Abstract
Background/Aim
The liver’s response to injury is fibrosis, and when chronic, cirrhosis. Age is a critical factor impacting many immune-mediated processes, potentially including the liver’s wounding response to injury.
Methods
The effects of age on acute and chronic liver injury were evaluated using a carbon tetrachloride model in mice. Lymphocyte and macrophage populations were assessed by flow cytometry and immunohistochemical analysis.
Results
Acute liver injury was greater in 18-month old (old) mice than in 9-month old (middle aged) mice as judged by changes in aminotransferases. Similarly, 18-month old livers had a significantly greater fibrogenic response to injury than did 9-month old livers after chronic injury (assessed by col1α1 mRNA expression, morphometric analysis and hydroxyproline measurement). Interestingly, livers from young mice (6 weeks old) also exhibited an increase in fibrogenesis compared to 9-month old mice, albeit not to the same degree as in old mice. Consistent with a role for macrophages in fibrogenesis, the number of liver macrophages in young and 9-month old mice increased, while in chronically injured 18-month old livers, the number of macrophages was reduced, and was less than in the livers of young and 9-month old injured livers.
Conclusions
Our data indicate that the fibrogenic response to injury varies substantially with age, and moreover that macrophage recruitment and dynamics may be an important component in differential age-associated fibrotic disease.
Keywords: extracellular matrix, collagen, cirrhosis, smooth muscle actin, aminotransferase
Introduction
Studies during the 1960’s indicated that older male rats (12-months old) developed greater fibrosis than younger rats (1 and 6-months old) long term exposure to carbon tetrachloride (CCl4) (1, 2). Interestingly, this apparent age effect was limited to male rats, and was not observed in female rats. This difference between male and female animals has been attributed to an age-specific decrease in the antioxidant defense system (3–6), which has been reported to be prominent in male rats but not in female rats or in mice (3). It was also observed that very old rats (30 months) exhibited less acute necrosis induced by chemical injury, but less regeneration following acute injury (5) than did younger rats (2 and 12-months old). A more recent study using male rats demonstrated that 12, 18, and 24-month old rats had greater acute liver injury in response to intraperitoneal injection of ethanol than did younger (3 and 6 month old) rats (7) as judged by serum alanine aminotransferase (ALT) levels. Further, histological analysis revealed that necrosis was greater in the 12 to 24-month old livers compared to younger livers after acute injury. Mechanisms underlying these findings have not been clearly elucidated.
In this study, we hypothesized that intrinsic immune responses in mice may explain divergent responses to injury, and moreover fibrogenesis occurring after said injury. We therefore utilized quantitative measures of liver injury and fibrosis to assess the effect of age on acute and chronic liver injury in mice. We found that while “older” livers appeared to be more susceptible to acute injury than “younger” livers, the chronic fibrotic response to a given injury was profoundly greater in older mice than in younger mice. This profound increase in fibrosis was accompanied by a substantial loss of macrophages in the chronically injured old liver, consistent with the concept that macrophages play a key role in fibrolysis during the recovery phase (8–12). Interestingly, we observed that very young livers (mice aged 6-weeks at the beginning of the seven week study) showed a moderately increased fibrotic response compared to middle aged (9-month old) mice. Unlike the old mice, the increased fibrosis observed in very young mice was not accompanied by a decrease in the number of hepatic macrophages, suggesting that the fibrotic response in very young and old mice may be differentially regulated. However, the increased fibrosis was considerably more pronounced in old mice than in very young mice.
Materials and Methods
Animals
Six week and nine month old male BALB/c mice were purchased from Harlan, Inc. (Indianapolis, IN). Eighteen-month-old male BALB/c mice were purchased from the National Institute on Aging (Bethesda, MD). Animal care conformed to the standards of the National Society of Medical Research (“Principles of Laboratory Animal Care”) and “The Guide for the Care and Use of Laboratory Animals” (NIH publication No. 86–23, revised 1985). Experiments were approved by the Duke University Institutional Animal Care and Use Committee.
Hepatic injury was induced by carbon tetrachloride (CCl4) (Sigma Chemical Co., St. Louis, MO) gavage. A mixture of CCl4 and corn oil (1:1, v) or corn oil alone as a control was given by oral gavage at a dose of 1.5mL/kg body weight once per week for six weeks. Animals were placed under very anesthesia (Tech 3 vaporizor, Harvard Apparatus, Holliston, MA) for the gavage procedure.
Animals were sacrificed by CO2 inhalation 7 days following the sixth gavage unless otherwise noted. Blood (600 – 1200 μl) was obtained from each animal through the portal vein immediately following sacrifice. The liver was removed and samples were snap-frozen in liquid nitrogen, fixed in 10% neutral buffered formalin (VWR International, West Chester, PA) or assayed immediately using procedures described below.
Lymphocyte isolation
Livers (gall bladders removed) were pressed through a 280 μm screen (Bellco Glass, Inc., Vineland, NJ) and incubated with 0.02% collagenase D and 400 units/ml DNase I in 20 mL Hank’s Balanced Salt Solution (HBSS) containing 20 mM HEPES, pH 7.4, at 37°C for 30 minutes with occasional shaking. The slurry was filtered through a 70 μm nylon filter (BD Biosciences-Discovery Labware, Bedford, MA) and centrifuged at 300 × g at 4°C for 5 minutes. The cell pellet was resuspended in HBSS (Gibco BRL, Grand Island, NY) with 33% Percoll (Sigma Chemical Co.) (30 milliliters total) and centrifuged at 800 × g at 4°C for 20 minutes. The upper layer and interface containing parenchymal cells, dead cells and debris was discarded and the pellet containing lymphocytes and red blood cells was washed one time with 50 milliliters HBSS by centrifugation at 300 × g at 4°C for 5 minutes. To lyse red blood cells, the pellet was resuspended in 20 milliliters of freshly prepared ACT buffer (125 mM NH4Cl and 17 mM Tris, pH 7.3) and incubated at 4°C for 10 min with occasional shaking. After incubation, 30 milliliters of HBSS was added to the cells and lymphocytes were centrifuged at 300 × g at 4°C for 5 minutes. To remove remaining debris the pellet was resuspended in 10 milliliters of HBSS, layered over 3 milliliters lymphocyte separation medium (ICN Biomedicals, Inc., Aurora, OH) and centrifuged at 1500 × g at 4°C for 15 minutes. The resulting interface contained the lymphocyte fraction and was recovered and washed one time with HBSS by centrifugation at 300 × g at 4°C for 5 minutes. Cell concentrations were determined using a Coulter Multisizer IIE (Beckman Coulter, Inc., Brea, CA).
