Abstract
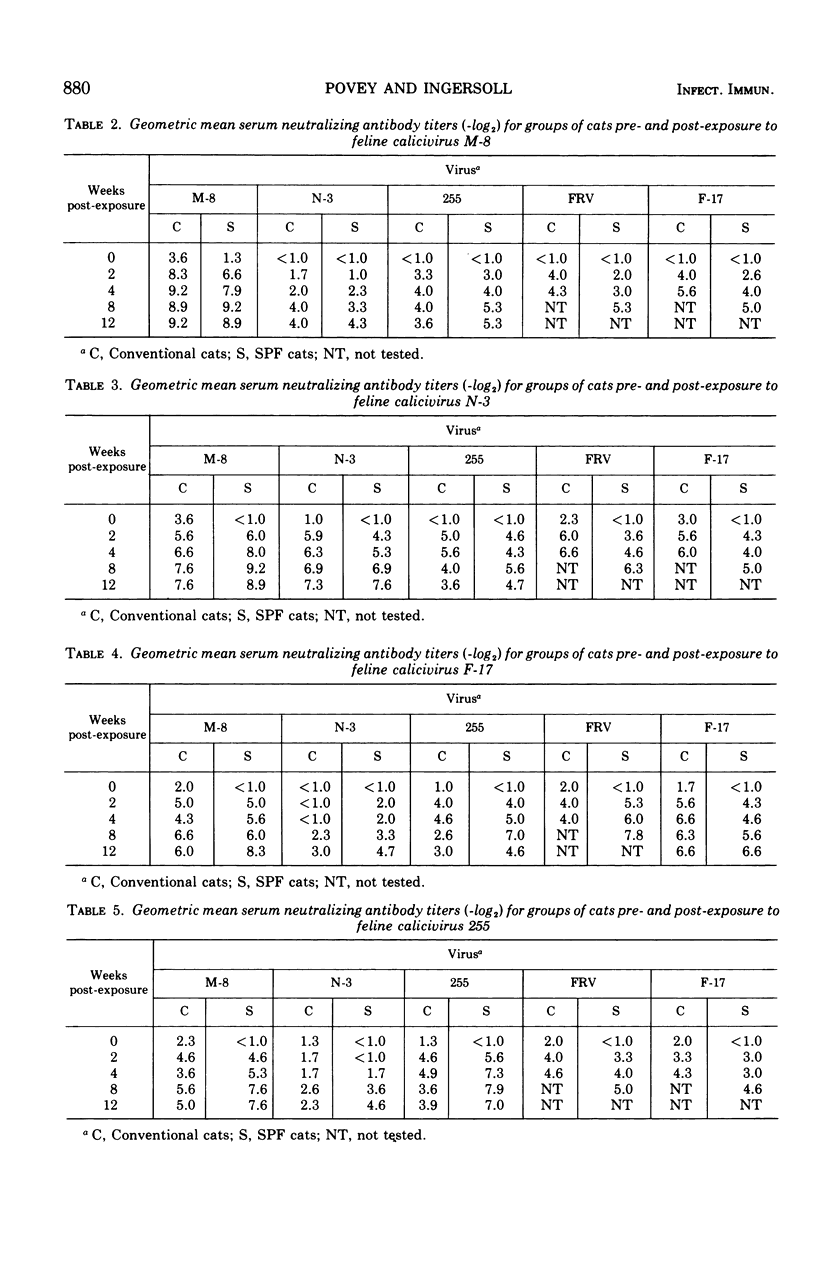
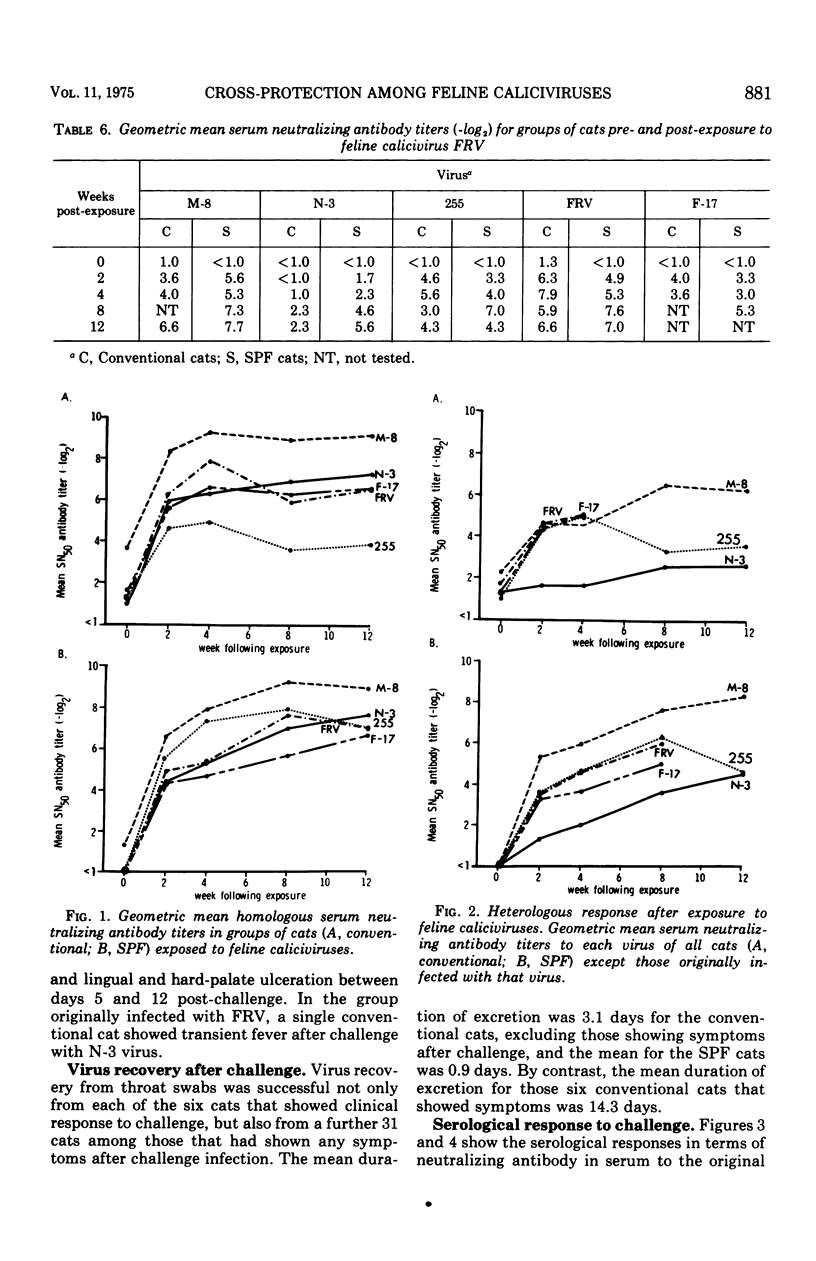
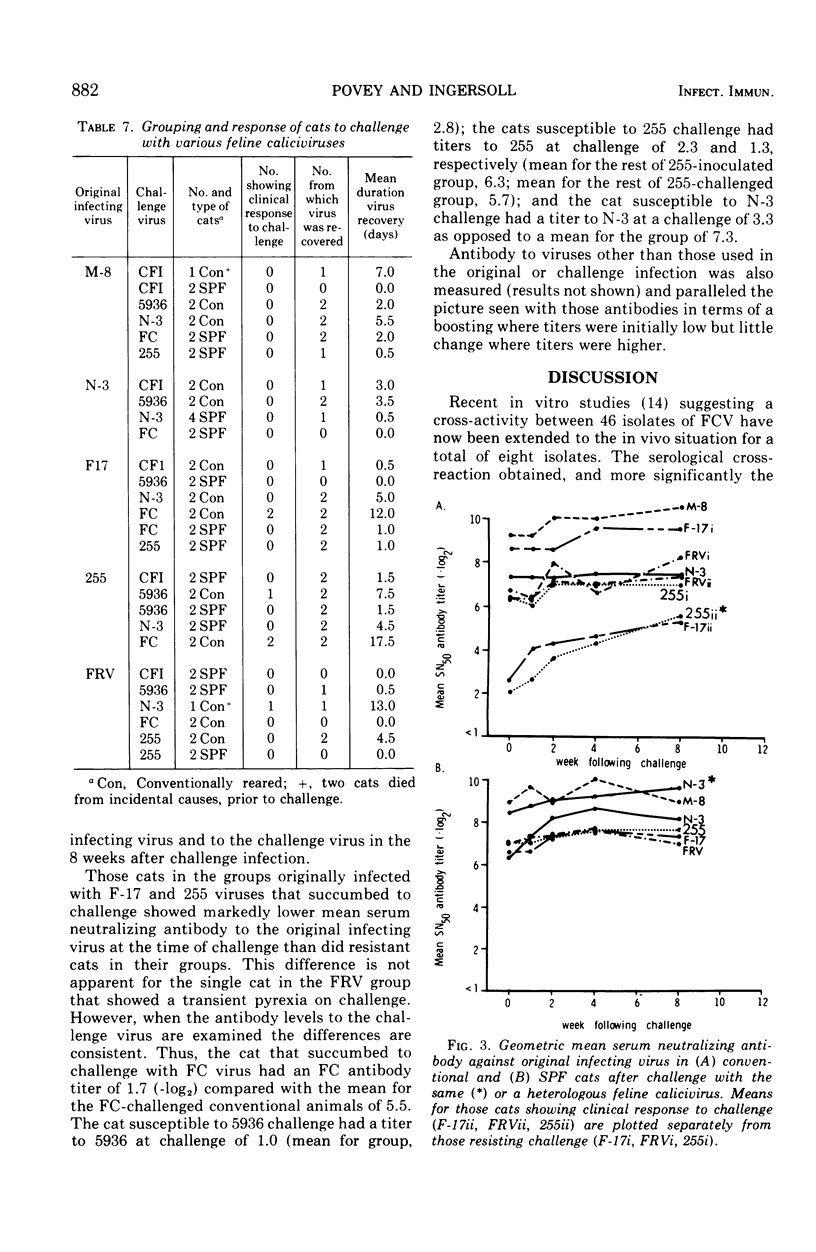
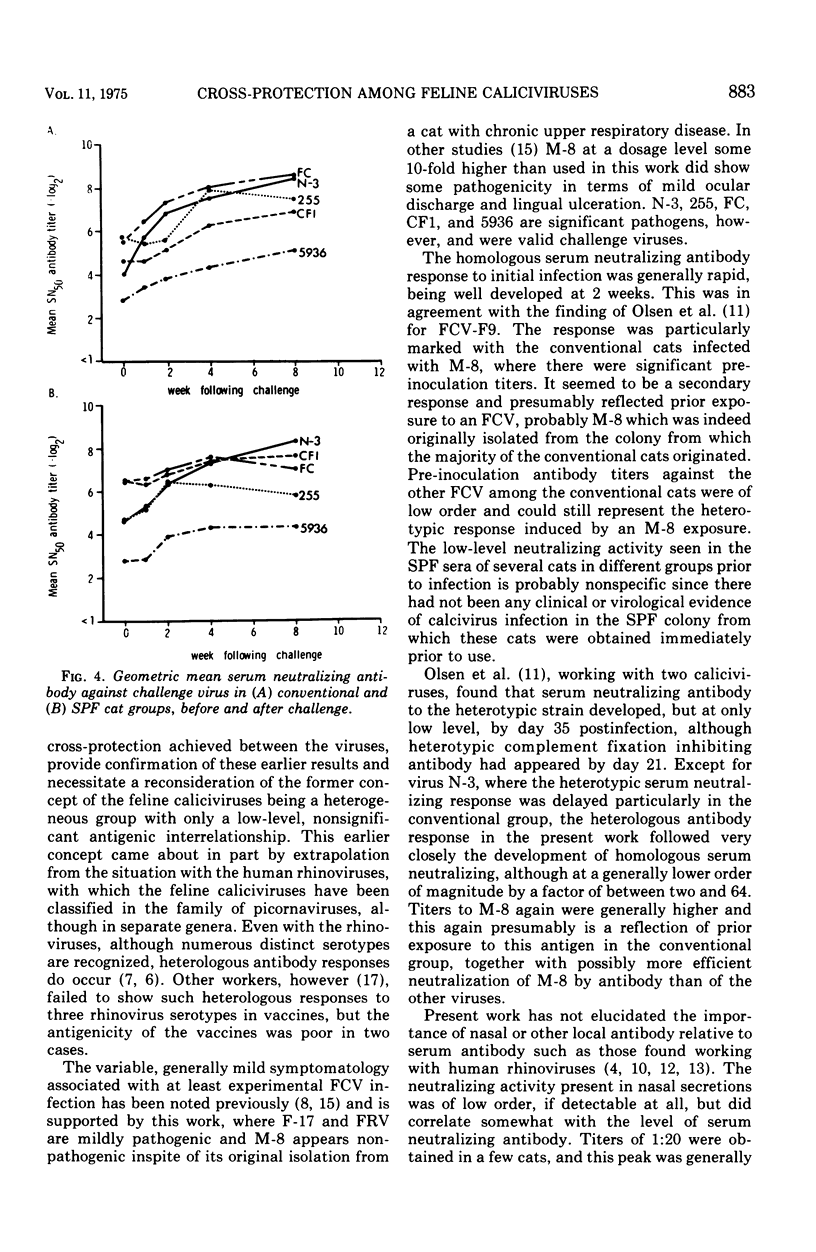
Each of five groups of specific-pathogen-free and conventionally reared cats was infected with a different strain of feline calicivirus. Two of the strains were pathogenic, producing characteristically fever, depression, loss of appetite, buccal ulceration, and occasionally increased ocular and nasal secretion. Two of the other strains were midly pathogenic and associated with fever or buccal ulceration or both; the fifth strain was nonpathogenic. The two pathogenic strains plus three others shown also to be pathogenic were used 3 months after the initial infection to challenge the cats in rearranged groupings. Of the 28 conventional cats challenged six (21.4%) showed at least a febrile response, although none of the 30 specific-pathogen-free cats showed any clinical signs. After challenge, virus was recovered from throat swabs of 37 or the 