Abstract
In the adult mammalian brain, bona fide neural stem cells were discovered in the subventricular zone (SVZ), the largest neurogenic niche lining the striatal wall of the lateral ventricles of the brain. In this region resides a subpopulation of astrocytes that express the glial fibrillary acidic protein (GFAP), nestin and LeX. Astonishingly, these GFAP-expressing progenitors display stem-cell-like features both in vivo and in vitro. Throughout life SVZ astrocytes give rise to interneurons and oligodendrocyte precursors, which populate the olfactory bulb and the white matter, respectively. The role of the progenies of SVZ astrocytes has not been fully elucidated, but some evidence indicates that the new neurons play a role in olfactory discrimination, whereas oligodendrocytes contribute to myelinate white matter tracts. In this chapter, we describe the astrocytic nature of adult neural stem cells, their organization into the SVZ and some of their molecular and genetic characteristics.
INTRODUCTION
In 1931, Santiago Ramon y Cajal stated “The Nature has granted us with a limited endowment of cerebral cells. This is a resource, large or little, which nobody can increase because the neuron is unable to reproduce itself” (Cajal’s speech for the “Archivo de la Palabra”, Spain). This statement soon became a dogma that was passionately supported by many other prominent neuroscientists. However, at the 1960’s, Joseph Altman (Altman, 1962) challenged this assumption by showing that the postnatal brain was not a quiescent organ incapable of neurogenesis. In a series of experiments Altman and colleagues, as well as other groups, described the presence of neurons labeled with tritiated thymidine (3H-TdR) in the subependymal zone lining the lateral wall of the lateral ventricles, which suggested the presence of neurogenesis in the adult brain (Altman and Gopal, 1965; Altman and Das, 1967; Kaplan and Hinds, 1977). Later studies from other groups also described ongoing neurogenesis in female canaries (Goldman and Nottebohm, 1983) and lizards (Pérez-Cañellas and García-Verdugo, 1996). Since 3H-TdR may also induce cell cycle arrest and apoptosis, these initial results were somehow criticized and could not unequivocally determine the presence of neurogenesis in the adult brain. This controversy was finally finished by Arturo Alvarez-Buylla and coworkers, whom in serial experiments not only demonstrated the presence of new born neurons (neuroblasts) in vivo, but also proved that the primary progenitor of these neuroblasts is a subpopulation of astrocytes resident in the lateral walls of the lateral ventricles of the adult mammalian brain. These newly generated cells migrate anteriorly and form functional circuits with the resident neurons in the olfactory bulb (Lois and Alvarez-Buylla, 1993, 1994b; Lois et al., 1996; Doetsch et al., 1997; Wichterle et al., 1997; Doetsch et al., 1999b; Sanai et al., 2004; Spassky et al., 2005; Jackson et al., 2006; Merkle et al., 2007).
Since the discovery of neurogenesis in the adult mammalian brain, a lot of research has been done in several brain regions to determine the presence new born neurons but, so far, it is well-accepted that this process is mainly confined to the subventricular zone (SVZ) at the forebrain and the subgranular zone (SGZ) in the dentate gyrus at the hippocampus (Reznikov, 1991; Luskin, 1993; Lois and Alvarez-Buylla, 1994a). The SVZ is the largest neurogenic niche in the adult mammalian brain (Luskin, 1993; Alvarez-Buylla and Garcia-Verdugo, 2002). In this region resides a subpopulation of glial cells with stem-cell-like features, both in vivo and in vitro, which can give rise to neurons, oligodendrocytes, NG2-glia and astrocytes (Doetsch et al., 1999c; Laywell et al., 2000; Imura et al., 2003; Morshead et al., 2003; Garcia et al., 2004; Menn et al., 2006; Gonzalez-Perez et al., 2009; Gonzalez-Perez and Quinones-Hinojosa, 2010; Gonzalez-Perez and Alvarez-Buylla, 2011). The role of the new born neurons and oligodendrocytes derived from the adult SVZ has not been fully elucidated, but some evidence indicates that the new neurons play a role in odor discrimination tasks (Gheusi et al., 2000), whereas SVZ oligodendrocyte precursors contribute to maintain the population of oligodendrocytes in the white matter (Menn et al., 2006; Gonzalez-Perez et al., 2009; Gonzalez-Perez and Alvarez-Buylla, 2011).
