 2.
The Interaction between Maternal Pre-Pregnancy BMI and Gestational Weight Gain on Infant Growth.
Abbreviations: BMI=Body Mass Index (kg/m2); GWG=Gestational Weight Gain (kg)
Representative curves are shown from the adjusted generalized least square model to illustrate that the shape of the infant growth curve is markedly different for infants of mothers who were obese prior to pregnancy. There was a significant 2-way interaction between GWG and maternal pre-pregnancy BMI (p=0.02). This interaction indicates that the effect of maternal GWG on infant growth in the first year is modified by pre-pregnancy BMI. The model was adjusted for estimated gestational age, exclusive breast-feeding for the first 6 months of life, maternal age, number of previous pregnancies, maternal smoking, levothyroxine use, hypertension, depression, insurance type, and gestational diabetes.
Discussion
Given that both maternal pre-pregnancy BMI and maternal gestational weight gain have been associated with early childhood growth patterns that predispose children to obesity, understanding the interaction of this effect is important for both understanding the complex determinants of and developing effective interventions for childhood obesity. Our results demonstrate that the combined effect of maternal pre-pregnancy obesity and excess maternal GWG resulted in a significant elevation of the infant growth curve, which was sustained throughout the first year of life. Furthermore, this is the first study, to our knowledge, that utilizes growth trajectory throughout the first year of life as a primary outcome to demonstrate a fundamentally different shape to the growth curve of infants of mothers who were morbidly obese prior to pregnancy.
Our results show, consistent with other studies, that maternal pre-pregnancy BMI has a direct relationship with infant growth15,16 and that the interaction between maternal pre-pregnancy BMI and maternal GWG is associated with rapid infant weight gain.17 These results add to the current literature by confirming this recently described interaction between maternal pre-pregnancy BMI and maternal GWG and by characterizing the resultant shape of infant growth throughout an infant’s first year of life.
Specifically, infants of mothers with elevated pre-pregnancy BMI had a phenotype of rapid weight gain in the first months of life, followed by a sustained elevation in weight-for-length percentile throughout the first year of life. By using growth trajectory to characterize infant growth, our results show that infants of mothers who were not obese prior to pregnancy tend to normalize their growth trajectory over time, a finding that would have been missed without studying growth trajectory. Thus it is the shape of the early infant curve that could prove to be a powerful predictor of later health and provide a tool to better understand the phenotype of at-risk early pediatric obesity.
The underlying biological or behavioral etiology that would explain the differing shape of the infant growth curve is unclear. Our data do not point to breast-feeding as a principal explanatory mechanism, as it was controlled for in our statistical modeling and it did not reach statistical significance. The finding that the growth curve separates so early in life for infants of morbidly obese mothers suggests an intra-uterine programming that may contribute to early growth. Based on these data, we suggest that future studies focus on the relationship between maternal-fetal biology and maternal feeding practices to further elucidate the complex determinants of early child growth. This line of research inquiry would provide guidance to prevent childhood obesity starting preconception and in-utero.
In addition, our results show excess gestational weight gain amplifies the dysregulated pattern of infant growth for mothers who were morbidly obese prior to pregnancy. Conversely, among women with a normal pre-pregnancy BMI, excess GWG was not a primary determinant of altered infant growth through the first year of life. Thus, these results support the 2009 IOM recommendations for gestational weight gain, especially for mothers who have a BMI ≥ 40kg/m2. Moreover, it underscores the importance of maternal post-partum weight loss, as returning to healthy BMI after pregnancy would be a potential opportunity to improve infant growth trajectory for subsequent children.
The finding that excess gestational weight gain amplifies the effect of pre-pregnancy obesity on infant growth in the first year of life points to pregnancy as an important time to consider pediatric obesity prevention. While the biological mechanism for this has been postulated for decades, research has only recently begun to describe the complex mechanisms underpinning a developmental plasticity towards an obese childhood phenotype.13,34 Noting that the growth curve in the first year of life is markedly different for infants of mothers who were obese prior to pregnancy compared to those infants exposed to normal pre-pregnancy BMI, it will be important for further research to better characterize whether interventions during the pre-conception or in utero periods will be most effective for pediatric obesity prevention.
This study has several limitations. As in any cohort study, the potential for residual confounding exists. To minimize this possibility, we used a strict set of inclusion and exclusion criteria to narrowly define our cohort that limits the generalizability of the findings. However, this study used objective measurements for height and weight within 365 days of conception and represents an improvement over related studies, which previously used self-reported pre-pregnancy BMI measures.8,35,36 The anthropometric measurements were taken as a part of routine care, and not specifically for the purposes of this study. Likewise all of the other variables of interest were abstracted from the EMR. As such, there was no ability to validate these measurements prospectively. This is likely a non-differential misclassification bias, however, and would likely bias the results towards the null. We also had a relatively low number of mothers with class 3 obesity prior to pregnancy. Because of our strict inclusion criteria, the final sample represented <10% of initial sample of maternal-child pairs from the pediatric primary care clinic. This introduces the potential for a selection bias. Finally, the population studied was from a single institution, and had a relatively low percentage of Hispanic mothers, limiting the generalizability of our findings for this group.
