Abstract
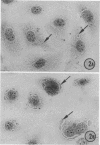
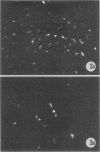
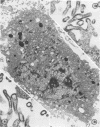
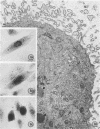
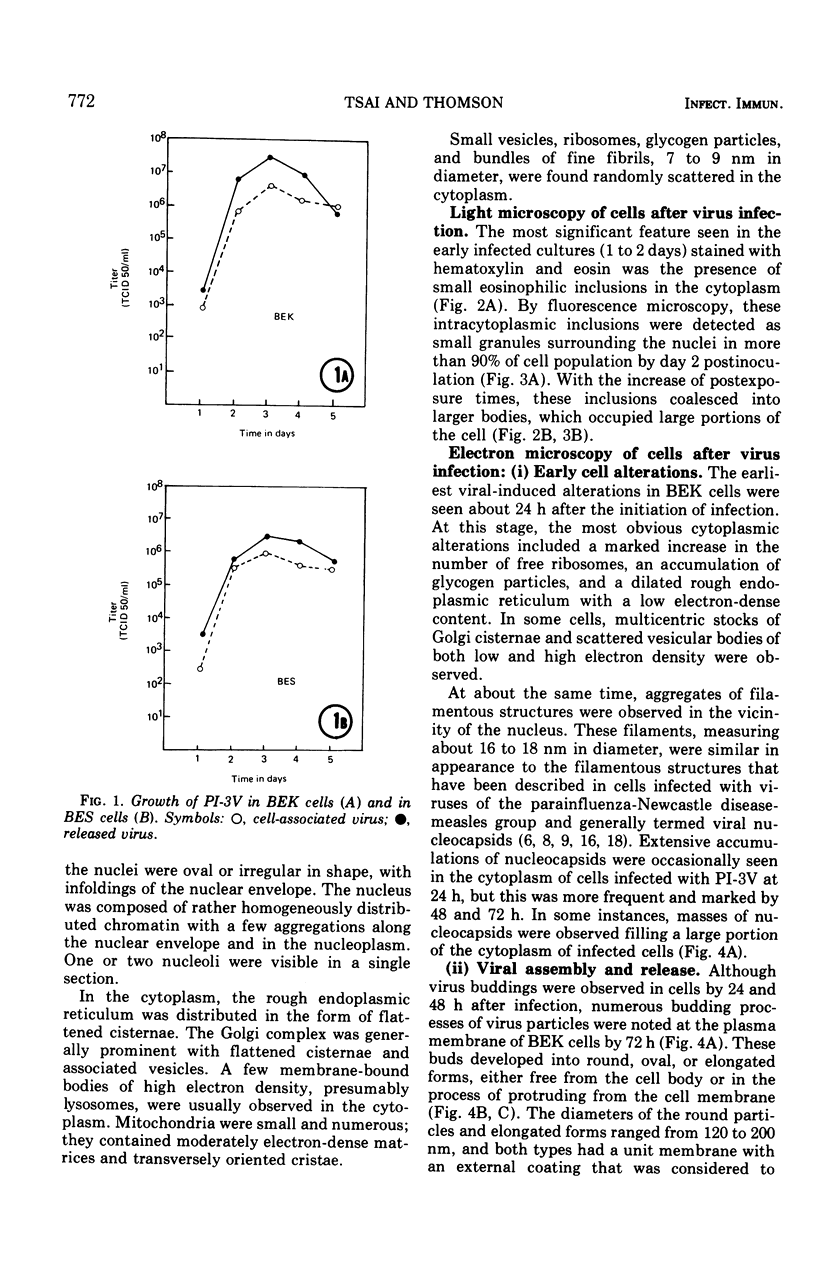
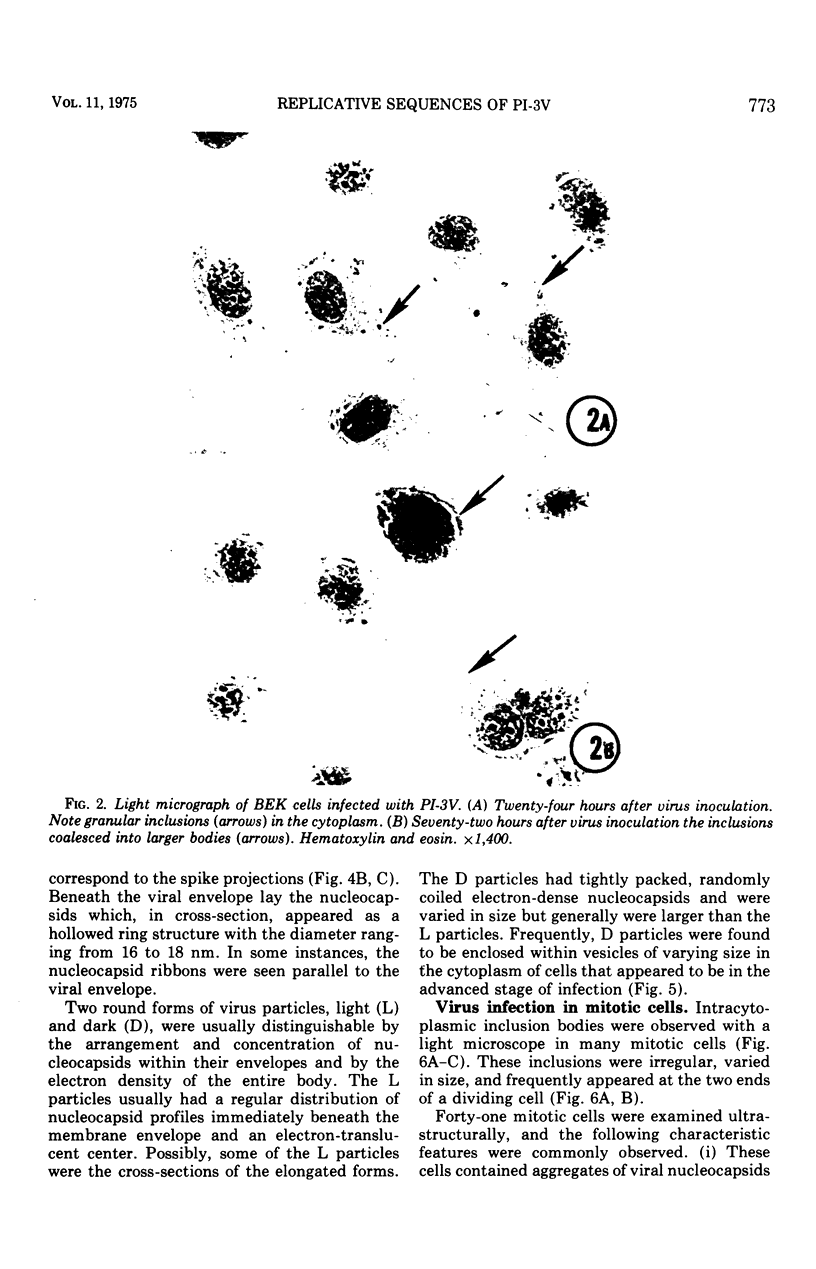
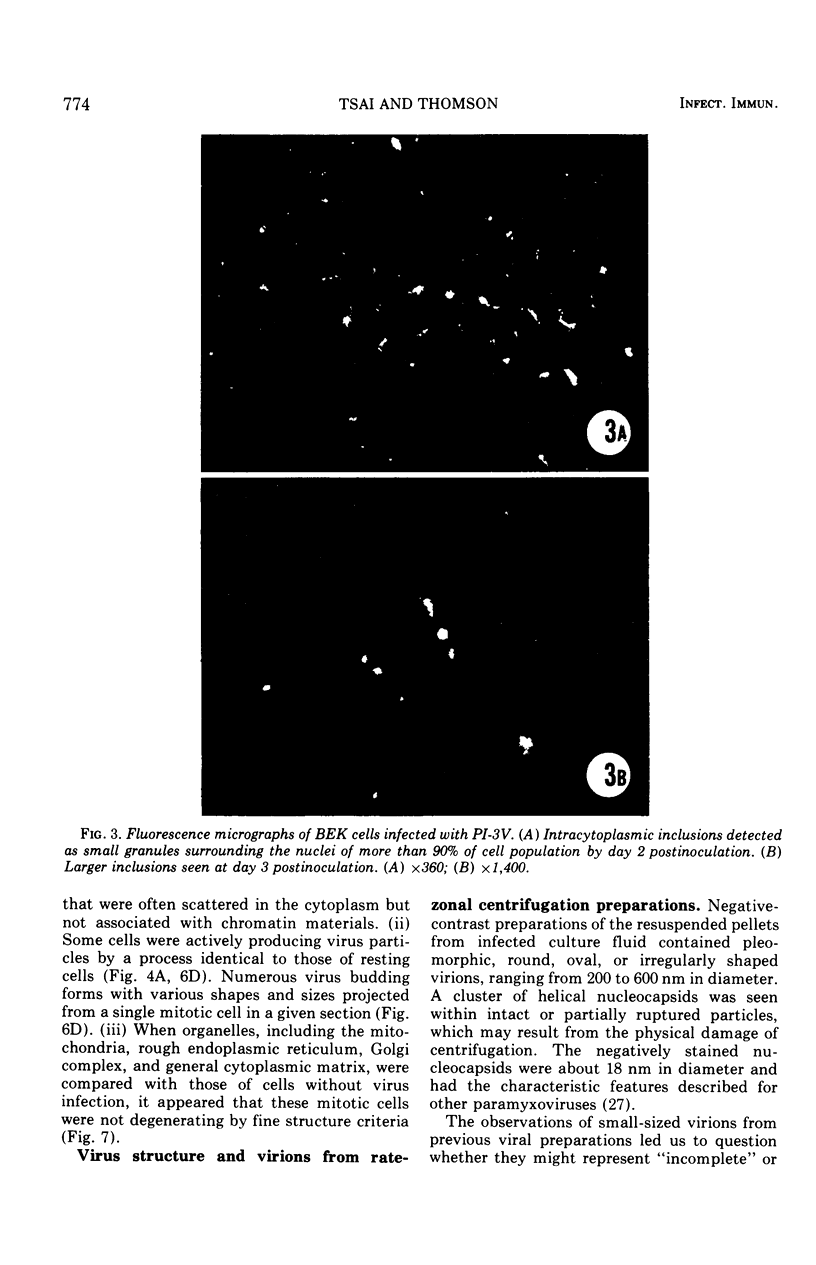
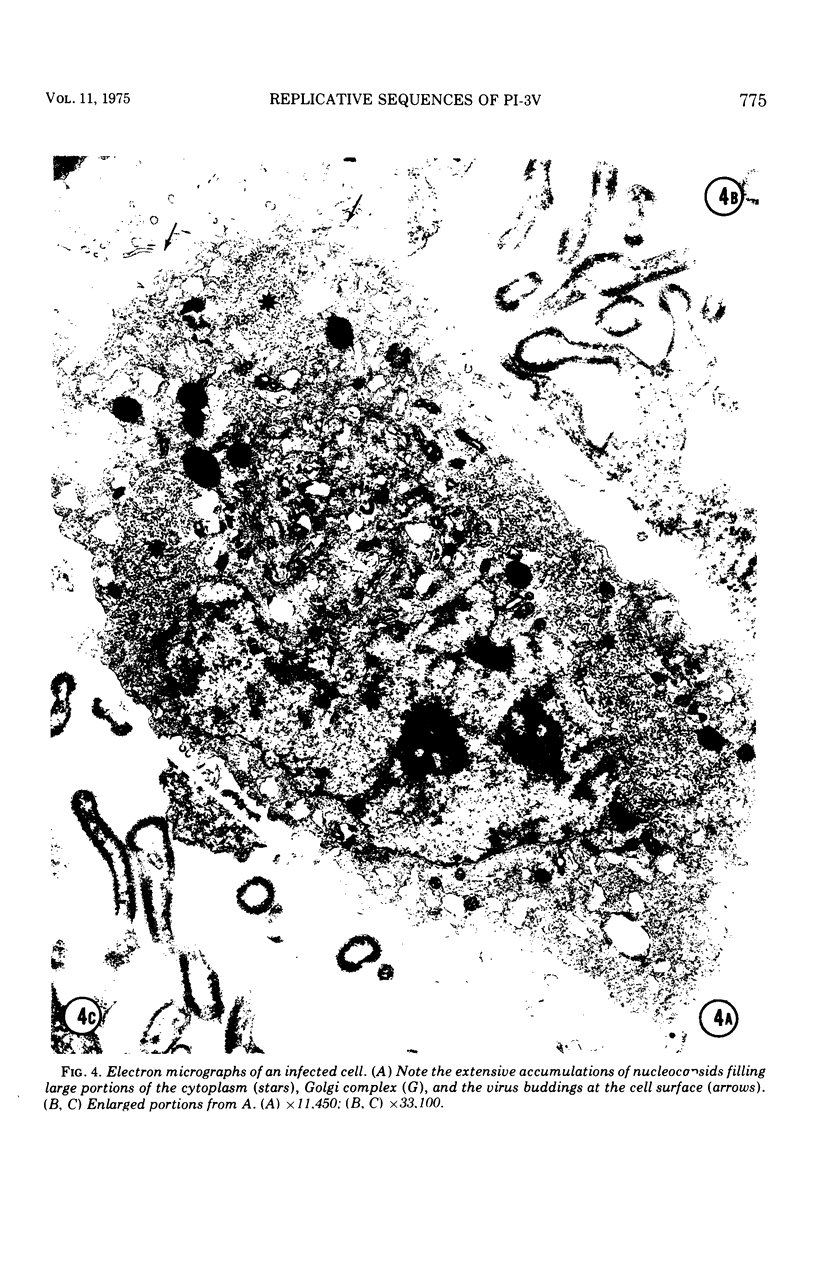
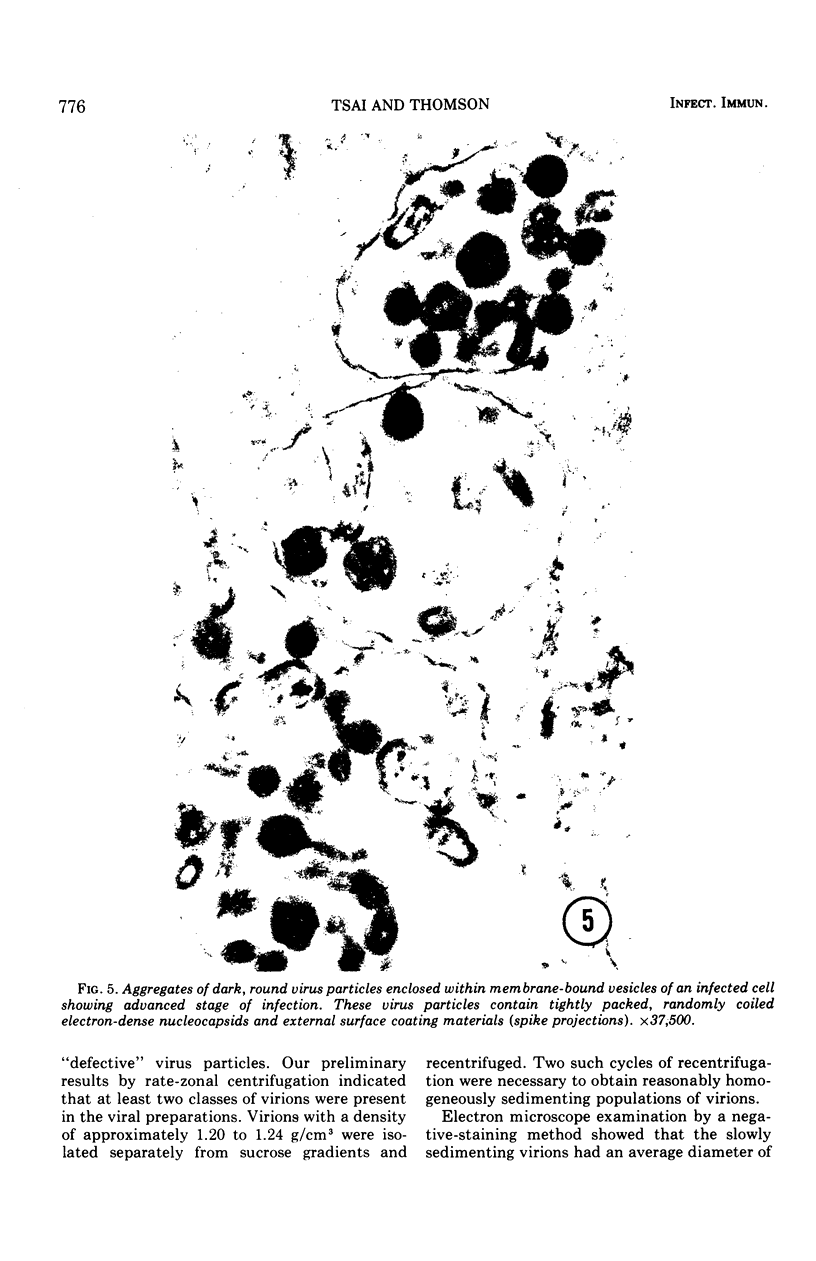
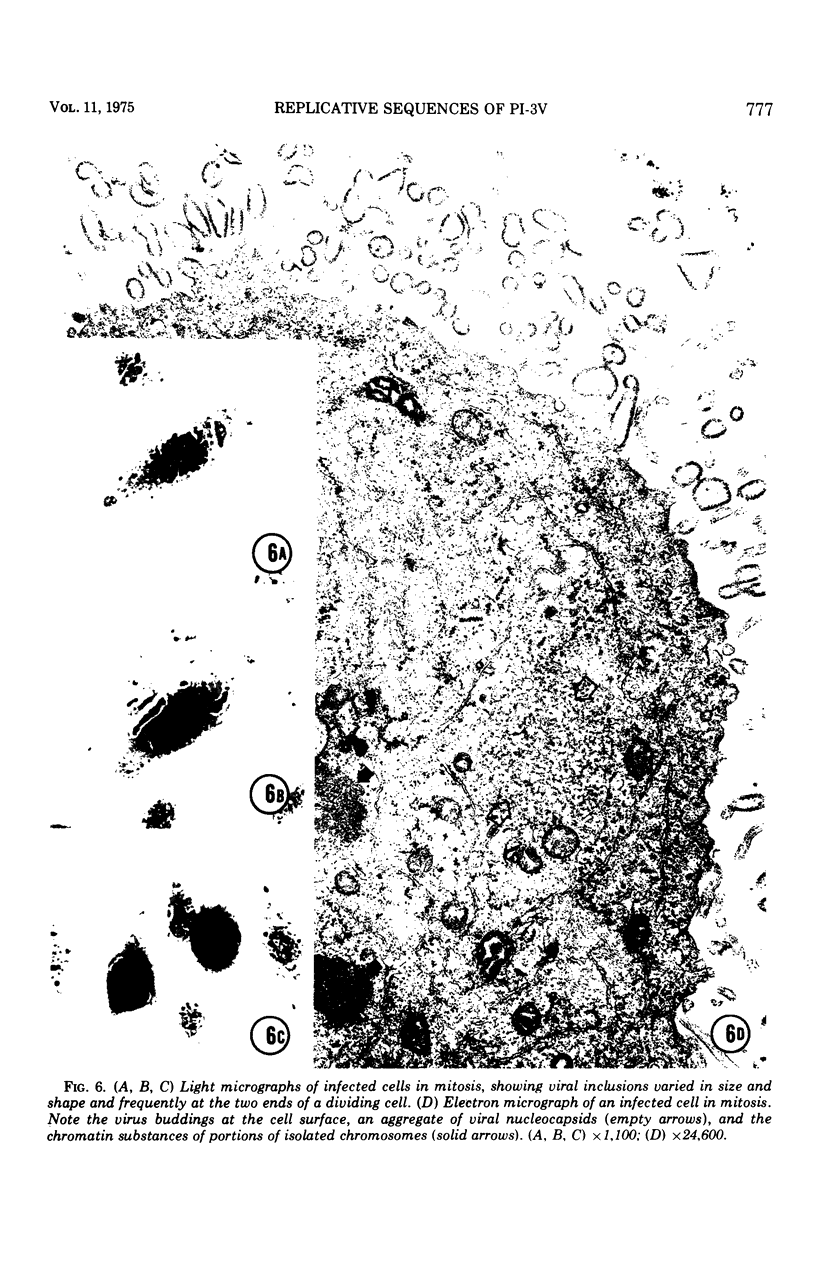
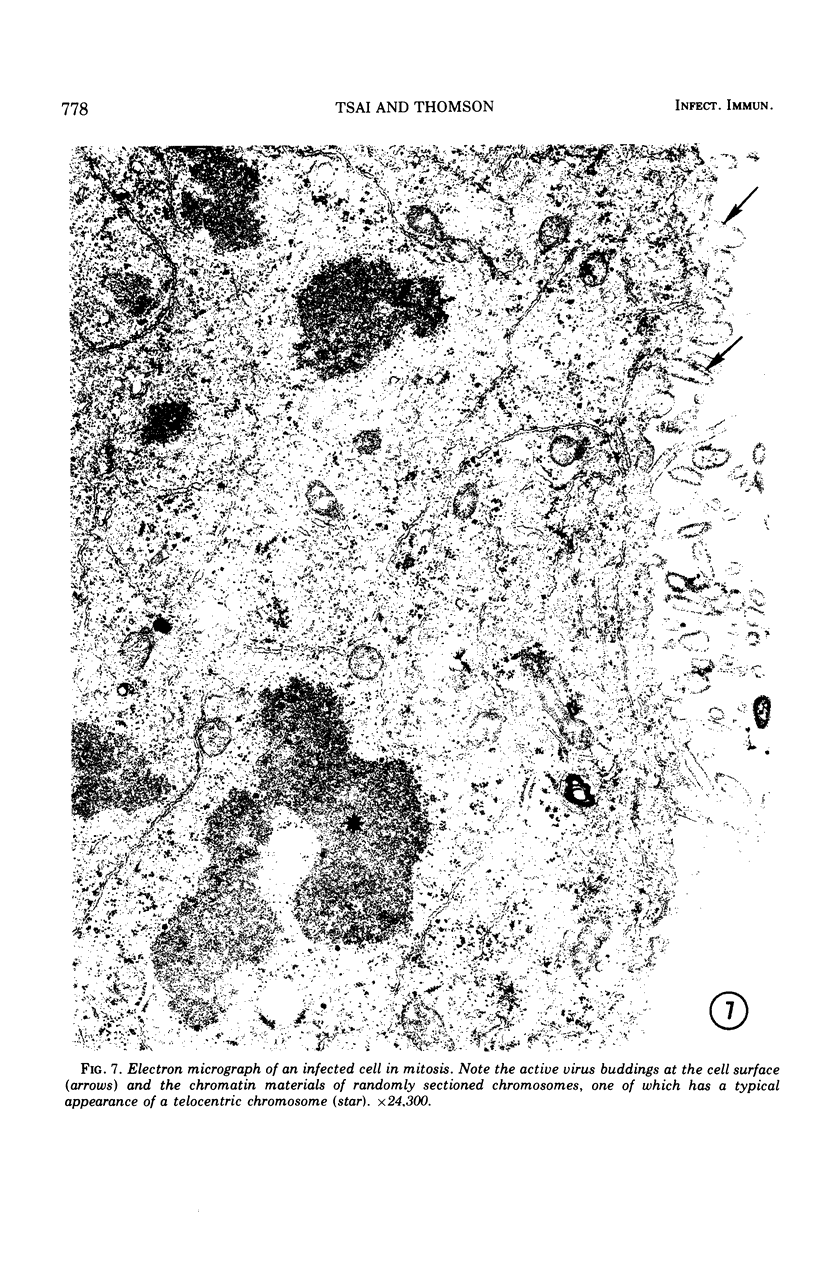
Replicative sequences of a bovine strain of parainfluenza type 3 virus in bovine embryonic kidney and spleen cell cultures were investigated by light and fluorescence microscopy and by ultrathin section and negative-contrast electron microscopy. Observations from light and fluorescence microscopy showed that intracytoplasmic inclusions were