In contrast to the SVZ progenitors, the precursor cells found in the SGZ of dentate gyrus in the hippocampus appear not to be bona fide neural stem cells, because they only give rise to neurons in vivo. In fact, some studies showed that adult SGZ progenitors were not multipotent self-renewing progenitors (Seaberg and van der Kooy, 2002; Bull and Bartlett, 2005). Therefore, SGZ neurogenic progenitors are not considered neural stem cells, but neuronal precursor cells (Song et al., 2002; Jagasia et al., 2006). In this chapter, we discuss the characteristics and functions of adult neural stem cells per se, as well as, we describe the evidence that demonstrated the astrocytic identity of multipotent cells resident within the adult SVZ.
Neural stem cells in the adult brain
Early experiments in vitro found that tissue harvested from the SVZ contained a subpopulation of multipotent precursor cells, which under growth-factor-enriched conditions were able to produce self-renewing clones (Reynolds and Weiss, 1992; Morshead et al., 1994; Weiss et al., 1996). These clones proliferated and gave rise to spherical cell aggregates known as ‘neurospheres’, which if subsequently subjected to growth factor removal and serum-free conditions, formed neurons, oligodendrocytes, and astrocytes, suggesting that these self-renewing cells are also multipotent. In consequence, the neurosphere assay has been extensively utilized as a method to indicate the presence of stem cells in tissue samples. However, more recent evidence indicates that this assay might not accurately identify stem cell capacity in vivo. Therefore in vivo experiments are always required to fully demonstrate the ‘stemness’ potential in the mammalian brain. The capacity of SVZ progenitor cells to behave as putative stem cells in vivo was subsequently demonstrated in rodents and humans (Doetsch et al., 1999b; Laywell et al., 2000; Sanai et al., 2004).
The presence of bona fide neural stem cells in the adult brain has been demonstrated through multiple experimental approaches. Primary precursors were identified in vivo by using deoxythymidine or bromodeoxyuridine (BrdU) as cell proliferation markers. Thus, it was found that a subpopulation of astrocytes remain labeled into the SVZ and SGZ after long survival times, which suggested that these glial cells corresponded to stem cells (Doetsch et al., 1999b; Alvarez-Buylla et al., 2002). These multipotent astrocytes are denominated as type-B cells, which typically express the glial fibrillary acidic protein (GFAP) and have very well-defined ultrastructural characteristics (see Chapter 5). The first evidence indicating that SVZ astrocytes are neural stem cells was provided by Fiona Doetsch et al., she discovered that a subpopulation of astrocytes that survived to an intracerebroventricular administration of a cytotoxic drug (cytosine-β-D-arabinofuranoside, Ara-C) was able to fully regenerate all germinal progenitors within the SVZ over a period of 14 days (Doetsch et al., 1999b; Doetsch et al., 1999a). After that, also using Ara-C to identify the primary progenitor in the SGZ, Betina Seri et al. found that SGZ radial astrocytes (named type-D cells) were capable of reconstituting the germinal layers in the dentate gyrus (Seri et al., 2001). Later reports confirmed these observations indicating that the GFAP-expressing astrocytes are candidate stem cells in the SVZ (Chiasson et al., 1999; Capela and Temple, 2002; Garcia et al., 2004; Spassky et al., 2005). Interestingly, astrocytes collected from multiple brain regions before postnatal day 10 may behave as neural stem cells in vitro. After that time, only the SVZ astrocytes retain this ‘stemness’ capacity (Lim and Alvarez-Buylla, 1999; Laywell et al., 2000).