ELISPOT detection of cytokine secretion
Wells of a 96-well MultiScreen-IP plate, Immobilon-P membrane (Millipore Corp., Bedford, MA) were coated with 100 μL of either anti-mouse IFN-γ monoclonal antibody or anti-mouse IL-4 monoclonal antibody (Pierce Biotechnology Inc., Rockford, IL) at 5 μg/mL in phosphate buffer saline (PBS) (Gibco BRL) overnight at 4°C. Wells were washed 3 times with 200 μl of PBS and filled with blocking buffer (PBS with 3% bovine serum albumin and 5% heat-inactivated fetal calf serum). Wells were blocked for up to 3 hours at room temperature and washed with ELISPOT medium (RPMI-1640 containing 20 mM HEPES, 10% heat-inactivated fetal calf serum, 1 mM L-glutamine, 100 μg/ml penicillin, 100 units/ml streptomycin, 8.0 mM NaOH, 1 mM sodium pyruvate, and 1x of each MEM Amino Acids Solution and MEM Non-essential Amino Acids Solution) (Gibco BRL). Isolated mouse lymphocytes (described above) were plated at various concentrations in 200 μl/well of ELISPOT media containing 10 ng/mL recombinant mouse IL-2 (R&D Systems, Minneapolis, MN) and 10 μg/ml Concanavalin A (Sigma Chemical Co.) and incubated for 24 to 48 hours at 37°C with 5% CO2. Wells were washed 3 times with 200 μl with PBS containing 5% fetal calf serum and 0.05% Tween-20 and 100 μl/well of either biotin-labeled anti-mouse IFN-γ monoclonal antibody or biotin-labeled anti-mouse IL-4 monoclonal antibody (Pierce Biotechnology) was added at a concentration of 2 μg/ml in blocking buffer overnight at 4°C. Wells were washed 3 times with 200 μl PBS containing 0.05% Tween-20 and 100 μl/well of horseradish peroxidase-conjugated avidin diluted 1:100 in PBS containing 0.05% Tween-20 was incubated for 1–2 hours at room temperature. Wells were washed 4 times with 200 μl PBS containing 0.05% Tween-20 and 100 μl/well of AEC chromogen substrate (BD Biosciences-Pharmingen, San Diego, CA) prepared as directed. Wells were developed for 10–30 minutes and then washed several times with tap water to stop the reaction. Images of each well were captured using an ImmunoSpot Image Analyzer system (Resolution Technology Inc., Columbus, OH) and spots were counted manually.
Hydroxyproline measurement
Hydroxyproline content in liver tissue was measured as described (13). In brief, frozen mouse liver tissue was homogenized in 6 N HCl at 20μL/mg and hydrolyzed under N2 gas at 110°C for 16 hours. Hydrolysates were filtered with 0.2 μm syringe filters and aliquots of 25 μL were evaporated under vacuum. Sediments were dissolved in 600 μl of 50% isopropanol. Standards of 4-hydoxyproline (Sigma Chemical Co.) from 0 to 1.6 μg/mL were made in 50% isopropanol. One hundred microliters of chloramine-T solution (0.56 mL of 15% N-chloro-p-toluenesulfonamide in dH2O (Sigma Chemical Co.) and 9.44 mL acetate-citrate buffer (0.42 M sodium acetate, 0.13 M sodium citrate, 26 mM citric acid in 39.5% isopropanol, pH 6.0) was added to samples and standards and incubated at room temperature for 10 minutes protected from light. Five hundred microliters of Ehrlich’s Reagent (3 milliliters of Ehrlich stock (10 g 4-(dimethylamino)benzaldehyde,11 milliliters of 60% perchloric acid) (Sigma Chemical Co.) and 8 milliliters of isopropanol) was added to samples and standards and incubated at 50°C for 90 minutes protected from light. Samples and standards were run in triplicate. Sample absorbance was read at 558 nm and the concentration of hydroxyproline was determined by comparison of unknowns with a standard curve.
RNA isolation, reverse transcription and quantitative PCR
Total RNA was isolated from liver tissue using RNeasy silica-gel binding membranes (Qiagen, Valencia, CA) as per the manufacturer’s directions. Briefly, frozen liver tissue (10–30 mg) was homogenized and passed through an RNA binding membrane. Genomic DNA was removed using DNase. Total RNA was eluted in dH2O and quantified by measuring absorbance at 260 nm. mRNA was reverse transcribed using M-MLV reverse transcriptase (Gibco BRL) and random hexamer primers (Roche Diagnostics Corp., Indianapolis, IN) as per the manufacturer’s directions. Real-time PCR was performed with SYBR green detection dye with a Mx3000 Real-Time PCR System (Stratagene, Cedar Creek, TX). Target cDNA was normalized using GAPDH cDNA as a control for each sample and an external standard was used for normalization between runs. Primers for quantitative detection are; mouse GAPDH forward, GTC GTG GAT CTG ACG TGC C, mouse GAPDH reverse, TGC CTG CTT CAC CAC CTT CT; mouse pro-alpha 1 collagen forward, TGC TTT CTG CCC GGA AGA, mouse pro-alpha 1 collagen reverse, TGG GTC CTC GAC TCC TAC ATC T; mouse fibronectin ED-A forward, GGG CAA GTT TCC AGG TAC AG, mouse fibronectin ED-A reverse, CAG CTC TGC AGT GTC GTC TT, mouse TGF-β1 forward, CCT GCA AGA CCA TCG ACA TG, mouse TGF-β1 reverse, GGA CTG GCG AGC CTT AGT TT. Primers are listed 5′ to 3′.
Flow cytometry
Isolated liver lymphocytes (described above) were incubated with mouse serum for 5 minutes at 4°C and the following fluorochrome-conjugated anti-mouse antibodies (BD Biosciences Pharmingen) for 30 minutes at 4°C in PBS with 1% bovine serum albumin: anti-CD3e-FITC and anti-CD3e-APC (clone 145-2c11), anti-CD4-FITC (clone GK1.5), anti-CD4-PE (clone RM4-5), anti-CD8a-Cy-Chrome (clone 53–6.7), anti-CD11b-APC (clone M1/70), anti-CD45R/B220-PE (clone RA3-6B2) and anti-Pan-NK cells-FITC (clone DX5). Optimum concentrations of antibodies and cells were determined experimentally for each pair and appropriate isotype controls were used. Cells were washed with PBS and resuspended at 1×106 cells/ml in PBS with 0.4% paraformaldehyde. Multiparameter flow cytometric analysis was performed using a FACS Vantage Cell Sorter and results were analyzed using Cell Quest software (BD Biosciences-Immunocytometry Systems, San Jose, CA) at the Duke Human Vaccine Institute Flow Cytometry Core Facility (Duke University, Durham, NC).
Alanine aminotransferase measurement
Alanine aminotransferase was measured from serum in the Carl Clinical Laboratories (Duke University Medical Center, Durham, NC) by standard methods (14).