58 cats (63.8%) including the six which showed symptoms, but the duration of the excretion of virus was significantly less than that seen with the initial infection. The homologous and heterotypic antibody responses correlated well with the clinical protection, or lack of it, seen on challenge. The results provide further evidence for significant cross-relationships between feline caliciviruses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew P. T., Gillespie J. H. Feline viruses. I. Characterization of four isolates and their effect on young kittens. Cornell Vet. 1968 Apr;58(2):248–265. [PubMed] [Google Scholar]
- Buscho R. F., Perkins J. C., Knopf H. L., Kapikian A. Z., Chanock R. M. Further characterization of the local respiratory tract antibody response induced by intranasal instillation of inactivated rhinovirus 13 vaccine. J Immunol. 1972 Jan;108(1):169–177. [PubMed] [Google Scholar]
- Bürki F. Picornaviruses of cats. Arch Gesamte Virusforsch. 1965;15(5):690–696. doi: 10.1007/BF01245215. [DOI] [PubMed] [Google Scholar]
- Crandell R. A. A description of eight feline picornaviruses and an attempt to classify them. Proc Soc Exp Biol Med. 1967 Oct;126(1):240–245. doi: 10.3181/00379727-126-32412. [DOI] [PubMed] [Google Scholar]
- Fleet W. F., Douglas R. G., Jr, Cate T. R., Couch R. B. Antibody to rhinovirus in human sera. II. Heterotypic responses. Proc Soc Exp Biol Med. 1968 Feb;127(2):503–509. doi: 10.3181/00379727-127-32725. [DOI] [PubMed] [Google Scholar]
- HAMRE D., CONNELLY A. P., Jr, PROCKNOW J. J. VIROLOGIC STUDIES OF ACUTE RESPIRATORY DISEASE IN YOUNG ADULTS. 3. SOME BIOLOGIC AND SEROLOGIC CHARACTERISTICS OF SEVENTEEN RHINOVIRUS SEROTYPES ISOLATED OCTOBER, 1960, TO JUNE, 1961. J Lab Clin Med. 1964 Sep;64:450–460. [PubMed] [Google Scholar]
- Hoover E. A., Kahn D. E. Lesions produced by feline picornaviruses of different virulence in pathogen-free cats. Vet Pathol. 1973;10(4):307–322. doi: 10.1177/030098587301000404. [DOI] [PubMed] [Google Scholar]