Organization of the subventricular zone
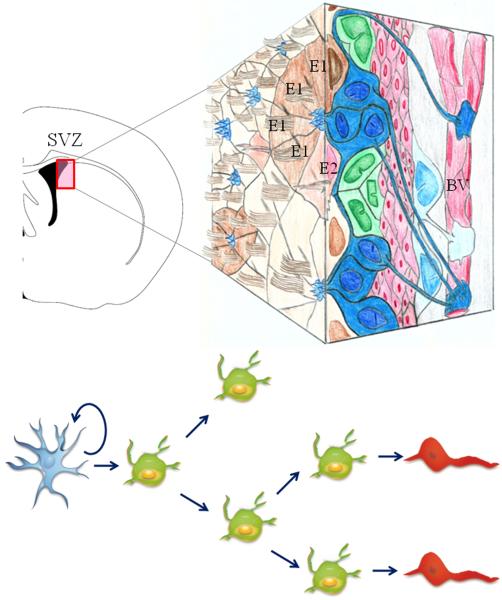
The subventricular zone, the largest niche of adult neural stem cells, is located along the lateral walls of the lateral ventricles in the forebrain (Figure 1) (Doetsch et al., 1997; Doetsch, 2003). To have a complete view of the SVZ as a neurogenic niche, we have to study not only the cell organization and ultrastructure of the SVZ progenitors, but also their interrelationships with surrounding microenvironment. Thus, some authors have denominated the SVZ niche as the ventricular-subventicular (VZ-SVZ) compartment. For the purposes of this chapter we will refer to this region simply as the SVZ. The adult SVZ has a center-surround cell arrangement, denominated as ‘pinwheel’ organization, for ependymal cells (type-E cells) and astrocytes (type-B cells) within ventricular walls (Mirzadeh et al., 2008). These pinwheel-like compartments contain the apical endings of type-B cells and of multiciliated (type-E1) and bi-ciliated (type-E2) ependymal cells (Figure 1). Remarkably, SVZ astrocytes extend two cell processes: a petite cilium that contact the ventricular lumen and a long basal process attached to blood vessels (Figure 1)(Mirzadeh et al., 2008; Shen et al., 2008). Some authors have proposed that these contacts are important to regulate adult neurogenesis through signals derived from extracellular matrix proteins, soluble factors or blood-vessel secretion (Leventhal et al., 1999; Mercier et al., 2002; Shen et al., 2008; Tavazoie et al., 2008). Some of these chemical mediators include the CXC chemokine, the stromal-derived factor 1 (SDF-1), the epidermal (EGF), the platelet-derived (PDGF) and the fibroblast (FGF) growth factors (Reynolds and Weiss, 1992; Vescovi et al., 1993; Gritti et al., 1995; Craig et al., 1996; Gritti et al., 1999; Jackson et al., 2006; Kokovay et al., 2010).
Figure 1.
The adult subventricular zone (SVZ) is lining the striatal wall of the lateral ventricles in the brain (coronal section left). A 3-D model of the SVZ is shown to the right. This region contains astrocytic neural stem cells (type-B cells depicted in blue). Type-B cells give rise to rapidly dividing intermediate progenitor cells (type-C cells depicted in green), which in turn produce migrating neuroblasts (type-A cells depicted in red). Type-B astrocytes have a long basal process contacting blood vessels (BV) and an apical ending at the ventricle surface. Note the pinwheel-like organization composed of multiciliated (Type-E1) and bi-ciliated (Type-E2) ependymal cells encircling apical surfaces of astrocytes. Reprinted from Brain Research Reviews, Vol 67, Gonzalez-Perez & Alvarez-Buylla, Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor, page 148, Copyright 2011, with permission from Elsevier.
Interestingly, the cell organization of the adult human SVZ is quite different to that found in rodents and most of the mammalians. In the human SVZ, astrocytes are not adjacent to the ependymal, instead, they accumulate into the parenchyma and form a ‘ribbon’ of cells separated from the ependymal layer by a gap that is largely devoid of cell bodies (Sanai et al., 2004; Quinones-Hinojosa et al., 2006).
SVZ astrocytes as neural stem cells
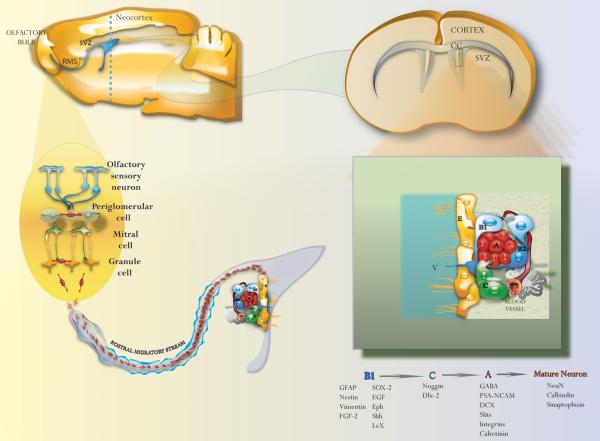
The adult SVZ contains slowly dividing type-B cells, the putative neural stem cells, which are a subpopulation of astrocytes that expresses nestin, GFAP and LeX (Doetsch et al., 1999b; Rietze et al., 2001; Capela and Temple, 2002; Garcia et al., 2004). Type-B astrocytes divide to self-renew and generate highly proliferating transit-amplifying progenitors (type-C cells), which in turn produce neuroblasts (type-A cells) (Figure 1 and 2) (Doetsch et al., 1997; Doetsch et al., 1999b). The cytoplasmic processes of type B cells sheath type-A cells and contribute to form tangential neuroblast chains. The confluence of neuroblast chains forms a brain structure, called the rostral migratory stream (RMS), which goes through the olfactory tract and connects the anterior SVZ with the olfactory bulb (Figure 2) (Lois and Alvarez-Buylla, 1994b; Lois et al., 1996). Interestingly, type-A cells follow a gradient of signaling proteins dissolved in the cerebrospinal fluid (Sawamoto et al., 2006; Gonzalez-Perez, 2012). Once in the olfactory bulb, type-A cells differentiate into mature neuronal cells that continually replace interneurons in the glomerular and the mitral cell layers (Lois and Alvarez-Buylla, 1993, 1994b; Merkle et al., 2007).