detected as small granules surrounding the nuclei of more than 90 percent of the cell population by day 2 postinoculation. With the increase of postexposure times, these inclusions coalesced into larger bodies which occupied large portions of the cell. Ultrastructurally, the first sign of virus development was the appearance of aggregates of viral nucleocapsids in the vicinity of the nucleus. With the concomitant accumulation of viral nucleocapsids in the cytoplasm, the virus maturation was expressed by budding processes through the cell membrane into round, oval, or elongated forms. Eosinophilic inclusions were demonstrable in many mitotic cells. Ultrastructurally, these cells were observed to produce virus particles by a process identical to that of resting cells. Virions, prepared from infected culture fluid and negatively stained, appeared to be pleomorphic and their diameter ranged from 200 to 600 mm. The virions were separated, by rate-zonal centrifugation, into two subclasses in a sucrose gradient (15 to 60 percent, wt/wt). The slowly sedimenting virions had a density approximately 1.20 gm/cm3 and an average size of 200 nm in diameter, whereas the faster-sedimenting virions had a density of 1.24 gm/cm3 and average diameter of 400 nm.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANG F. B. The development of Newcastle disease virus in cells of the chorio-allantoic membrane as studied by thin section. Bull Johns Hopkins Hosp. 1953 Apr;92(4):309–329. [PubMed] [Google Scholar]