Morphometric analysis of hepatic collagen content
Mouse livers fixed in 10% neutral buffered formalin were embedded in paraffin and 4 μm sections were stained with picrosirius red (which detects collagen) as previously described (15, 16). Briefly, sections were deparaffinized, stained with 0.1% picrosirius red and 0.1% Fast Green FCF in saturated picric acid (Sigma Chemical Co.) for 30 min. Sections were washed briefly in water, dehydrated to 100% xylene and mounted with Permount (VRW International). Collagen was quantified using digital morphometric analysis. A minimum of 10 random fields (20x magnification) per liver section and 2 sections per liver were captured (DS-L1 camera and ACT-1 software, Nikon Inc., Melville, NY), and the amount of picrosirius red stained liver was measured with MetaVue software (Molecular Devices, Sunnyvale, CA). Fibrosis was expressed as the percentage of stained area in each field.
Immunohistochemical staining
For immunohistochemistry of liver sections, ten micron paraffin embedded sections were rehydrated, washed with PBS, heated to 37°C, and then exposed to 1:4 diluted proteinase K (1 mg/mL) for 30 seconds, rinsed with PBS, then after blocking with a dilute milk solution, incubated with anti-F4/80 primary antibody (Abcam, Cambridge, MA), washed in PBS, and antigen detected with a Vectastatin ABC Staining Kit as per the manufacturer’s instructions (Vector Laboratories, Burlingame, CA). Sections were washed, counterstained with hematoxylin for 1 minute, dehydrated, and mounted (Gel/Mount™; BiØmedia). Images were obtained with a Nikon TE300 microscope (Nikon, Inc., Melville, NY). Control specimens were identical to experimental specimens except they were exposed to irrelevant isotype matched antibody.
Statistics
Results are expressed as mean ± SD. All experiments were repeated with greater than 6 animals per group. All data were analyzed using a 2-way ANOVA, followed by post-hoc t-tests. Differences were considered significant when p < 0.05.
Results
Liver injury following acute injury as a function of age
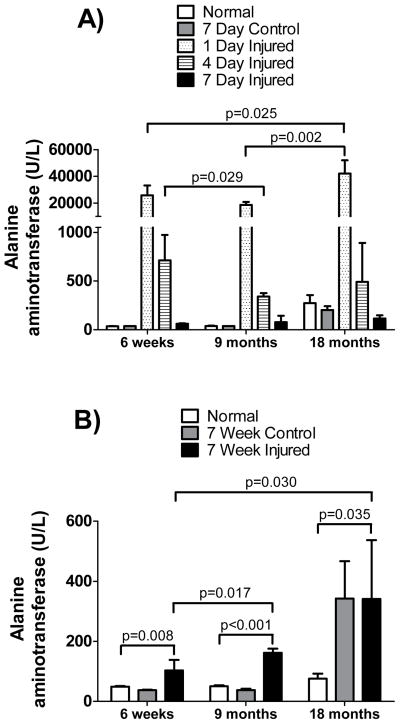
To determine the effect of age on the extent of liver injury following CCl4, ALT serum levels in mice of various ages were evaluated (Figure 1). ALT levels in all mice, regardless of age, rose acutely following CCl4 (p < 0.05). ALT levels in old (18-months) mice rose significantly more than levels in 6-week old and 9-month old mice at one-day post- CCl4 exposure (p = 0.025 and 0.0016, respectively). Interestingly, in the 18-month old mice but not in any of the younger mice, exposure to oil alone over a period of 7 weeks (i.e., no CCl4; “control” in Figure 1B) led to an increase in ALT levels. Evidence of hepatocellular necrosis 1 and 4 days after administration of CCl4 paralleled biochemical abnormalities. These data indicate that the older mice are more susceptible to acute liver injury than are younger mice.
Figure 1. Serum alanine aminotransferase levels after acute and chronic injury in different aged mice.
Mice aged 6-weeks, 9-months or 18-months of age were exposed to CCl4 as in Methods. In (A), CCl4 was administered once and serum was collected 1, 4 or 7 days after a single dose. Groups included untreated (normal) mice, mice which received 1 dose of corn oil (control) at 1.5ml/kg and mice which received 1 dose of CCl4 (injured) diluted in corn oil (1:1, v) at 1.5 ml/kg body weight (n ≥ 8 in each group). In (B), groups included untreated (normal) mice, mice which received 1 dose per week of corn oil (control) at 1.5ml/kg for 6-weeks at 7 day intervals and mice which received 1 dose per week of CCl4 (injured) diluted in corn oil (1:1, v) at 1.5ml/kg for 6-weeks. Alanine aminotransferase levels were measured in serum from blood drawn 7 days after the 6th dose (n ≥ 6 in each group). The normal, untreated, mice were the same mice in both graphs. Data in each graph were analyzed separately as in Methods, and significant p-values are shown.
Mean serum ALT levels in the youngest mice (6-weeks old at the beginning of the study) rose higher than those of middle-aged (9-month old) mice (Figure 1A). Although the difference in ALT levels between 6-week old and 9-month old mice at one-day post exposure was not significant (p = 0.070), the difference in ALT levels at 4 days post exposure was significant (p = 0.029).
Serum ALT levels during chronic liver injury were elevated in all mice (Figure 1B) (p < 0.05), regardless of age. Eighteen-month old mice and 9-month old mice had higher serum ALT levels than did 6-week old mice (p = 0.030 and 0.017, respectively), although the difference between 18-month old mice and 9-month old mice receiving CCl4was not significant. We identified an increase in serum ALT levels in 18-month old mice receiving oil only (control in Figure 1B) compared to younger mice (p < 0.05). The observation that oil and CCl4 exposed 18-month old mice had similar serum ALT levels may reflect the idea that liver necrosis in the CCl4 mice was limited by advanced fibrosis of those livers. This idea is consistent with the data described in the sections below.
A substantial number of mice died as a result of CCl4 toxicity. The mortality rate of chronically injured 6-week and 9-month old mice was 27% (4 out of 15) and 25% (5 out of 20), respectively during the course of the study. The mortality rate in 6-week and 9-month controls (oil fed) was 7% (1 out of 14) and 0% (0 out of 16), respectively. Mortality in 18-month old mice chronically exposed to CCl4 was 50% (7 out of 14) during the course of the study. However, it is likely that some of the 18-month old mice died of causes other than CCl4 toxicity, since the mortality in the 18-month old group fed oil only (i.e., no CCl4) was 38% (6 out of 16) during the course of the study.