Conclusion
The interaction between maternal pre-pregnancy BMI and maternal gestational weight gain shapes infant growth in the first year of life. Further work will clarify the complex relationship between the underlying biological mechanisms that may predispose children to develop obesity and the behavioral and environmental influences that may contribute to an already dysregulated growth trajectory in infancy. What is clear, however, is that counseling pregnant women, especially those that are already obese, about the importance of appropriate gestational weight gain and post-partum weight loss represents an underutilized opportunity for a public health intervention to prevent pediatric obesity.
Supplementary Material
Supplemental Table 1: Recommended Gestational Weight Gain based on 2009 IOM Guidelines9
Supplemental Figure 1: Infant Growth Trajectory stratified by Maternal Pre-Pregnancy BMI.
Abbreviations: BMI=Body Mass Index (kg/m2); GWG=Gestational Weight Gain (kg) Expected infant growth trajectory based on the adjusted generalized least square model is presented for four representative values of maternal pre-pregnancy BMI. There was a significant 2-way interaction between GWG and maternal pre- pregnancy BMI (p=0.02). This interaction indicates that the effect of maternal GWG on infant growth in the first year is modified by pre-pregnancy BMI. The analysis was adjusted for estimated gestational age, exclusive breast-feeding for the first 6 months of life, maternal age, number of previous pregnancies, maternal smoking, levothyroxine use, hypertension, depression, insurance type, and gestational diabetes. The range of maternal gestational weight gain is shown for each BMI category.
What’s New.
The combined effect of maternal obesity prior to pregnancy and excess maternal gestational weight gain results in rapid infant weight gain and sustained dysregulation of infant growth through the first year of life, suggesting new approaches to pediatric obesity prevention.
Acknowledgements
The project described was supported by a young-investigator award from the Academic Pediatric Association and the Maternal-Child Health Bureau.
Preliminary data from this manuscript were presented at the 2014 Pediatric Academic Societies meeting in Vancouver, BC.
It was also supported, in part, by award number U01 HL03620-03 from the National Heart, Lung, and Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Development and the Office of Behavioral and Social Sciences Research as well as by CTSA award number UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Dr. Heerman is supported by a T-32 grant through Vanderbilt University (5T32HD060554-05).
Dr. Shintani’s and Dr. Barkin’s work is supported in part by grant P30DK092986.
The data management system REDCap was supported by a grant from NCATS/NIH (UL1 TR000445).
We would also like to acknowledge Ioana Danciu, Jana Shirey-Rice, Michelle DeRaneiri, and Pat Gideon for their help with chart abstraction, and Dr. Warren Lambert for his assistance with conceptualizing the preliminary data analysis plan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA : the journal of the American Medical Association. 2012 Feb 1;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taveras EM, Rifas-Shiman SL, Sherry B, et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Archives of pediatrics & adolescent medicine. 2011 Nov;165(11):993–998. doi: 10.1001/archpediatrics.2011.167. [DOI] [PubMed] [Google Scholar]
- 3.Ekelund U, Ong K, Linne Y, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) The American journal of clinical nutrition. 2006 Feb;83(2):324–330. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 4.Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA : the journal of the American Medical Association. 2009 Jun 3;301(21):2234–2242. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- 5.Ekelund U, Ong KK, Linne Y, et al. Association of weight gain in infancy and early childhood with metabolic risk in young adults. The Journal of clinical endocrinology and metabolism. 2007 Jan;92(1):98–103. doi: 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- 6.Laitinen J, Jaaskelainen A, Hartikainen AL, et al. Maternal weight gain during the first half of pregnancy and offspring obesity at 16 years: a prospective cohort study. BJOG : an international journal of obstetrics and gynaecology. 2012 May;119(6):716–723. doi: 10.1111/j.1471-0528.2012.03319.x. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005 Oct 27;353(17):1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 8.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009 Apr;123(4):1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute of Medicine . Weight Gain During Pregnancy; Reexamining the Guidelines. Vol. 2009. National Research Council; Committee to Reexamine IOM Pregnancy Weight Guidelines; Washington, D.C.: May 28, 2009. [Google Scholar]