- Baldwin D. E., Marshall R. G., Wessman G. E. Experimental infection of calves with myxovirus parainfluenza-3 and Pasteurella haemolytica. Am J Vet Res. 1967 Nov;28(127):1773–1782. [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Portner A., Kingsbury D. W. Sendai virus replication: an ultrastructural comparison of productive and abortive infections in avian cells. J Gen Virol. 1970 Dec;9(3):169–177. doi: 10.1099/0022-1317-9-3-169. [DOI] [PubMed] [Google Scholar]
- Donnelly W. H., Yunis E. J. The morphogenesis of virulent Newcastle disease virus in the chick embryo. An ultrastructural study. Am J Pathol. 1971 Jan;62(1):87–110. [PMC free article] [PubMed] [Google Scholar]
- Feller U., Dougherty R. M., Di Stefano H. S. Morphogenesis of Newcastle disease virus in chorioallantoic membrane. J Virol. 1969 Nov;4(5):753–762. doi: 10.1128/jvi.4.5.753-762.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMDY A. H., TRAPP A. L., GALE C., KING N. B. Experimental transmission of shipping fever in calves. Am J Vet Res. 1963 Mar;24:287–294. [PubMed] [Google Scholar]
- HEDDLESTON K. L., REISINGER R. C., WATKO L. P. Studies on the transmission and etiology of bovine shipping fever. Am J Vet Res. 1962 May;23:548–553. [PubMed] [Google Scholar]
- HETRICK F. M., CHANG S. C., BYRNE R. J., HANSEN P. A. THE COMBINED EFFECT OF PASTEURELLA MULTOCIDA AND MYXOVIRUS PARAINFLUENZA-3 UPON CALVES. Am J Vet Res. 1963 Sep;24:939–947. [PubMed] [Google Scholar]
- Holmes K. V., Klenk H. D., Choppin P. W. A comparison of immune cytolysis and virus-induced fusion of sensitive and resistant cell types. Proc Soc Exp Biol Med. 1969 Jun;131(2):651–657. doi: 10.3181/00379727-131-33945. [DOI] [PubMed] [Google Scholar]