Fibrosis following liver injury as a function of age
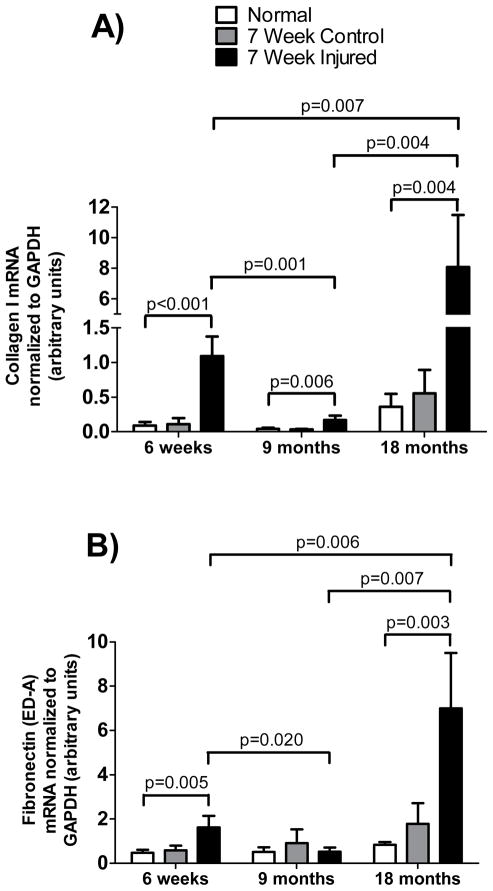
To determine the extent of fibrogenesis in chronically injured livers as a function of age, we assessed type I collagen and fibronectin (cellular) mRNA levels in livers of mice following 6-weeks of CCl4 induced injury (Figure 2). Type I collagen mRNA (Figure 2A) and fibronectin mRNA (Figure 2B) were more elevated in 18-month old mice than in 6-week old mice (p = 0.0066 and 0.0056 respectively). Similarly, type I collagen mRNA and fibronectin mRNAs were more elevated in 6-week old mice than in 9-month old mice (p = 0.0070 and 0.020 respectively), although the elevation in type I collagen mRNA and fibronectin mRNA levels in the 18-month old mice far exceeded those of either the 6-week old or the 9-month old mice. These data demonstrate the fibrogenesis is ongoing in the chronically injured liver, and that this process is profoundly greater in the old liver than in younger livers.
Figure 2. Detection of type I collagen and fibronectin mRNA in different aged mice.
Exposure to CCl4 began at 6-weeks, 9-months or 18-months of age as in Figure 1. Groups included untreated (normal) mice, mice which received 1 dose per week of corn oil (control) at 1.5ml/kg for 6-weeks and mice which received 1 dose per week of CCl4 (injured) diluted in corn oil (1:1, v) at 1.5ml/kg for 6-weeks. Livers were harvested on the 7th day after the 6th dose. Total RNA was extracted and subjected to RT-PCR as in Methods. Type I collagen (A) and EDA fibronectin (B) mRNAs were measured (n ≥ 8 for each group). Data in each graph were analyzed separately using a 2-way ANOVA as in Methods and post-hoc t-tests were run comparing peak responses as a function of age, and significant p-values are shown.
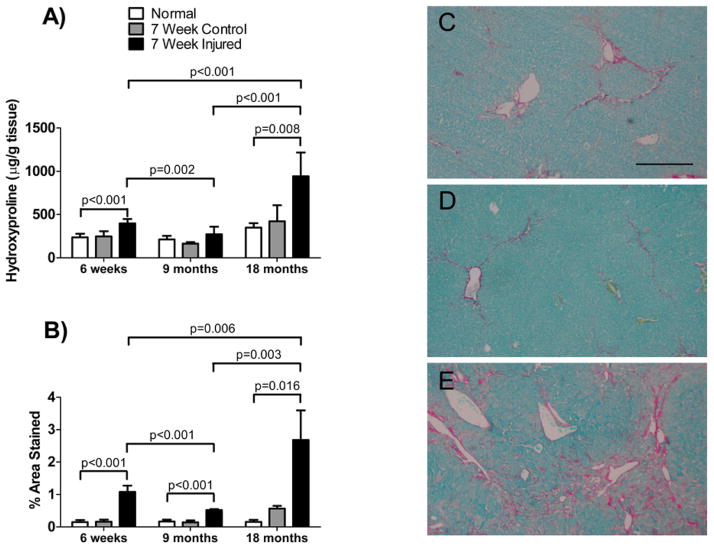
We also quantitated fibrosis by measuring hydroxyproline content and by morphometric analysis of picrosirius red stained collagen in injured livers (Figure 3). Fibrosis in the chronically injured livers of old mice was significantly greater (p < 0.05) than the injury in younger mice (both 6-week old and 9-months old). Similarly, fibrosis in chronically injured 6-week old livers was significantly greater than in 9-month old livers.
Figure 3. Collagen content after chronic injury in livers of different aged mice.
Mice were as in Figure 2. In (A), hydroxyproline was measured as in Methods (n = 6 in all groups). In (B), mouse livers were subjected to morphometric analysis of collagen content as in Methods (n ≥ 9 in each group). In (C, D and E) are shown representative picrosirius red stained liver sections from 6-week, 9-month and 18-month old mice respectively. The bar shown in (C) represents 100 microns. Data in each graph were analyzed separately using a 2-way ANOVA as in Methods, and significant p-values are shown..