Figure 2.
Cellular organization of the adult SVZ, rostral migratory stream (RMS) and the olfactory bulb. Neuroblast (type-A cells) in the SVZ migrate to the olfactory bulb via the RMS. Migrating neuroblasts differentiate into granular and periglomerular interneurons in the olfactory bulb. B1: Type-B1 cell; C: Type-C cell; A: Type-A cell; V: Ventricle; CC: Corpus callosum; RMS: Rostral migratory stream; SVZ: Subventricular zone. Reprinted from Current Immunology Reviews, Vol 6, Gonzalez-Perez et al., Immune System Modulates the Function of Adult Neural Stem Cells, Page 169, Copyright 2010, with permission from Bentham Science Publishers Ltd..
Remarkably, type-B stem cells are not a homogeneous population; instead, it seems they comprise restricted subpopulations with diverse neurogenic potential (Merkle et al., 2007). In addition to interneurons, type-B astrocytes of the SVZ produce oligodendrocyte precursor cells that help maintain the oligodendrocyte population in the corpus callosum, fimbria fornix and striatum (Menn et al., 2006; Gonzalez-Perez et al., 2009; Gonzalez-Perez and Quinones-Hinojosa, 2010; Gonzalez-Perez and Alvarez-Buylla, 2011).
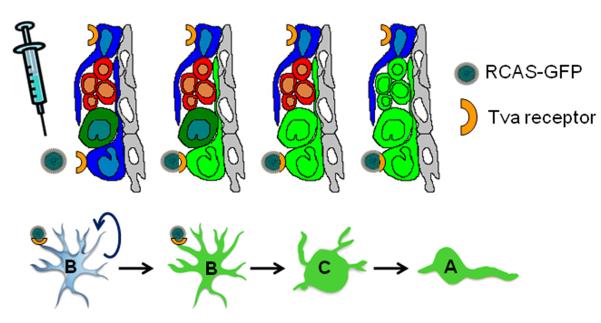
The identification of type-B cells in the SVZ as primary neural progenitors of new neurons and oligodendrocytes was demonstrated by labeling SVZ astrocytes in transgenic mice denominated as GFAP-tva mice (Figure 3) (Doetsch et al., 1999b; Doetsch et al., 2002; Menn et al., 2006). These animals express the receptor for an avian leukosis retrovirus (RCAS) controlled by the GFAP gene promoter (Holland and Varmus, 1998). Thus, the progeny of dividing astrocytes can be permanently traced by injecting engineered avian RCAS retroviruses carrying an inheritable reporter gene, which drives the expression of green fluorescent protein. With this method, it has been demonstrated that type-B astrocytes in vivo can generate both new neurons and new oligodendrocytes, and both of them become permanent and functional populations of cells in the adult brain (Doetsch et al., 1999b; Menn et al., 2006; Gonzalez-Perez et al., 2009). Interestingly, parenchymal astrocytes from cortex, striatum, septum or white matter appear not to have multipotential properties.
Figure 3.
Schematic representation of the RCAS/GFAP-tva system. After injection of the RCAS-GFP virus, proliferating astrocytes incorporate into their genome the gene of green fluorescent protein, which is inherited to their progeny. With this system only dividing GFAP-expressing cells are permanently label and can be efficiently traced throughout life. Reprinted from Brain Research Reviews, Vol 67, Gonzalez-Perez & Alvarez-Buylla, Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor, page 150, Copyright 2011, with permission from Elsevier.