- Kahn D. E., Gillespie J. H. Feline viruses. X. Characterization of a newly-isolated picornavirus causing interstitial pneumonia and ulcerative stomatitis in the domestic cat. Cornell Vet. 1970 Oct;60(4):669–683. [PubMed] [Google Scholar]
- Knopf H. L., Perkins J. C., Bertran D. M., Kapikian A. Z., Chanock R. M. Analysis of the neutralizing activity in nasal wash and serum following intranasal vaccinaion with inactivated type 13 rhinovirus. J Immunol. 1970 Mar;104(3):566–573. [PubMed] [Google Scholar]
- Olsen R. G., Kahn D. E., Hoover E. A., Saxe N. J., Yohn D. S. Differences in acute and convalescent-phase antibodies of cats infected with feline picornaviruses. Infect Immun. 1974 Aug;10(2):375–380. doi: 10.1128/iai.10.2.375-380.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J. C., Tucker D. N., Knope H. L., Wenzel R. P., Hornick R. B., Kapikian A. Z., Chanock R. M. Evidence for protective effect of an inactivated rhinovirus vaccine administered by the nasal route. Am J Epidemiol. 1969 Oct;90(4):319–326. doi: 10.1093/oxfordjournals.aje.a121076. [DOI] [PubMed] [Google Scholar]
- Perkins J. C., Tucker D. N., Knopf H. L., Wenzel R. P., Kapikian A. Z., Chanock R. M. Comparison of protective effect of neutralizing antibody in serum and nasal secretions in experimental rhinovirus type 13 illness. Am J Epidemiol. 1969 Dec;90(6):519–526. doi: 10.1093/oxfordjournals.aje.a121098. [DOI] [PubMed] [Google Scholar]
- Povey R. C., Hale C. J. Experimental infections with feline caliciviruses (picornaviruses) in specific-pathogen-free kittens. J Comp Pathol. 1974 Apr;84(2):245–256. doi: 10.1016/0021-9975(74)90065-6. [DOI] [PubMed] [Google Scholar]
- Povey R. C. Serological relationships among feline caliciviruses. Infect Immun. 1974 Dec;10(6):1307–1314. doi: 10.1128/iai.10.6.1307-1314.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Draper C., Stons P. B., Tyrrell D. A. Absence of heterologous antibody responses in human volunteers after rhinovirus vaccination. (Brief report). Arch Gesamte Virusforsch. 1969;28(1):89–92. doi: 10.1007/BF01250850. [DOI] [PubMed] [Google Scholar]