- Hotchin J. Transient virus infection: spontaneous recovery mechanism of lymphocytic choriomeningitis virrus-infected cells. Nat New Biol. 1973 Feb 28;241(113):270–272. doi: 10.1038/newbio241270a0. [DOI] [PubMed] [Google Scholar]
- Howe C., Morgan C., de Vaux St Cyr C., Hsu K. C., Rose H. M. Morphogenesis of type 2 parainfluenza virus examined by light and electron microscopy. J Virol. 1967 Feb;1(1):215–237. doi: 10.1128/jvi.1.1.215-237.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund S., Morein B. A morphological study of outfolded and released parainfluenza type 3 virus. J Gen Virol. 1973 Nov;21(2):359–369. doi: 10.1099/0022-1317-21-2-359. [DOI] [PubMed] [Google Scholar]
- McLean A. M., Doane F. W. The morphogenesis and cytopathology of bovine parainfluenza type 3 virus. J Gen Virol. 1971 Sep;12(3):271–279. doi: 10.1099/0022-1317-12-3-271. [DOI] [PubMed] [Google Scholar]
- Nakai T., Shand F. L., Howatson A. F. Development of measles virus in vitro. Virology. 1969 May;38(1):50–67. doi: 10.1016/0042-6822(69)90127-5. [DOI] [PubMed] [Google Scholar]
- Numazaki Y., Karzon D. T. Density separable fractions during growth of measles virus. J Immunol. 1966 Oct;97(4):458–469. [PubMed] [Google Scholar]
- RECZKO E., BOEGEL K. [Electron microscopy studies on the behavior of a parainfluenza-3 virus isolated from calves in calf kidney cell culture]. Arch Gesamte Virusforsch. 1962;12:404–420. [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Ultrastructure of measles virus in cultures of hamster cerebellum. J Virol. 1969 Aug;4(2):169–181. doi: 10.1128/jvi.4.2.169-181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson R. G., Benson M. L., Savan M. Pneumonic pasteurellosis of cattle: microbiology and immunology. Can J Comp Med. 1969 Jul;33(3):194–206. [PMC free article] [PubMed] [Google Scholar]
- WALKER D. L., HINZE H. C. A carrier state of mumps virus in human conjunctiva cells. I. General characteristics. J Exp Med. 1962 Nov 1;116:739–750. doi: 10.1084/jem.116.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATERSON A. P., HURRELL J. M. The fine structure of the parainfluenza viruses. Arch Gesamte Virusforsch. 1962;12:138–142. doi: 10.1007/BF01258760. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Katz M., Müller D. Subacute sclerosing panencephalitis: a review. Curr Top Microbiol Immunol. 1972;57:1–38. doi: 10.1007/978-3-642-65297-4_1. [DOI] [PubMed] [Google Scholar]