Immune milieu associated with liver injury as a function of age
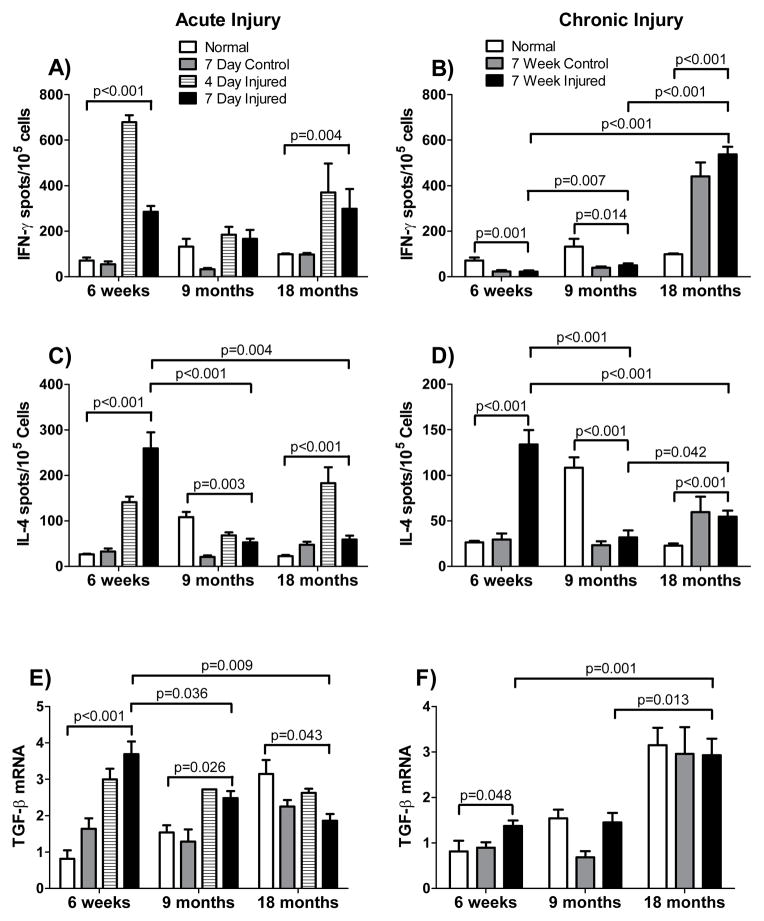
We hypothesized that age-associated differences in the susceptibility and response to chronic liver injury may be due, at least in part, to age-dependent differences in immune status. As part of our evaluation of the immune milieu following liver injury, we evaluated the levels of IFN-γ producing cells, IL-4 producing cells, and TGF-β mRNA in acutely and chronically injured livers (Figure 4). Substantial age-associated differences in these cytokines were observed in both acutely and chronically injured livers. The levels of IFN-gamma producing cells increased in 6-week old and 18-month old acutely injured mouse livers but not in 9-month old mouse livers. Chronic injury, on the other hand, was associated with increased levels of IFN-γ producing cells in 18-month old mice only, with significant (p < 0.05) decreases (relative to the uninjured liver of the same age) of IFN-γ producing cells in 6-week old and 9-month old mice. Similarly, levels of IL-4 producing cells varied as a function of the type of injury and the age of the injured mouse (Figure 4). For example, levels of IL-4 secreting cells increased dramatically in chronically injured 6-week old livers, to a lesser extent in chronically injured 18-month old livers, and not at all in 9-month old chronically injured livers. TGF-β mRNA levels were significantly increased in chronically injured 6-week old livers (p < 0.05), but not in chronically injured 9-month old livers. Although levels of TGF-β mRNA did not increase in the chronically injured 18-month old livers compared to the normal 18-month old liver, levels of TGF-β mRNA were elevated in all 18-month old livers compared to the 6-week and 9-month animals (p < 0.02). Since TGF-β is an important cytokine involved in fibrosis, these data are consistent with the idea that the age-dependent immune milieu may be associated with the increased fibrosis observed in old livers. Further, these data suggest that, to the extent that fibrotic injury processes are immune mediated, those processes are fundamentally distinct in very young (6-week) mice compared to old (18-month) mice.
Figure 4. Cytokine production by liver lymphocytes of different aged mice after acute and chronic injury.
In (A, C, and E), CCl4 was administered to mice aged 6-weeks, 9-months or 18-months and livers were harvested 4 or 7 days after a single dose. Groups included untreated (normal) mice, mice which received 1 dose of corn oil (control) at 1.5ml/kg and mice which received 1 dose of CCl4 (injured) diluted in corn oil (1:1, v) at 1.5 ml/kg body weight (n ≥ 6 in each group). In (B, D and F), exposure to CCl4 began at 6-weeks, 9-months or 18-months of age. Groups included untreated (normal) mice, mice which received 1 dose per week of corn oil (control) at 1.5ml/kg for 6-weeks and mice which received 1 dose per week of CCl4 (injured) diluted in corn oil (1:1, v) at 1.5ml/kg for 6-weeks. Livers were harvested on the 7th day after the 6th dose (for B and D n ≥ 9 in each group, for F n ≥ 5 in each group). In (A and B), lymphocytes were isolated from livers taken 4 days and 7 days after treatment. In (C and D), lymphocytes were isolated from livers harvested 7 days after the 6th dose of CCl4 and ELISPOT assays to detect IFN-γ (A/B) or to detect or IL-4 (C/D) were performed as in Methods. In (E) and (F), liver tissue was harvested 4 days (E) and 7 (F) days after the first or last dose of CCl4 was administered, respectively and TGF-β mRNA was measured by RT-PCR as in Methods, normalized to the level of GAPDH mRNA; data are presented graphically. In (F), liver tissue was harvested 7 days after the 6th dose and TGF-β mRNA was measured as in (E). Data in each graph were analyzed separately using a 2-way ANOVA and were found to be significantly different (p < 0.05) for both age and treatment group or for the interaction between the two, with the exception of the data in graph 4F, where age but not treatment affected the result. Post-hoc t-tests were run comparing changes associated with peak responses at a given age, and significant p-values are shown.
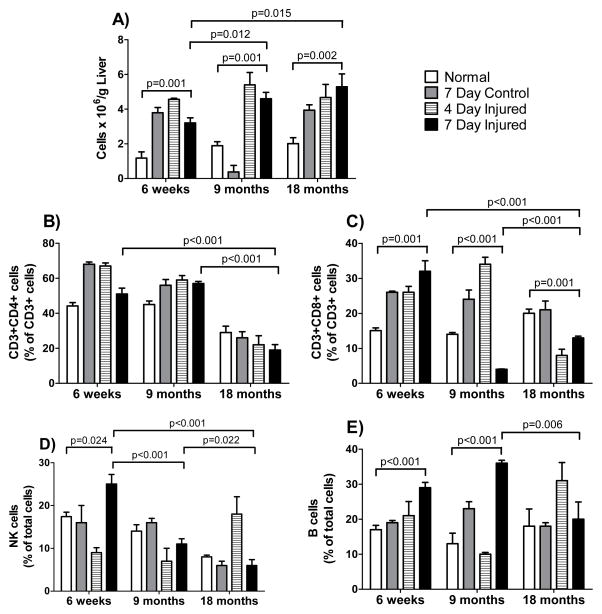
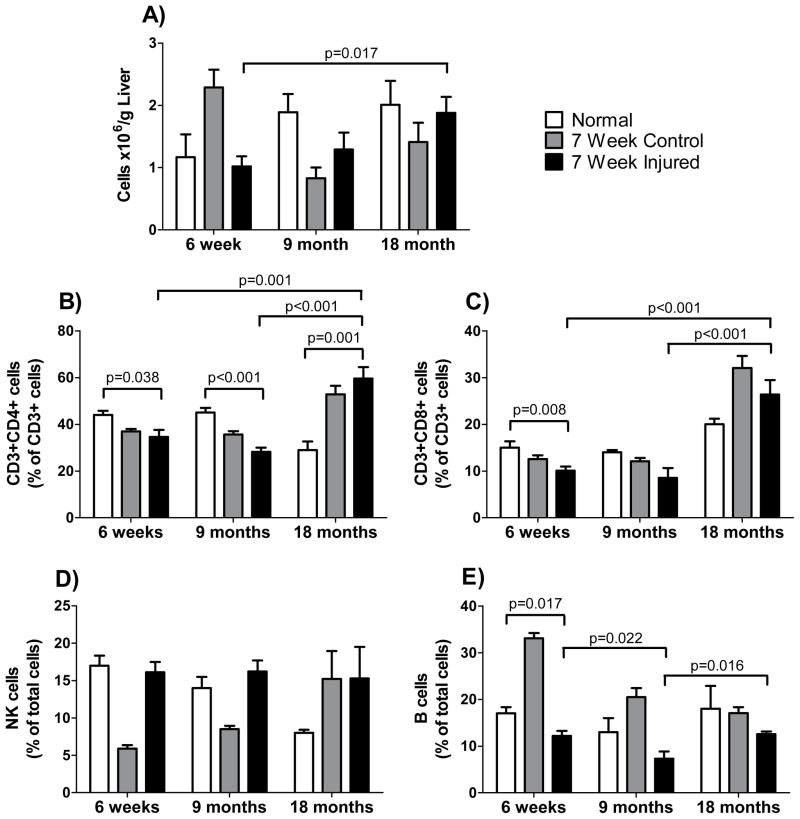
To further explore of the age-dependent immune milieu associated with chronic liver injury, the number of total cells, CD3+/CD4+ cells, CD3+/CD8+ cells, B-cells, NK cells, and macrophages was evaluated in normal mice and in mice with acute and with chronic liver injury. A number of significant (p < 0.05) changes in the immune cell populations were observed. Transient increases in the CD3+CD8+ cell, NK cell and B cell populations were observed following acute liver injury across all age groups (Figure 5), but no significant increases of these populations were identified in chronically injured livers (Figure 6). This could suggest that variations in lymphocyte responses to acute injury as a function of age trigger later reactions involved in chronic injury. Increases in the CD3+CD4+ population were observed in 18-month old mice following chronic liver injury, while a decrease was observed in 6-week old and 9-month old mice with chronic liver injury (Figure 6).