- 10.Hochberg Z, Feil R, Constancia M, et al. Child health, developmental plasticity, and epigenetic programming. Endocr Rev. 2011 Apr;32(2):159–224. doi: 10.1210/er.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004 Jan 29;427(6973):411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet. 2010 Sep 18;376(9745):984–990. doi: 10.1016/S0140-6736(10)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oken E, Gillman MW. Fetal origins of obesity. Obesity research. 2003 Apr;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 14.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. American journal of obstetrics and gynecology. 2007 Apr;196(4):322, e321–328. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PloS one. 2013;8(4):e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nohr EA, Bech BH, Vaeth M, Rasmussen KM, Henriksen TB, Olsen J. Obesity, gestational weight gain and preterm birth: a study within the Danish National Birth Cohort. Paediatric and perinatal epidemiology. 2007 Jan;21(1):5–14. doi: 10.1111/j.1365-3016.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- 17.Li N, Liu E, Guo J, et al. Maternal prepregnancy body mass index and gestational weight gain on offspring overweight in early infancy. PloS one. 2013;8(10):e77809. doi: 10.1371/journal.pone.0077809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Group WHOMGRS WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006 Apr;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 20.Leroy J. ZSCORE06. Stata. 2011 [Google Scholar]
- 21.de Jongh BE, Paul DA, Hoffman M, Locke R. Effects of Pre-pregnancy Obesity, Race/Ethnicity and Prematurity. Maternal and child health journal. 2013 Jun 26; doi: 10.1007/s10995-013-1296-8. [DOI] [PubMed] [Google Scholar]
- 22.Matias SL, Dewey KG, Quesenberry CP, Jr., Gunderson EP. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. The American journal of clinical nutrition. 2013 Nov 6; doi: 10.3945/ajcn.113.073049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Rossem L, Taveras EM, Gillman MW, et al. Is the association of breastfeeding with child obesity explained by infant weight change? International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2011 Jun;6(2-2):e415–422. doi: 10.3109/17477166.2010.524700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frederick IO, Williams MA, Sales AE, Martin DP, Killien M. Pre-pregnancy body mass index, gestational weight gain, and other maternal characteristics in relation to infant birth weight. Maternal and child health journal. 2008 Sep;12(5):557–567. doi: 10.1007/s10995-007-0276-2. [DOI] [PubMed] [Google Scholar]
- 25.Ong KK, Preece MA, Emmett PM, Ahmed ML, Dunger DB, Team AS. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Pediatric research. 2002 Dec;52(6):863–867. doi: 10.1203/00006450-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Healton CG, Vallone D, McCausland KL, Xiao H, Green MP. Smoking, obesity, and their co-occurrence in the United States: cross sectional analysis. Bmj. 2006 Jul 1;333(7557):25–26. doi: 10.1136/bmj.38840.608704.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Shankaran S. Effects of alcohol use, smoking, and illicit drug use on fetal growth in black infants. The Journal of pediatrics. 1994 May;124(5 Pt 1):757–764. doi: 10.1016/s0022-3476(05)81371-x. [DOI] [PubMed] [Google Scholar]
- 28.Orbach H, Matok I, Gorodischer R, et al. Hypertension and antihypertensive drugs in pregnancy and perinatal outcomes. American journal of obstetrics and gynecology. 2013 Apr;208(4):301, e301–306. doi: 10.1016/j.ajog.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. American journal of public health. 2000 Feb;90(2):251–257. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong L, Liu E, Guo J, et al. Relationship between maternal fasting glucose levels at 4-12 gestational weeks and offspring growth and development in early infancy. Diabetes research and clinical practice. 2013 Nov 2; doi: 10.1016/j.diabres.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiologic reviews. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 32.Harrell FE. Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. Springer; New York: 2001. [Google Scholar]
- 33.Atkinson. A note on the generalized information criterion for choice of a model. Biometrika. 1980;67:413–418. [Google Scholar]
- 34.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976 Aug 12;295(7):349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 35.Baker JL, Michaelsen KF, Rasmussen KM, Sorensen TI. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. The American journal of clinical nutrition. 2004 Dec;80(6):1579–1588. doi: 10.1093/ajcn/80.6.1579. [DOI] [PubMed] [Google Scholar]
- 36.Hinkle SN, Sharma AJ, Swan DW, Schieve LA, Ramakrishnan U, Stein AD. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. The Journal of nutrition. 2012 Oct;142(10):1851–1858. doi: 10.3945/jn.112.161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Recommended Gestational Weight Gain based on 2009 IOM Guidelines9
Supplemental Figure 1: Infant Growth Trajectory stratified by Maternal Pre-Pregnancy BMI.
Abbreviations: BMI=Body Mass Index (kg/m2); GWG=Gestational Weight Gain (kg) Expected infant growth trajectory based on the adjusted generalized least square model is presented for four representative values of maternal pre-pregnancy BMI. There was a significant 2-way interaction between GWG and maternal pre- pregnancy BMI (p=0.02). This interaction indicates that the effect of maternal GWG on infant growth in the first year is modified by pre-pregnancy BMI. The analysis was adjusted for estimated gestational age, exclusive breast-feeding for the first 6 months of life, maternal age, number of previous pregnancies, maternal smoking, levothyroxine use, hypertension, depression, insurance type, and gestational diabetes. The range of maternal gestational weight gain is shown for each BMI category.