After these initial reports, other studies using viral transfection or mutant mice have fully demonstrated that GFAP-expressing astrocytes are neural stem cells in the adult mammalian brain. Conditional ablation of GFAP-expressing cells produces a complete loss of neurogenesis (Imura et al., 2003; Morshead et al., 2003). Interestingly, a recent study using a Cre-lox-based strategy demonstrated that radial glial cells (multipotent neural precursors in the developing brain) and SVZ zone astrocytes (multipotent neural precursors in the adult brain) belong to the same lineage (Merkle et al., 2004). This study showed that radial glial cells generate SVZ astrocytes, which act as adult neural stem cells both in vivo and in vitro. Taken together, these findings confirm that SVZ astrocytes function as neural stem cells in the adult brain
CONCLUSION
A subpopulation of astrocytes derived from the SVZ acts as neural stem cells both in vivo and in vitro. These findings have changed our perception about glia and though we have greatly advanced our understanding of neural stem cells, there are still many unanswered questions. For instance, what are the fundamental characteristics that distinguish neurogenic astrocytes from the vast population of non-neurogenic astrocytes elsewhere in the brain? What genetic profiles induce SVZ astrocytes to act as a stem cell? Can SVZ astrocytes be induced to generate a specific repertoire of neural cell types? Addressing these questions may shed light on the genetic and molecular basis of stem-cell behavior in SVZ astrocytes, which in turn will allow designing novel therapies against neurodegenerative diseases.
ACKNOWLEDGEMENTS
This work was supported by grants from the Consejo Nacional de Ciencia y Tecnologia (CONACyT; CB-2008-101476) and The National Institute of Health and the National Institute of Neurological Disorders and Stroke (NIH/NINDS; R01 NS070024). I also thank to Jimena Rocha-Espejel for her technical assistance.
REFERENCES
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J, Gopal DD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and Histological Studies of Postnatal Neurogenesis. I. A Longitudinal Investigation of the Kinetics, Migration and Transformation of Cells Incorporating Tritiated Thymidine in Neonate Rats, with Special Reference to Postnatal Neurogenesis in Some Brain Regions. J Comp Neurol. 1967;126:337–390. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull. 2002;57:751–758. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, Van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. JNeurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, Van der Kooy D. Adult Mammalian Forebrain Ependymal and Subependymal Cells Demonstrate Proliferative Potential, but only Subependymal Cells Have Neural Stem Cell Characteristics. JNeurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. JNeurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. ProcNatlAcadSciUSA. 1999a;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular Zone Astrocytes Are Neural Stem Cells in the Adult mammalian Brain. Cell. 1999b;97:1–20. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999c;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. ProcNatlAcadSciUSA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez O. [Hydrocephaly does not modify neuroblast chain migration in the subventricular zone (SVZ)] Gac Med Mex. 2012;148:130–136. [PubMed] [Google Scholar]
- Gonzalez-Perez O, Quinones-Hinojosa A. Dose-dependent effect of EGF on migration and differentiation of adult subventricular zone astrocytes. Glia. 2010;58:975–983. doi: 10.1002/glia.20979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez O, Alvarez-Buylla A. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res Rev. 2011;67:147–156. doi: 10.1016/j.brainresrev.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Epidermal Growth Factor Induces the Progeny of Subventricular Zone Type B Cells to Migrate and Differentiate into Oligodendrocytes. Stem Cells. 2009;27:2032–2043. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti A, Cova L, Parati EA, Galli R, Vescovi AL. Basic fibroblast growth factor supports the proliferation of epidermal growth factor-generated neuronal precursor cells of the adult mouse CNS. NeurosciLett. 1995;185:151–154. doi: 10.1016/0304-3940(95)11247-t. [DOI] [PubMed] [Google Scholar]
- Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CRR, Vescovi AL. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. JNeurosci. 1999;19:3287–3297. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. ProcNatlAcadSciUSA. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The Predominant Neural Stem Cell Isolated from Postnatal and Adult Forebrain But Not Early Embryonic Forebrain Expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Jagasia R, Song H, Gage FH, Lie DC. New regulators in adult neurogenesis and their potential role for repair. Trends Mol Med. 2006;12:400–405. doi: 10.1016/j.molmed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci U S A. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman S. Endothelial Trophic Support of Neuronal Production and Recruitment from the Adult Mammalian Subependyma. MolCellNeurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994a;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994b;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Garcia AD, Sofroniew MV, van Der Kooy D. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur J Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, Van der Kooy D. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Pérez-Cañellas MR, García-Verdugo JM. Adult neurogenesis in the telencephalon of a lizard: A [3H]thymidine autoradiographic and bromodeoxyuridine immunocytochemical study. Dev Brain Res. 1996;93:49–61. doi: 10.1016/0165-3806(96)00014-4. [DOI] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Reznikov KY. Cell proliferation and cytogenesis in the mouse hippocampus. Adv Anat Embryol Cell Biol. 1991;122:1–74. doi: 10.1007/978-3-642-76447-9. [DOI] [PubMed] [Google Scholar]
- Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, Tessier-Lavigne M, Okano H, Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, Van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron. 1997;18:779–791. doi: 10.1016/s0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]