Figure 5. Lymphocyte populations after acute injury in different aged mice.
CCl4 was given to mice aged 6-weeks, 9-months, or 18-months as in Figure 1. Groups included untreated (normal) mice, mice that received 1 dose of corn oil (control) at 1.5 mL/kg and mice that received 1 dose of CCl4 (injured) diluted in corn oil (1:1, v) at 1.5 mL/kg body weight. Liver tissue was harvested and cells were isolated either 4 or 7 days after CCl4; in (A), total lymphocytes were measured by flow cytometry as in Methods (n ≥ 6 in each group). In (B–E), specific lymphocyte populations were characterized with specific antibodies by flow cytometry as in Methods (n ≥ 6 in each group). Data in each graph were analyzed separately using a 2-way ANOVA as in Methods, and significant p-values are shown.
Figure 6. Lymphocyte populations after chronic injury in different aged mice.
Exposure to CCl4 began at 6-weeks, 9-months or 18-months of age. Groups included untreated (normal) mice, mice which received 1 dose per week of corn oil (control) at 1.5ml/kg for 6-weeks and mice which received 1 dose per week of CCl4 (injured) diluted in corn oil (1:1, v) at 1.5ml/kg for 6-weeks. Liver tissue was harvested and cells were isolated on the 7th day after the 6th dose of CCl4. In (A), total lymphocytes were measured by flow cytometry as in Methods (n ≥ 6 in each group). In (B–E), specific lymphocyte populations were characterized with specific antibodies by flow cytometry as in Methods (n ≥ 6 in each group). Data in each graph were analyzed separately using a 2-way ANOVA as in Methods, and significant p-values are shown.
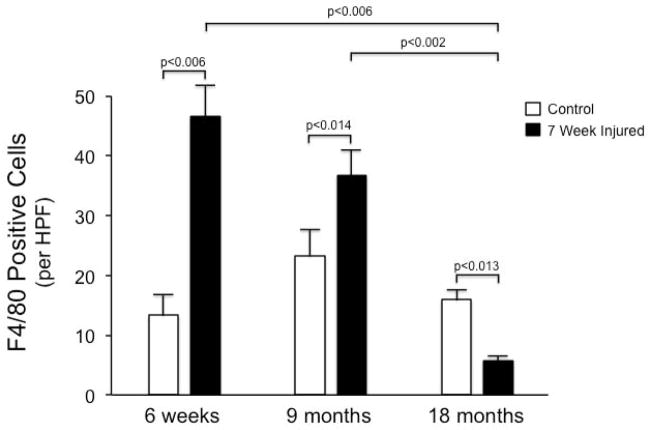
Since macrophages appear to play key roles in both fibrogenesis and fibrolysis in the liver, we explored macrophages in each acutely and chronically injured livers. The level of macrophages in normal 18-month old livers was greater than that in either 6-week old or 9-month old livers (Figure 7). Changes in the levels of CD11 positive cells following chronic injury were dramatically age-dependent: A two-fold increase in CD11 positive cells was observed following chronic injury of 6-week old and 9-month old mice (p = 0.0045 and 0.0135, respectively, compared to normal). In contrast, a two-fold decrease in CD11 positive cells following chronic injury to 18-month old livers was observed (p = 0.0009 compared to normal). Liver macrophages, assessed by F4/80 immunostaining of chronically injured livers revealed an increase in macrophages following chronic injury of 6-week old and 9-month old mice, and a substantial decrease in macrophages in 18 month old mice (Figure 8).
Figure 7. CD11 populations in the liver after acute and chronic injury of different aged mice.
In (A), mice aged 6-weeks, 9-months or 18-months were given one dose of CCl4. Macrophages were isolated with flow cytometry as in Methods (using anti-mouse CD11b-APC-conjugated antibody (clone M1/70)) from livers 4 and 7 days after CCl4 (n ≥ 6 in each group). In (B), macrophages were isolated with anti-mouse CD11b-APC-conjugated antibody as in (A) in mice aged 6-weeks, 9-months or 18-months who were given CCl4 or corn oil weekly for 6-weeks (n ≥ 6 in each group). Data in each graph were analyzed separately using a 2-way ANOVA and the data in graph 7A were found to be significantly different (p < 0.05) for treatment group but not age, whereas the data in graph 7B were significantly different (p < 0.05) for both age and treatment group and for the interaction between the two. Post-hoc t-tests compared changes associated with peak responses at a given age, and significant p-values are shown.
Figure 8.
F4/80 positive cells are reduced in chronically injured old livers.
Immunohistochemistry was performed as in Methods to detect F4/80 positive cells in chronically injured, different aged, livers as in Figure 1. F4/80 positive cells were counted in a blinded fashion (10 random fields for each specimen), and quantitative data presented graphically (n ≥3 each group); significant p-values are shown.
Discussion
The immune system appears to play a key role in both acute and chronic liver injury. Macrophages, B and T cells, and perhaps even other cells may be linked to the fibrogenic and fibrolytic processes (9, 17–24). For example, macrophages can secrete PDGF and TGF-β1, which can stimulate hepatic stellate cells and myofibroblasts, inducing a fibrogenic phenotype in those cells (9, 25–27). Additionally, macrophages may play a role in the fibrolytic process by secreting TRAIL, TNF-α, and other pro-apoptotic mediators (9, 28, 29), thus directly inducing apoptosis of hepatic stellate cells, which in turn leads to increased fibrolysis through downregulation of extracellular matrix production and TIMPs (30). Given the important role of the immune system in fibrosis, it is reasonable to hypothesize that changes in the immune system as a function of age play a key role in the fibrotic response to injury exhibited by old livers.
Our data demonstrate that (a) old livers are more susceptible to injury and that (b) there is more fibrosis in old livers following a given injury. Following exposure to CCl4, acute liver injury as measured by serum ALT levels was approximately twice as high in 18-month old mice as in 9-month old mice. However, chronically injured livers of 18-month old mice had nearly 50-fold greater collagen mRNA and 10-fold greater fibronectin mRNA expression than livers of comparable 9-month old mice, indicating substantial, ongoing fibrogenesis in the chronically injured, old liver. Direct measures of fibrosis (hydroxyproline quantification and picrosirius red staining) indicated a four to five fold increase in fibrosis of 18-month old chronically injured livers compared to livers from comparably treated 9-month old mice.
Taken together, these results demonstrate the profound propensity for the “old” liver to become fibrotic in the face of injury. Although old livers appear to be somewhat more susceptible to injury, the most remarkable feature of the old liver appears to be the dramatic increase in fibrosis. In the experimental model used, acute injury in old livers was greater than that in younger livers by a factor of two, whereas the fibrosis observed after chronic injury was greater by a factor of four to five. These data may provide a potential explanation for the clinical observation that older livers exposed to hepatitis C develop fibrosis more rapidly than “younger” livers (31, 32), and further that advanced donor liver age in liver transplantation for hepatitis C is associated with more rapid fibrosis progression (33–36).
Interestingly, very young livers appeared to also be more susceptible to a fibrogenic response than did middle aged livers. Of note is the fact that, although the youngest mice used in this study were relatively young (6-weeks old) at the onset of the study, their age had more than doubled by the completion of the study. Thus, studies on even younger animals could demonstrate even greater fibrosis in response to chronic liver injury than was observed in this study. However, such studies would necessarily be conducted on animals other than mice because of the technical difficulty associated with study of mice less than 6-weeks old.
Given the evolving body of literature surrounding the role of macrophages in fibrosis (37–42), our study raises a number of further questions. Previous data suggest that the role of macrophages in fibrogenesis is complex, with the potential for macrophages to stimulate (37–39), as well as ameliorate fibrosis (39–42). The possibility that macrophages secrete metalloproteases important in fibrolysis is an attractive mechanistic explanation for the observations reported here (39–41). Therefore, our work further highlights several new lines of investigation relevant to the further understanding of the role of age-associated differences in macrophages (e.g. perhaps in macrophage-depleted animals). Ex vivo examination of the activity of macrophages derived from young and old mice would also be attractive.
In summary, the effect of age on the fibrotic liver response is paralleled by numerous differences in the immune systems of young and old mice. We observed substantial age-dependent differences in cytokines involved in the fibrotic response, including TGF-β, as well as changes in the number of cellular components, including T-cells, NK cells, CD11 positive cells, and F4/80 macrophages. Perhaps most striking was the loss of macrophages in the chronically injured old liver - in contrast to the increases observed in younger livers. These findings in lead us to speculate that in chronic injury in the aged liver, macrophages participating in the initial liver injury response may be involved in fibrogenesis, whereas macrophages participating in the repair process following injury may be critical for fibrolysis. Thus, the data raise the possibility that there is (a) increased fibrogenesis at early stages of chronic injury, with a higher number of pro-fibrogenic macrophages in older livers compared to younger livers, and (b) decreased fibrolysis at later time points in chronic injury, with lower numbers of pro-fibrolytic macrophages in older livers compared to younger livers.
Acknowledgments
Funding
This work was supported by funds from the National Institutes of Health (AI-51445 to BHC, and R01 DK 50574 to DCR) and the National Institute of Aging, who supplied aged mice.
Abbreviations
- CCl4
carbon tetrachloride
- IFN-γ
interferon-γ
Footnotes
Author contributions
BHC, AEH, and DCR were directly involved in study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. All authors were involved in acquisition of data; analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
Financial Interests
The authors certify that we have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, major honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product.
References
- 1.REUBER MD, GLOVER EL, DOVE LF. Hepatic lesions in aged rats given carbon tetrachloride. Gerontologia. 1969;15(1):7–13. doi: 10.1159/000211670. [DOI] [PubMed] [Google Scholar]
- 2.REDDY DG, KRISHNAMURTHY KR, BHASKAR GR. Carbon tetrachloride cirrhosis in rats. 1. The influence of age, sex, and gonadectomy; 2. The changes in the livers of the fetuses born to mothers exposed to carbon tetrachloride. Archives of Pathology & Laboratory Medicine. 1962;74:73–80. [PubMed] [Google Scholar]
- 3.KITANI K. Aging and the liver: functional aspects. Archives of Gerontology and Geriatrics. 1994;19(2):145–58. doi: 10.1016/0167-4943(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 4.SANZ N, DIEZ FC, ALVAREZ A, CASCALES M. Age-dependent modifications in rat hepatocyte antioxidant defense systems. Journal of Hepatology. 1997;27(3):525–34. doi: 10.1016/s0168-8278(97)80358-3. [DOI] [PubMed] [Google Scholar]
- 5.SANZ N, DIEZ FC, ALVAREZ AM, FERNANDEZ SL, CASCALES M. Age-related changes on parameters of experimentally-induced liver injury and regeneration. Toxicology & Applied Pharmacology. 1999;154(1):40–9. doi: 10.1006/taap.1998.8541. [DOI] [PubMed] [Google Scholar]
- 6.SANZ N, DIEZ FC, CASCALES M. Aging delays the post-necrotic restoration of liver function. Biofactors. 1998;8(1–2):103–9. doi: 10.1002/biof.5520080118. [DOI] [PubMed] [Google Scholar]
- 7.GIAVAROTTI L, D’ALMEIDA V, GIAVAROTTI KA, AZZALIS LA, RODRIGUES L, CRAVERO AA, et al. Liver necrosis induced by acute intraperitoneal ethanol administration in aged rats. Free Radical Research. 2002;36(3):269–75. doi: 10.1080/10715760290019282. [DOI] [PubMed] [Google Scholar]
- 8.DANENBERG HD, FISHBEIN I, GAO J, MONKKONEN J, REICH R, GATI I, et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106(5):599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- 9.DUFFIELD JS, FORBES SJ, CONSTANDINOU CM, CLAY S, PARTOLINA M, VUTHOORI S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. Journal of Clinical Investigation. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LEIBOVICH SJ, ROSS R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. American Journal of Pathology. 1975;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- 11.MARIANI TJ, SANDEFUR S, ROBY JD, PIERCE RA. Collagenase-3 induction in rat lung fibroblasts requires the combined effects of tumor necrosis factor-alpha and 12-lipoxygenase metabolites: a model of macrophage-induced, fibroblast-driven extracellular matrix remodeling during inflammatory lung injury. Molecular Biology of the Cell. 1998;9(6):1411–24. doi: 10.1091/mbc.9.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ZHANG Y, MCCLUSKEY K, FUJII K, WAHL LM. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. Journal of Immunology. 1998;161(6):3071–6. [PubMed] [Google Scholar]
- 13.JAMALL IS, FINELLI VN, QUE HEESS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Analytical Biochemistry. 1981;112(1):70–5. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 14.REJ R. Aminotransferases in disease. Clinics in Laboratory Medicine. 1989;9(4):667–87. [PubMed] [Google Scholar]
- 15.JUNQUEIRA LC, BIGNOLAS G, BRENTANI RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochemical Journal. 1979;11(4):447–55. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 16.ROCKEY DC, CHUNG JJ. Endothelin antagonism in experimental hepatic fibrosis. Implications for endothelin in the pathogenesis of wound healing. J Clin Invest. 1996;98(6):1381–8. doi: 10.1172/JCI118925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WINWOOD PJ, SCHUPPAN D, IREDALE JP, KAWSER CA, DOCHERTY AJ, ARTHUR MJ. Kupffer cell-derived 95-kd type IV collagenase/gelatinase B: characterization and expression in cultured cells. Hepatology. 1995;22(1):304–15. [PubMed] [Google Scholar]
- 18.SHI Z, WAKIL AE, ROCKEY DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10663–8. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ZHANG-HOOVER J, SUTTON A, VAN ROOIJEN N, STEIN-STREILEIN J. A critical role for alveolar macrophages in elicitation of pulmonary immune fibrosis. Immunology. 2000;101(4):501–11. doi: 10.1046/j.1365-2567.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NOVOBRANTSEVA TI, MAJEAU GR, AMATUCCI A, KOGAN S, BRENNER I, CASOLA S, et al. Attenuated liver fibrosis in the absence of B cells. Journal of Clinical Investigation. 2005;115(11):3072–82. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SAFADI R, OHTA M, ALVAREZ CE, FIEL MI, BANSAL M, MEHAL WZ, et al. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127(3):870–82. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 22.WYNN TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nature Reviews Immunology. 2004;4(8):583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.JEONG WI, PARK O, GAO B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134(1):248–58. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CONNOLLY MK, BEDROSIAN AS, MALLEN-ST CLAIR J, MITCHELL AP, IBRAHIM J, STROUD A, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J Clin Invest. 2009;119(11):3213–25. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FRIEDMAN SL, ARTHUR MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. Journal of Clinical Investigation. 1989;84(6):1780–5. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PINZANI M, GESUALDO L, SABBAH GM, ABBOUD HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. JClinInvest. 1989;84:1786–93. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GRESSNER AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22(2 Suppl):28–36. [PubMed] [Google Scholar]
- 28.FISCHER R, CARIERS A, REINEHR R, HAUSSINGER D. Caspase 9-dependent killing of hepatic stellate cells by activated Kupffer cells. Gastroenterology. 2002;123(3):845–61. doi: 10.1053/gast.2002.35384. [DOI] [PubMed] [Google Scholar]
- 29.SONG E, OUYANG N, HORBELT M, ANTUS B, WANG M, EXTON MS. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cellular Immunology. 2000;204(1):19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- 30.ARTHUR MJ, MANN DA, IREDALE JP. Tissue inhibitors of metalloproteinases, hepatic stellate cells and liver fibrosis. Journal of Gastroenterology & Hepatology. 1998;13 (Suppl):S33–8. doi: 10.1111/jgh.1998.13.s1.33. [DOI] [PubMed] [Google Scholar]
- 31.POYNARD T, RATZIU V, CHARLOTTE F, GOODMAN Z, MCHUTCHISON J, ALBRECHT J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. Journal of Hepatology. 2001;34(5):730–9. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 32.SERRA MA, RODRIGUEZ F, DEL OLMOJA, ESCUDERO A, RODRIGO JM. Influence of age and date of infection on distribution of hepatitis C virus genotypes and fibrosis stage. Journal of Viral Hepatitis. 2003;10(3):183–8. doi: 10.1046/j.1365-2893.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- 33.WALI M, HARRISON RF, GOW PJ, MUTIMER D. Advancing donor liver age and rapid fibrosis progression following transplantation for hepatitis C. Gut. 2002;51(2):248–52. doi: 10.1136/gut.51.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.BERENGUER M, PRIETO M, SAN JUANF, RAYON JM, MARTINEZ F, CARRASCO D, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients.[Erratum appears in Hepatology. 2003 Feb;37(2):489] Hepatology. 2002;36(1):202–10. doi: 10.1053/jhep.2002.33993. [DOI] [PubMed] [Google Scholar]
- 35.RUSSO MW, GALANKO JA, ZACKS SL, BEAVERS KL, FRIED MW, SHRESTHA R. Impact of donor age and year of transplant on graft survival in liver transplant recipients with chronic hepatitis C. American Journal of Transplantation. 2004;4(7):1133–8. doi: 10.1111/j.1600-6143.2004.00470.x. [DOI] [PubMed] [Google Scholar]
- 36.MACHICAO VI, BONATTI H, KRISHNA M, AQEL BA, LUKENS FJ, NGUYEN JH, et al. Donor age affects fibrosis progression and graft survival after liver transplantation for hepatitis C. Transplantation. 2004;77(1):84–92. doi: 10.1097/01.TP.0000095896.07048.BB. [DOI] [PubMed] [Google Scholar]
- 37.ZIMMERMANN HW, SEIDLER S, NATTERMANN J, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5(6):e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LIASKOU E, ZIMMERMANN HW, LI KK, et al. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology. 2013;57(1):385–98. doi: 10.1002/hep.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.RAMACHANDRAN P, IREDALE JP. Macrophages: central regulators of hepatic fibrogenesis and fibrosis resolution. Journal of hepatology. 2012;56(6):1417–9. doi: 10.1016/j.jhep.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 40.POPOV Y, SVERDLOV DY, BHASKAR KR, et al. Macrophage-mediated phagocytosis of apoptotic cholangiocytes contributes to reversal of experimental biliary fibrosis. American journal of physiology Gastrointestinal and liver physiology. 2010;298(3):G323–34. doi: 10.1152/ajpgi.00394.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.GADD VL, MELINO M, ROY S, et al. Portal, but not lobular, macrophages express matrix metalloproteinase-9: association with the ductular reaction and fibrosis in chronic hepatitis C. Liver international : official journal of the International Association for the Study of the Liver. 2013;33(4):569–79. doi: 10.1111/liv.12050. [DOI] [PubMed] [Google Scholar]
- 42.SUH YG, KIM JK, BYUN JS, et al. CD11b(+) Gr1(+) bone marrow cells ameliorate liver fibrosis by producing interleukin-10 in mice. Hepatology. 2012;56(5):1902–12. doi: 10.1002/hep.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]